Radiation therapy is frequently employed in treating breast cancer and is thus available in virtually all radiation centers. Given the commonality of breast cancer across the world, management of this disease occupies a large volume of patient care in a radiation oncology clinic. As science advances and innovative approaches are validated in the literature, breast cancer management in the modern era has become more complex and specialized. A multidisciplinary approach employing input from a diverse group of specialties bringing their knowledge base to the table contributes to an elevated standard of care.
In our institution, multidisciplinary care is practiced in two main forums: (1) multidisciplinary cancer conferences in which new cancer cases are presented in a prospective fashion seeking input on management across disciplines, and (2) multidisciplinary clinics in which a newly diagnosed patient meets with their cancer team of physicians and healthcare extenders in a single setting to provide a streamlined recommendation. Both approaches work to provide the patient with a personalized care plan and a consensus recommendation. In both settings, the current breast cancer management guidelines from the National Comprehensive Cancer Network are discussed as validation for the treatment plan and patients are screened for potential eligibility into available clinical research trials, which may also provide an alternative treatment pathway. We also have standardized treatment pathways among our group to ensure that we are providing consistent care to patients across our 11 sites of care, and accommodate innovation with rapid introduction across our system.
Radiation Therapy Background
Radiation is a natural phenomenon present throughout the universe in the form of electromagnetic radiation and particles emitted from radioactive decay. Naturally occurring radiation was first described by Becquerel in 1896. The first described human-made radiation was announced by William Roentgen in 1895. Marie and Pierre Curie, co-discovering both radium and polonium as natural radioactive elements, shared the 1903 Nobel Prize with Henri Becquerel. Radioactivity measurements have been described using the Becquerel and Curie in their honor. In 1920, the Curie Institute in Paris was formed for medical research. Ironically Curie died in 1934 at the age of 66 of probable aplastic anemia, likely a side effect of chronic radiation exposure. Roentgen, who initially discovered high-energy electromagnetic energy emanating from an electrified vacuum tube, coined the discovery as “X-rays” and won the Nobel Prize in physics in 1901. Roentgen is considered the father of radiology ( Fig. 8.1 ).

In the mid-1900s, cobalt-60 was used as a human-made radioactive source for delivery of radiation. However the penetrating energy of the cobalt-60 gamma rays was relatively low, thus limiting effective and safe radiation doses for deep-seated tumors in the chest, abdomen, and pelvis.
Brothers Russell and Sigurd Varian invented the linear accelerator in Palo Alto, California, which delivered higher penetrating radiation than cobalt therapy. Linear accelerators (LINACs) are used in virtually all cancer centers for treatment today and Varian remains an international leader in this technology. One current Varian model, the TrueBeam, has multiple features including beam shaping, arc and rotational radiation delivery, and built-in computed tomography (CT)-based imaging for radiation delivery ( Fig. 8.2 ). The system also allows for radiation delivery timed with respiratory gating, which is especially relevant to cancer treatment in the chest, breast, and abdominal areas.
Proton therapy has expanded in its use across the United States based on the physical feature that protons (positively charged particles) will deliver radiation treatment to a defined depth in tissue based on the selected beam energy (the Bragg Peak). Protons require significantly greater financial start-up investment, and for most nonpediatric applications have not proven clearly superior to high-energy photon (X-ray) treatment. Ongoing clinical trials will better define the role of proton therapy for more common cancers such as breast and prostate cancer.
Clinical radiation treatment requires the accurate measurement and delivery of radiation doses, which is defined by the term Gray (Gy) or centigray (cGy). Radiation delivery from an external body source is referred to as tele-therapy, whereas radiation delivery from an internalized source (using radioactive seeds, interstitial needles, or balloon) is referred to as brachytherapy.
It is the radiation oncologist who defines anatomical targets and avoidance tissue, typically from CT-based imaging, then defines fractional and total doses. A clinical dosimetrist is trained to incorporate the physician’s anatomic contouring into a treatment plan, and subsequently the medical physicist, specializing in radiation therapy, will review and approve the technical aspects of the plan. The radiation oncologist is ultimately responsible for supervising the patient’s treatment. The team approach for radiation delivery involves coordination and strict quality assurance.
Radiation Therapy Definitions
Radiation therapy energy produced by a linear accelerator will determine its effective penetration into tissue. Modern-day LINAC units typically deliver a maximum photon energy ranging between 6 and 20 million electron volts (MeV). In contrast, diagnostic X-ray imaging typically uses 50–150 kV energies, with this much lower energy range taking advantage of differences in X-ray interaction based on tissue density (i.e., bone versus soft tissue) by virtue of the photoelectric effect. LINAC treatment systems typically have a range of energies available for treatment, chosen during the treatment planning phase.
