Brain metastases are the most common intracranial neoplasm in adults. It is estimated that approximately 10% to 16% of patients with metastatic breast cancer will develop clinically evident brain metastases,1 while up to 30% of patients will have brain metastases identified at autopsy.2 Intraparenchymal metastases occur via hematogenous spread to the watershed area of the brain at the junction of the gray and white matter.3 The presence of metastases in the supratentorial and infratentorial compartments is in proportion to the relative weight and blood supply of these areas, with 85% occurring in the cerebral hemispheres, 10% to 15% in the cerebellum, and 1% to 3% in the brainstem.4 Leptomeningeal metastases are less common than intraparenchymal metastases and are found in 5% to 16% of patients at autopsy.1,2
Young age,5 estrogen receptor (ER)–negative status,6-8 the presence of pulmonary metastases,7 and HER-2 overexpression6,9 have all been described as risk factors for the development of brain metastases in patients with metastatic breast cancer. With improvements in systemic therapies and overall survival of patients with metastatic disease, the incidence of brain metastases appears to have increased over the last several years.10 In particular, with the introduction of trastuzumab, a humanized monoclonal antibody that binds to the extracellular segment of the HER-2/neu (erbB2) receptor and does not penetrate the blood-brain barrier,11 a higher incidence of central nervous system (CNS) metastases has been observed in patients receiving trastuzumab for metastatic breast cancer, ranging from 28% to 43%.9 This observation suggests that the incidence of CNS metastases may be higher in patients with HER-2-positive metastatic breast cancer whose systemic disease is well controlled with this newer agent.
While some patients may be asymptomatic, the most common presenting symptom is headache, which can occur in 24% to 48% of patients.12,13 Other presenting signs and symptoms include nausea/vomiting (4%), gait ataxia (7%), focal weakness (20%), seizures (12%), speech difficulty and/or visual changes (5%), or impaired cognitive function (14% to 34%).12,14,15 Symptoms can be a result of the metastatic tumor itself, depending on the location, or from surrounding vasogenic edema. Cranial neuropathies suggest leptomeningeal involvement or invasion into the cavernous sinus.
Although the clinical suspicion of new onset brain metastases is higher in patients with known metastatic breast cancer, any patient with a history of breast cancer who presents with such signs or symptoms should undergo additional evaluation with a thorough neurologic examination and diagnostic imaging.
Gadolinium-enhanced brain magnetic resonance imaging (MRI) is the best diagnostic test for brain metastases as it has been shown to be more sensitive than contrast-enhanced computed tomography (CT), particularly with respect to identifying lesions less than 10 mm and lesions in the posterior fossa.16 Most brain metastases appear hypointense to isointense with respect to gray matter on T1-weighted images and enhance in a solid or ring-like pattern following administration of intravenous gadolinium contrast. On T2-weighted images, metastatic lesions may appear relatively hypointense to normal brain while cystic or necrotic areas or surrounding vasogenic edema are hyperintense (Fig. 97-1).17
Figure 97-1
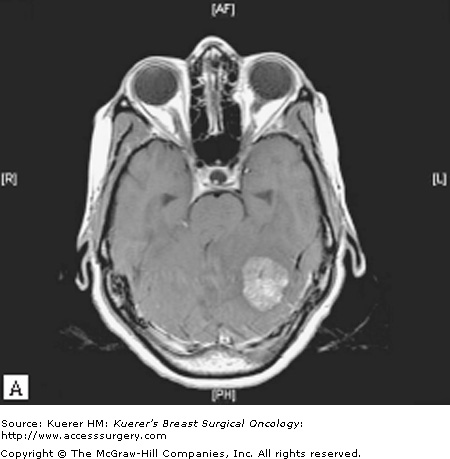
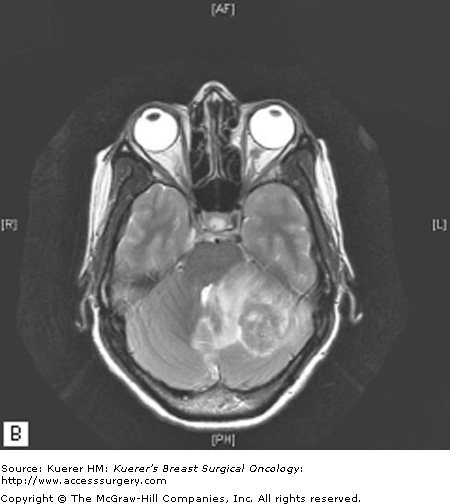
A. Axial T1-weighted MRI showing a heterogeneously enhancing left cerebellar hemisphere mass, which exerts significant mass effect with downward tonsillar herniation and obstructive hydrocephalus. B. Axial T2-weighted MRI showing surrounding vasogenic edema. This patient underwent surgical resection followed by postoperative radiotherapy.