Modern LINACs shape the radiation field using either stationary multiple beams or dynamic moving beams, all preplanned using dedicated computer and software technology. Intensity-modulated radiation therapy (IMRT) refers to the shaping of radiation fields using dynamic field-shaping technology and requires greater planning and quality assurance but may deliver better avoidance of normal tissue with treatment. Three-dimensional radiation therapy uses stationary and well-defined radiation field shapes, may be most appropriate for palliative cases, and dosimetry may equal IMRT involving large field treatment.
Imaging on the linear accelerator is always incorporated during a treatment course, as it confirms setup accuracy and may include daily or weekly CT/kV/MV depending upon clinical indications. The imaging is not for diagnostic purposes but rather to confirm set up immediately prior to radiation treatment.
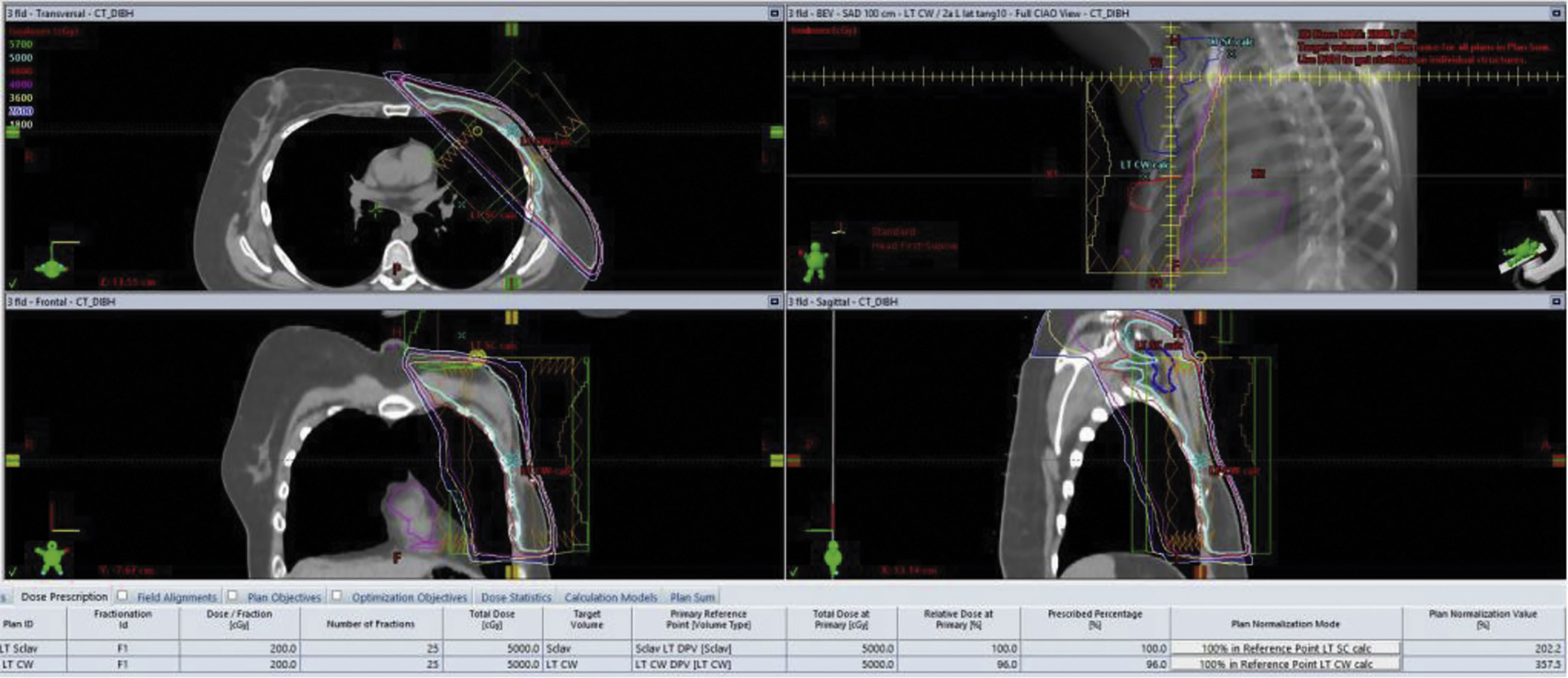
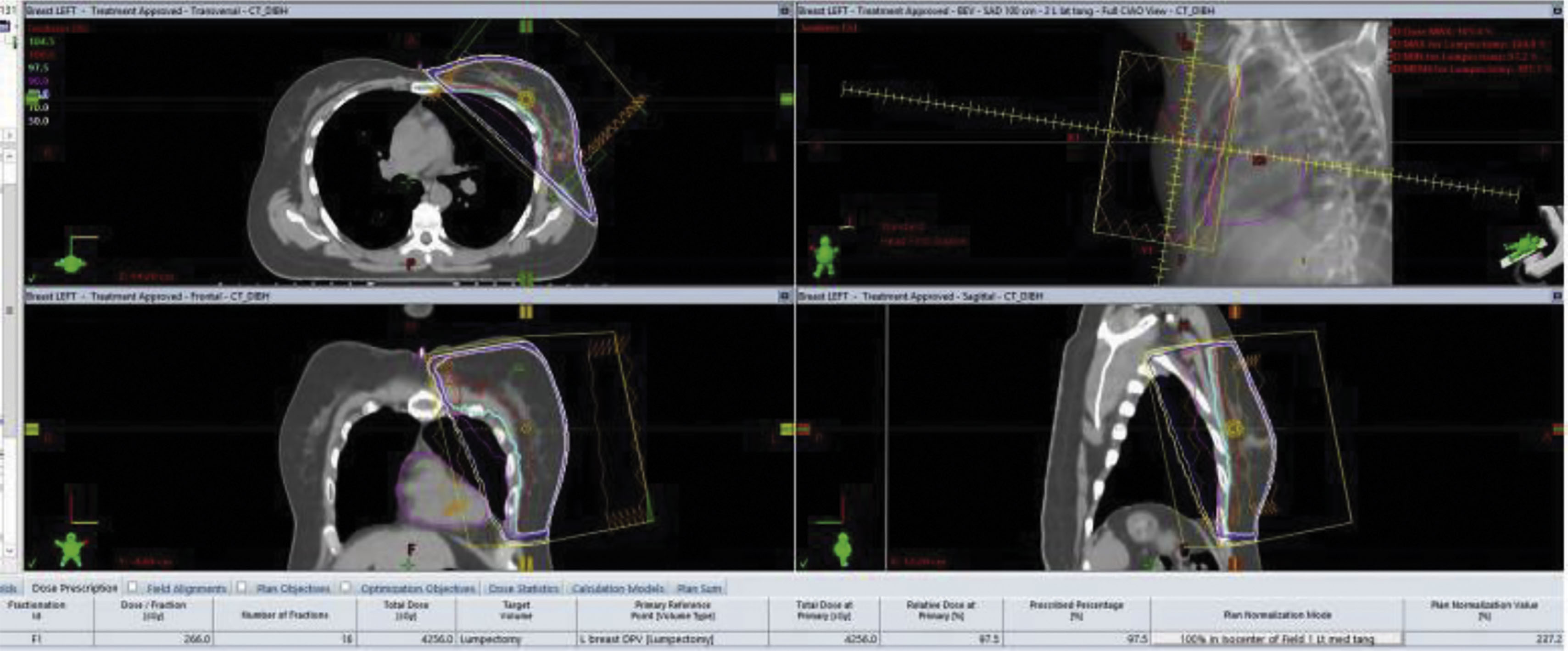
A radiation simulation is a setup session that precedes any radiation delivery and allows for the planning. Simulation typically occurs up to 1 week before treatment and may incorporate a CT scan for baseline imaging. In many clinical situations, a pre-existing positron emission tomography (PET) scan or magnetic resonance image (MRI) can be fused digitally with CT imaging to allow the radiation oncologist better definition of the treatment target and avoidance of normal tissue ( Fig. 8.3 ).
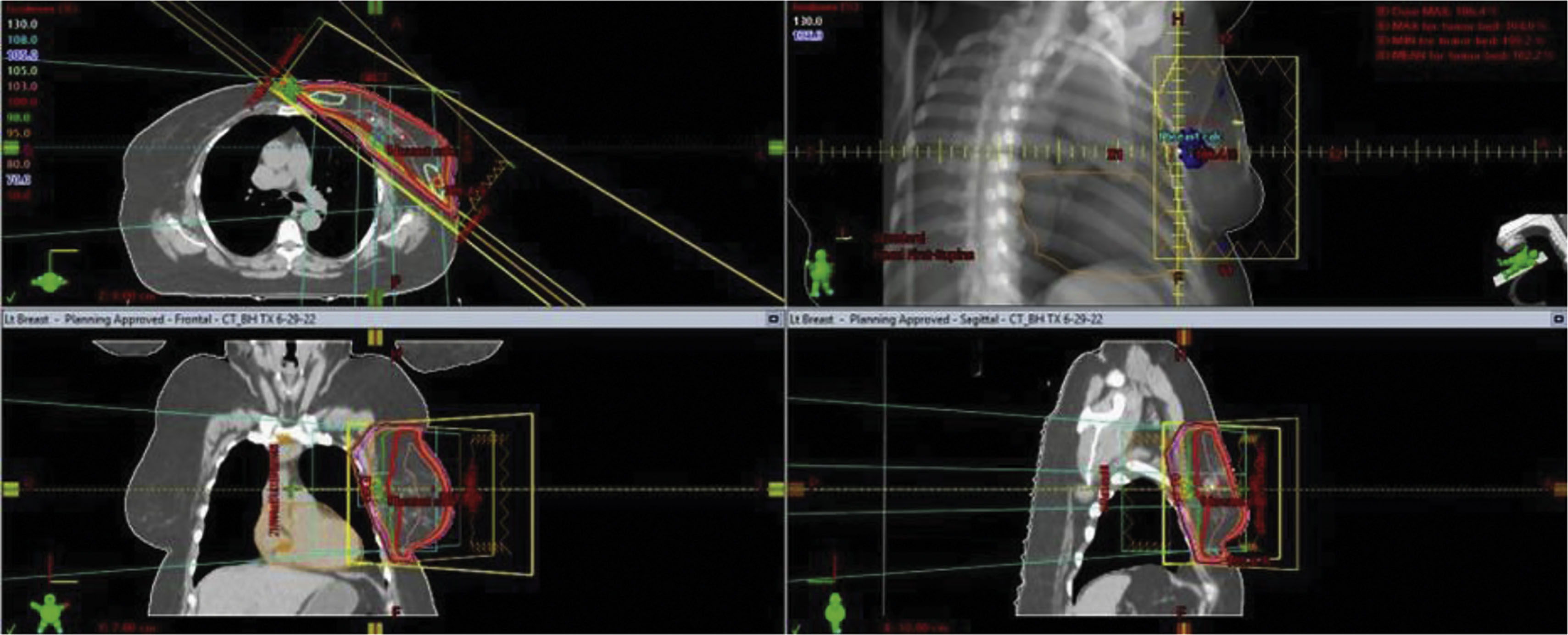
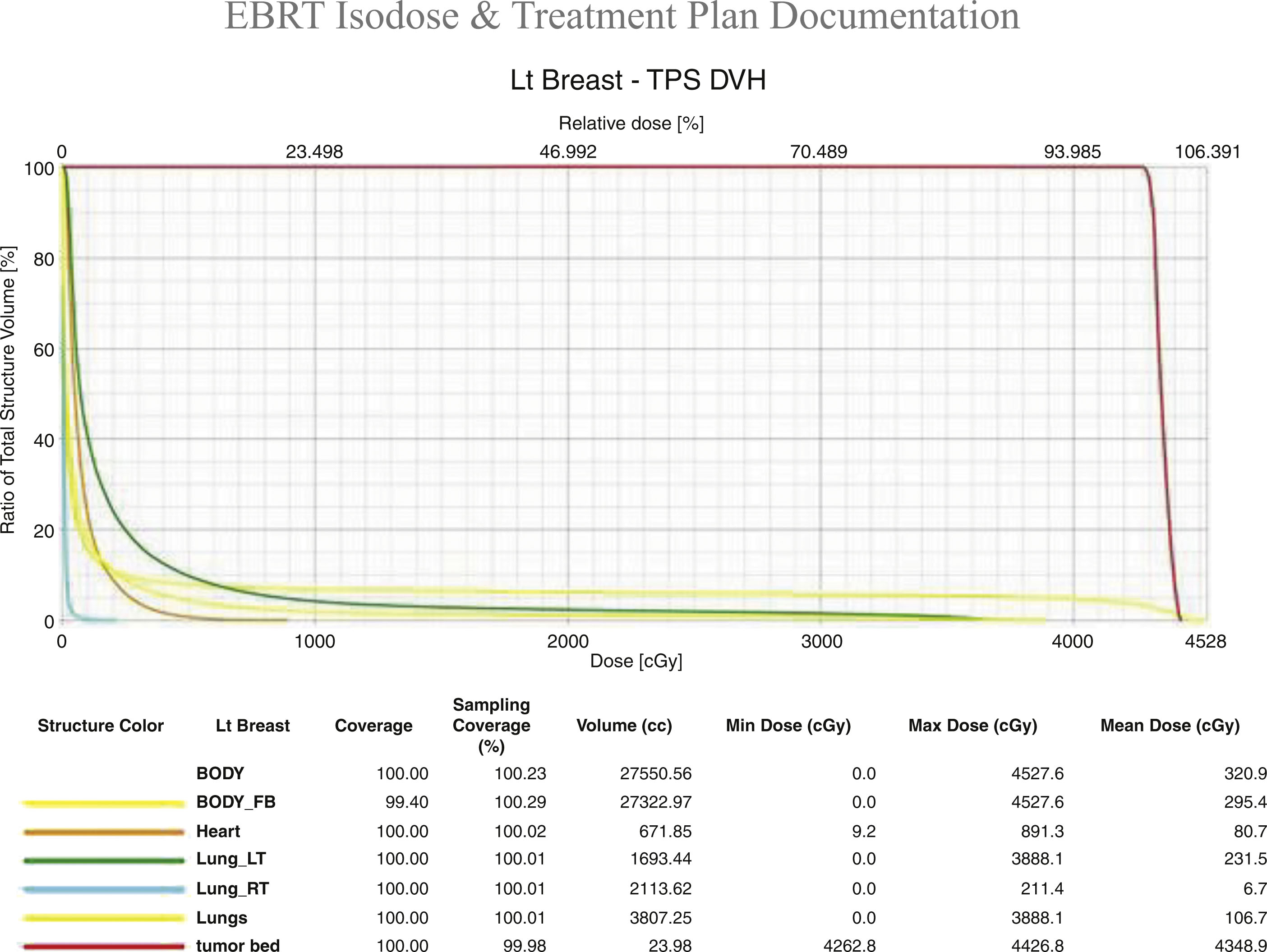
The radiation treatment course is defined not only by the target and avoidance of normal tissues but by the treatment dose per fraction of delivery and the total number of fractions ( Fig. 8.4 ). Both are important from a biological perspective and are defined by palliative versus curative, and preoperative or postoperative clinical situations. For example breast cancer treatment may be delivered using a 5 day per week 6-week course to a total maximum dose of 5000–6000 cGy. Alternatively, some courses can be completed in 16 fractions over just 3 weeks for a total dose of 4256 cGy. In some clinical situations the surgical bed requires additional radiation, possibly adding five more fractions to a dose of 1000 cGy. The radiation prescription is personalized to the patient’s situation, treatment intent, pathology, and according to the clinical trial protocol if enrolled.
Radiation Biology
The recognized target of radiation is the DNA within cells. Radiation can cause direct double-stranded breaks in DNA, resulting in eventual cell death. Alternatively, free radical formation (indirect effect) can produce permanent DNA damage and death. Free radical formation is enhanced by the presence of oxygen, meaning that hypoxic tumors are less effectively treated with radiation. Approximately 70% of radiation tumor kill is from the indirect effect.
Competent DNA repair is retained in most normal tissue but reduced in tumor cells, thus allowing for preferred tumor killing with radiation treatment. Radiation therapy tumor kill can be enhanced using concurrent chemotherapy as radiation sensitizers, and is commonly used in treating rectal cancer, lung cancer, and head neck cancer. Radiation sensitizers, however, are not typically used for treating breast cancer.
Different human tissues have different tolerances to radiation treatment. Rapidly growing tissue in the gastrointestinal system and head and neck mucosal surfaces respond briskly to radiation, whereas musculoskeletal tissue has minimal immediate effect. In addition, the total radiation dose to an organ is defined based on complication risks. Clinical studies and radiobiological models provide guidance for radiation oncology dose prescription.
Radiation therapy side effects are typically classified as acute or late. Acute side effects may include mucositis, dermatitis, nausea, and fatigue, dependent upon the target area, and are typically predictable with predictable recovery. Acute side effects are most often seen during radiation treatment. Late tissue toxicity may occur months to years after treatment, is limited to the tissue radiated, and may not be recognized, or alternatively can be quite severe. Examples would include secondary malignancies and normal tissue necrosis. Fortunately, with carefully prescribed radiation fields and dose delivery, late tissue effects are uncommon.