A diagnosis of breast cancer metastatic to the brain cannot be made solely on the acquired imaging studies, although the diagnosis is likelier in the setting of known metastatic breast cancer or if there are multiple enhancing lesions.18 In a patient with a history of breast cancer and no evidence of distant metastatic disease, a neurosurgical biopsy would be appropriate to establish the diagnosis.
Investigators from the Radiation Therapy Oncology Group (RTOG) have defined 3 prognostic groups based on a recursive partitioning analysis of various prognostic factors.19 Of 1200 patients with brain metastases from a variety of solid tumors, patients less than 65 years of age with a Karnofsky Performance Status (KPS) greater than 70 who have controlled primary tumor without evidence of extracranial disease had a median survival of 7.1 months after whole-brain radiotherapy. Patients with a KPS less than 70 had a median survival of only 2.3 months. All other patients had a median survival of 4.2 months. This prognostic index was validated using a phase III trial, confirming that age, performance status, status of the primary tumor, and the extent of metastatic disease are valid prognostic factors that can help guide treatment decisions.20
With more recent data demonstrating that the number of brain metastases is prognostic, a new index, the Graded Prognostic Assessment (GPA), has been developed to reflect age, KPS, the presence or absence of extracranial metastases, and the number of brain metastases.21 Additional predictors of survival in patients with brain metastases include longer disease-free interval22 and HER-2 amplification.23
An initial bolus of corticosteroids followed by a maintenance dose of 4 mg every 6 hours can help mitigate symptoms within several hours, particularly if there is a significant component of vasogenic edema as noted on the T2-weighted images. Because of significant side effects associated with corticosteroids, such as mood changes, insomnia, elevated blood sugar, weight gain, gastrointestinal bleeding, and increased susceptibility to infections,24 tapering of the steroid dose is often recommended during definitive treatment.
The incidence of seizure is influenced by the location of the metastatic lesion, with patients having a higher incidence when lesions are located in the parietal, frontal, or temporal lobes and lowest in the occipital lobe.25 Patients who present with seizure have an approximately 70% chance of having recurrent seizures, and antiepileptic drugs (AEDs) are often prescribed.25 The choice of AED in patients who will be undergoing radiotherapy is important, as there have been several reports of patients developing Stevens-Johnson syndrome while on phenytoin during whole-brain radiotherapy.26 In patients with metastatic brain lesions who have not experienced a seizure, AEDs are not effective in preventing first seizures. The prophylactic use of AEDs, therefore, should not be used routinely in such patients given the lack of efficacy and potential side effects.27
For patients with a single metastasis, complete surgical resection followed by whole-brain radiotherapy (WBRT) has been shown in a randomized trial to improve local control and overall survival compared to a needle biopsy plus radiotherapy.28 Randomized trials have also shown that combined modality therapy, complete surgical resection followed by whole-brain radiotherapy, improves local control compared to surgery alone (local recurrence of 10% vs 46%, p < .001)29 and improves survival compared to WBRT alone for patients with a single brain metastasis (median survival of 10 months vs 6 months, p = .04).30 It is therefore important to obtain a brain MRI for complete evaluation to determine whether or not a patient has a single metastasis or multiple metastases, as small lesions may otherwise be missed on a contrast-enhanced CT. The current accepted standard of care for patients with a single metastasis who are deemed appropriate surgical candidates with a good performance status and controlled extracranial disease is complete surgical resection followed by WBRT.
The role of surgery in patients with multiple brain metastases is not well established. While retrospective studies suggest improvement in outcome with surgery in patients with multiple metastases,31 there are no randomized data supporting combined modality therapy with surgery and WBRT over WBRT alone in these patients. Surgical resection is therefore limited to those patients with large lesions causing neurologic compromise secondary to mass effect or significant edema, or if a pathologic diagnosis is required.
Most chemotherapeutic agents have limited efficacy in the treatment of brain metastases because of the blood-brain barrier and resulting low CNS levels for agents delivered intravenously.32 As such, whole-brain radiotherapy is used to palliate symptoms and improve quality of life in patients with multiple brain metastases and, as previously mentioned, has been shown to decrease the rate of local recurrence in patients with a single brain metastasis after surgical resection.29 Multiple randomized trials have evaluated various treatment schedules, ranging from 10 gray (Gy) in a single fraction to 50 Gy in 20 fractions.33,34 While no study has demonstrated superior outcomes with one schedule over another, most patients in the United States receive 30 Gy in 10 daily fractions over 2 weeks, or 37.5 Gy in 15 daily fractions over 3 weeks.
Acute toxicities related to WBRT include skin erythema, hair loss, fatigue, nausea, and anorexia. Approximately 75% to 85% of patients with headaches or seizure activity will achieve some palliation of symptoms either during the treatment course or within 3 to 4 weeks posttreatment. Unfortunately, only 50% of patients with more severe neurologic signs or symptoms such as motor dysfunction will have a partial or complete response. Long-term toxicities of WBRT may include memory loss, lethargy, dementia, personality changes, and endocrine dysfunction.35
Stereotactic radiosurgery (SRS) refers to highly conformal, high-dose radiation that is delivered to a relatively small target using multiple beams with minimal exposure to normal tissues. This treatment was originally developed by a Swedish neurosurgeron, Lars Leksell, as a potential substitute for surgical resection.36 Several techniques are currently available for SRS: the Gamma Knife system (cobalt 60 source), linac (linear accelerator)-based radiosurgery, or the CyberKnife® Robotic Radiosurgery System (linear accelerator with an image-guided robotic system).
Although there are no randomized data comparing surgical resection of a single brain metastasis with SRS, the addition of SRS to WBRT had been shown to improve local control in patients with a single metastasis compared with WBRT alone.37,38 Given the concern about long-term neurocognitive dysfunction with the use of whole-brain radiotherapy, questions remain as to whether a focal therapy such as SRS could provide adequate initial treatment in patients with limited CNS disease and whether WBRT can be deferred without compromising outcomes. Several retrospective series found no difference in survival between patients treated with SRS alone and those treated with WBRT plus SRS,39,40 yet approximately 50% of patients treated with SRS alone developed additional brain metastases. The only published phase III trial investigating SRS plus WBRT versus SRS alone in 132 patients with limited (≤4) brain metastases reported no difference in overall survival between the 2 treatment groups (median survival of 7.5 months vs 8.0 months in the WBRT + SRS and SRS alone groups, respectively; p = .42).41 The 12-month rate of brain tumor recurrence, however, was significantly higher in the SRS-alone group (76.4%) compared with the WBRT + SRS group (46.8%; p < .001). Although intracranial relapse is more common when WBRT is omitted from the initial treatment in patients with ≤4 brain metastases, the use of up-front WBRT with SRS did not improve overall survival, suggesting that early detection of recurrence and early salvage with WBRT may prevent neurologic complications and neurologic death. The use of SRS alone without up-front WBRT is currently controversial, with some advocating that patients with multiple metastases should receive WBRT up front with SRS reserved for recurrences.42 Future studies are required before SRS alone is considered the standard of care in patients with limited brain metastases.