Assessing Patients Appropriate for Radiation Therapy
Radiation therapy may be indicated in the following clinical scenarios:
- 1.
As adjuvant treatment following breast-conserving surgery (BCS);
- 2.
As adjuvant treatment following mastectomy, when the patient has documented increased risk for local recurrence based on clinical/pathologic factors;
- 3.
For palliative inoperable disease with pain/bleeding control;
- 4.
Locally recurrent malignancy in a radiation-naïve patient or previously radiated patient.
A multidisciplinary discussion following the initial biopsy-confirmed breast cancer is our standard format prior to discussing treatment recommendations with the patient. For the radiation oncologist, pathologic confirmation of malignancy is required. Histological confirmation of invasive versus in situ disease is important as well as tumor receptor (Estrogen [ER]/Progesterone [PR]) findings and HER2 status. Cancer staging may also include CT or PET scan. Documentation of collagen vascular disease and autoimmune disease as well as any prior breast radiation may represent contraindications to radiation therapy. This latter information should be available prior to a decision regarding breast conservation as it may influence a preference for mastectomy.
In the early multidisciplinary evaluation discussion, an experienced breast surgeon should assess the likelihood of achieving negative margins following breast-conservation surgery. If multicentric disease is present or suspected by the radiologist, mastectomy may be the preferred surgical option. In addition cosmetic considerations following adequate surgical resection are important, and in some cases mastectomy with reconstruction provides a better cosmetic outcome than breast conservation with radiation.
A staging workup may include PET scan, brain MRIs, and so on when appropriate depending on the clinical stage. Oncotype DX and other predictive studies may be obtained and will determine whether adjuvant chemotherapy will be beneficial/indicated. Unless neoadjuvant chemotherapy (NACT) is indicated, adjuvant guidelines sequence chemotherapy following surgical recovery and prior to any radiation treatment. We currently recommend any adjuvant hormonal antiendocrine therapy (e.g., aromatase inhibitors, tamoxifen) to follow radiation treatment. In younger fertile patients pregnancy testing is virtually mandatory and, when positive, may represent a barrier for offering radiation therapy without some delay until the pregnancy has been completed.
Based on initial workup and pathology, the patient meets with the radiation oncologist to discuss the indication for adjuvant breast radiation therapy. In the situation of a postmenopausal/elderly patients with low-risk (ER-positive, node-negative, margin clear) disease, omitting radiation therapy may be considered if the patient is committed to the prescribed course of antiendocrine therapy. Technical decisions by the radiation oncologist include volume of tissue irradiated, i.e., partial breast versus whole breast, and treatment of axillary/supraclavicular/internal mammary nodes. We also advocate cardiac-sparing radiation techniques when treating the (typically left) breast, as discussed later in this chapter. The spectrum of treatment duration can range from 1 week to 6 weeks dependent on the volume of tissue encompassed and stage of disease
Consent is also important, with relevant treatment risks being discussed, including acute toxicity, late-term toxicity, cosmetic outcome, financial cost to the patient, and logistics.
Consultation by a radiation oncologist prior to definitive surgery is strongly encouraged to avoid confusion and confirm expectations of treatment duration. In our experience this early meeting with the patient enhances the patient’s experience.
We will lay out our typical treatment process based on multidisciplinary decision-making later in this chapter.
General Clinical Radiation Therapy Concepts
Pathology is very important to the radiation oncologist, as a malignant diagnosis is required for radiation treatment. Upfront imaging including MRI and mammograms may be useful for radiation treatment planning. A radiation oncologist should be involved in the initial management discussion of newly diagnosed breast cancer patients and may influence decisions regarding breast conservation versus mastectomy, as well as extent of axillary lymph node staging. After initial surgery, pathology is again reviewed, and in some cases reoperation may be required to achieve negative margins. Again, the radiation oncologist should provide input at this decision point. Surgical clips placed at the time of initial/subsequent surgery may help define an area for radiation boosting and should be included in the surgical technique whenever possible. Patients who develop large treatment bed seromas may require tapping, which may delay radiation planning. We typically initiate radiation treatment within 8 weeks of surgery when chemotherapy is not indicated, or within 1 year of surgery if adjuvant multicycle chemotherapy is delivered.
Radiation Side Effects Following Treatment of Breast Cancer
Radiation side effects from breast radiation are limited to the radiated tissues, which includes the targeted breast/chest wall, lymph nodes, underlying lung, and skin. Contralateral breast tissue may be partially in field as well. For left-sided tumors cardiac tissue/coronary arteries/pericardium may be in field, requiring additional radiation planning for avoidance. For some right-sided breast cancer patients, the right coronary artery may be at risk for radiation exposure, requiring assessment at the time of planning.
Acute side effects of breast radiation include dermatitis in the radiation field. Tanning may also be a sequelae of radiation treatment as well as telangiectasia. Fibrosis may occur, especially, but not limited to, the surgical bed, and systemic fatigue is a transient side effect, although not typically as pronounced as with chemotherapy. Radiation pneumonitis is uncommon and can occur when larger volumes of underlying lung are included within the radiation field. Current techniques that limit lung volume minimize this risk. Patients who present with a dry cough post radiation may require a course of steroids (usually prednisone) with taper for management.
Late tissue toxicity from radiation could include lymphedema of the ipsilateral arm, but is only typically seen following extensive lymph node surgery.
Secondary malignancies from radiation are well documented in the literature. Radiation-induced cancer risk for all sites ranges between 0.2% and 1% per year in cancer survivors after radiotherapy. A bimodal distribution of secondary malignancies is noted, with the first peak within 3 years of treatment (typically leukemia in those receiving chemotherapy). A second peak typically involves solid tumors and occurs more than a decade out.
An increased risk of secondary malignancy was reported in a meta-analysis involving more than 42,000 breast cancer patients (some treated with radiation and some not).
Radiation-induced sarcomas typically originate 5–20 years after radiation and are typically more aggressive than spontaneously occurring sarcomas.
Ironically young patients with Hodgkin’s disease who received thoracic radiation, and unavoidably had exposed breast tissue, are reportedly 75 times more likely to develop invasive breast cancer than the general population. These patients were typically young at the time of successful treatment, and their long life expectancy produced a long latency period for cancer development.
An excellent review article is referenced for a more comprehensive description of complications from chest radiation.
Radiation Therapy Technical Definitions
Radiation therapy is given using highly calibrated LINACs that produce high-energy X-rays (called photons) that can be directed to an anatomically defined area. Various technical radiation deliveries are available, including three-dimensional delivery or IMRT, the latter of which effectively “shapes” the radiation field. IMRT or three-dimensional surface compensation technique represents appropriate considerations when treating the left breast, as it allows for avoidance of cardiac tissue and minimizes the volume of lung exposure, and is often delivered in conjunction with breath hold by the patient and automated positioning by the machine, further enhancing accuracy and cardiac tissue avoidance.
LINACs also produce electron beams, which are charged particles that interact with tissue at very limited depths. Electron beam treatment is typically used for boosting small volumes of the surgical tumor bed (a “boost”). Radiation therapy is typically given 5 days per week, each treatment called a “fraction” and delivered over a typically 10-minute time frame per fraction. Prior to the start of radiation, a treatment planning session is carried out, called a “simulation,” which includes a noncontrast CT scan, and this is sometimes done using a motion-monitoring technique ( Figs. 8.5 and 8.6 ). Permanent markers may be used on the patient’s breast/chest wall called “tattoos” to allow for reproducible daily set up. In addition, a body cast or immobilization device is used at the time of simulation, which is used for daily treatment. Once the simulation is complete planning follows, allowing the radiation oncologist to define the overall target, tissue avoidance, boost volume, and technical treatment aspects. A dosimetry plan is subsequently generated and reviewed by the radiation oncologist and approved following a medical physicist’s final technical review.
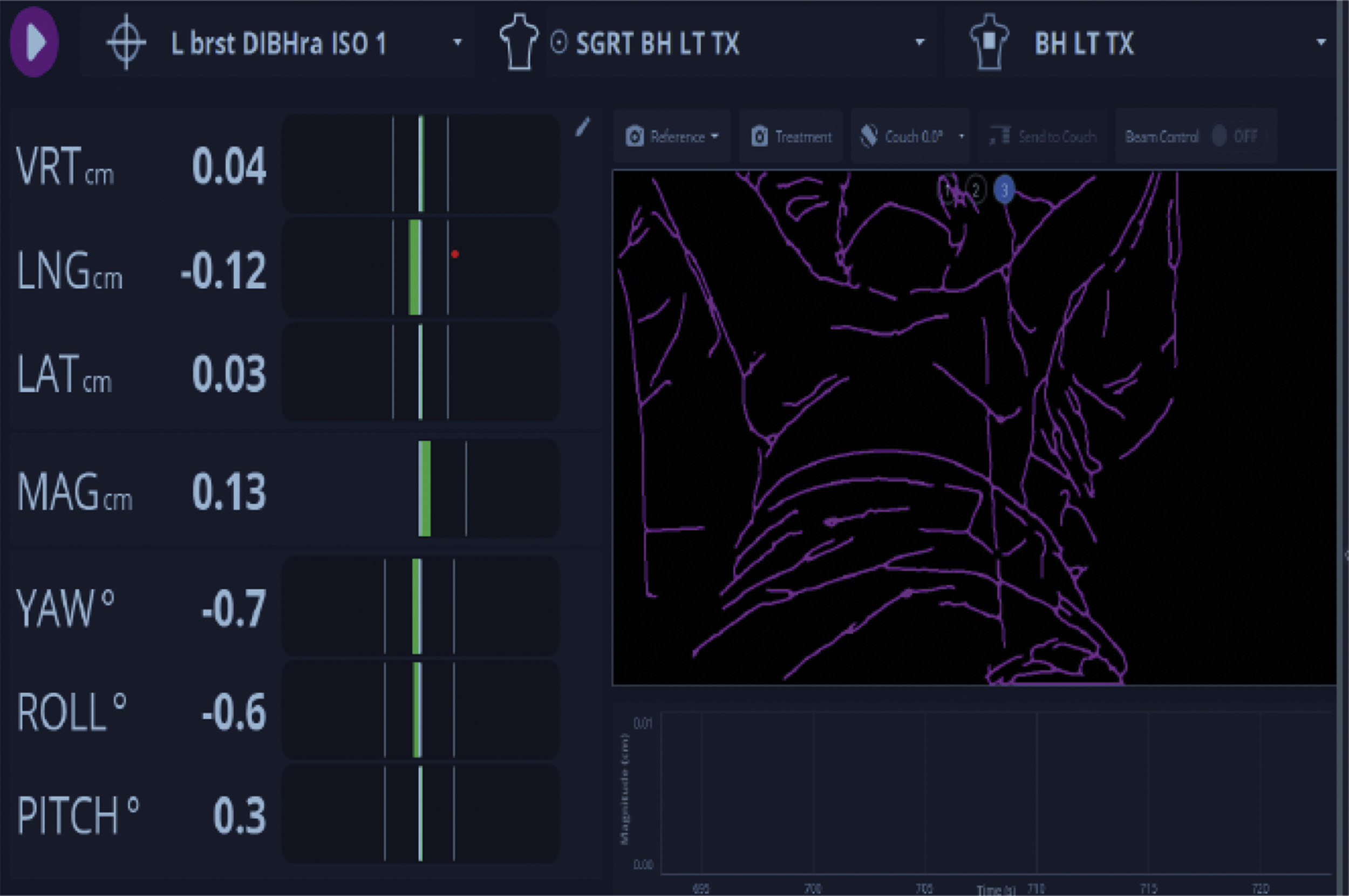
We typically schedule radiation planning simulation 1 week prior to the desired start of treatment. Imaging is obtained daily or weekly on the linear accelerator to confirm set up accuracy. The overall goal of our radiation therapy technology is to provide a highly calibrated and accurate, tightly anatomically delivered radiation dose covering a limited volume of normal tissue. Finally, clinical assessment of the patient is done on a weekly basis, more frequently if needed, to monitor patient tolerance or toxicity.
Ductal Carcinoma In Situ
Ductal carcinoma in situ (DCIS) is a heterogeneous disease, found in 20–25% of newly diagnosed breast cancer patients. Pathologists classify DCIS into low-, intermediate-, or high-grade categories, the latter demonstrating a higher likelihood of developing into invasive breast cancer. We recommend a surgical margin of at least 2 mm when possible, and surgical clips to define the surgical bed. Given the lower mortality of DCIS versus invasive breast cancer, studies were performed to determine the relative value of offering postoperative breast radiation. In the landmark NSABP B-17 trial, a standard course of breast radiation reduced the risk of local recurrence in the ipsilateral breast by more than 50%. A large meta-analysis published in 2010 showed a reduction of 15% at 10 years when using radiation for DCIS. The data does not show a survival benefit with radiation therapy postoperatively in these patients, possibly attributed to salvage options at the time of documented recurrence. We typically recommend a course of hypofractionation (e.g., 4256 cGy in 16 fractions or 4000 cGy in 15 fractions) with or without a boost to the primary surgical bed. We do not include lymph node stations in the radiation field, given their very low