In patients with a single metastasis treated with surgical resection, some retrospective studies suggest that SRS alone to the postoperative bed without WBRT can provide local control in up to 79% of patients at 1 year posttreatment, and that WBRT can be reserved for salvage treatment.43
Side effects of SRS depend on the location of the treated metastasis but can include headaches, nausea, exacerbation of existing neurologic deficits, or seizures, particularly in patients with temporal lobe lesions. Doses usually range between 15 and 24 Gy, depending on the size of the lesion, with SRS limited to tumors less than 3 cm.44 With larger tumors, the risk of radiation necrosis with high-dose radiosurgery increases significantly and fractionated treatment should be considered.
Future areas of research for breast cancer patients will need to address possible CNS prophylactic strategies with perhaps new agents that have improved penetration into the CNS, particularly for patients with HER-2-positive breast cancer who appear to have a higher incidence of brain metastases since the introduction of trastuzumab.
In summary, breast cancer patients who present with persistent headaches or neurologic compromise should undergo brain MRI. If there is a single brain lesion that appears suspicious for metastatic disease, surgical resection should be considered if the patient is an appropriate surgical candidate with a good performance status and controlled systemic disease. Whole-brain radiotherapy should be administered adjuvantly based on phase III data demonstrating a reduction of local recurrence from 46% to 10% (p < .001) without a difference in median survival (43 weeks vs 48 weeks, p = .39).29 Some retrospective series support using SRS alone to the postoperative bed,43 but frequent monitoring would be necessary to detect recurrences early for salvage treatment. If a patient with a single metastasis is not deemed to be a good surgical candidate or the lesion is considered unresectable given its location in eloquent areas of the brain that may result in significant postoperative neurologic sequelae, WBRT should be used with consideration of an SRS boost. SRS alone in patients with a single metastasis is used in some institutions but is still considered an area of investigation.
In patients with multiple brain metastases, surgical resection is reserved for those situations in which a confirmation of diagnosis is required, such as the first presentation of metastatic disease, or for those patients who may have a dominant lesion causing significant neurologic dysfunction, midline shift, or herniation. WBRT should otherwise be given with consideration of an SRS boost if there are upto 4 lesions or larger lesions that may not be well controlled with WBRT alone. SRS alone in patients with upto 4 metastatic lesions is controversial. Although it is not currently considered standard treatment, it is used in some situations with the expectation that close follow-up is necessary and WBRT may need to be given as salvage therapy.
Due to its high vascularity, the choroid is the most common location of ocular metastases from breast cancer, followed by the orbit, iris, ciliary body, optic nerve, and eyelid.45 Autopsy studies estimate a 9% to 37% incidence of choroidal metastases from breast cancer upon death.46,47 A prospective screening program of 120 patients with known disseminated breast cancer found that 5% had asymptomatic choroidal disease.48 The incidence of symptomatic metastases, however, is lower (1% to 2%).49 Breast cancer patients with metastatic disease to more than 1 organ and involvement of lung and brain are at higher risk of developing choroidal metastases.48 Although most patients presenting with choroidal lesions have known metastatic cancer, it has been reported to be the initial manifestation of breast cancer in 4% to 46% of patients or the initial site of metastatic disease in 12% to 31% of patients.48,50
The most common symptom is blurred vision, occurring in 75% to 91% of patients. Other presenting symptoms include visual field defects (22%), photopsia (15%), floaters (6%), or pain (1%).45,51 Choroidal metastases are often unilateral with an equal distribution between the right and left eyes, but can be bilateral in 30% to 40% of patients with multifocality noted in 32% to 50%.48,51
Patients with a history of breast cancer or known metastatic cancer who develop visual symptoms should be referred for a thorough ophthalmologic examination. Based on a screening program that detected asymptomatic lesions in 5% of patients with disseminated breast cancer, some clinicians recommend screening for high-risk patients.48 A-scan ultrasonography will reveal medium to medium-high internal reflectivity while B-scan ultrasonography will show acoustic solidity. Choroidal metastases usually appear as yellow, spotted subretinal lesions often with secondary retinal detachment (Fig. 97-2).48,51 A biopsy is considered only in patients with no detectable primary or other sites of metastatic cancer.52 Because the incidence of CNS disease increases after the development of ocular metastasis,45 a brain MRI should be obtained to determine whether there is any evidence of CNS involvement.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree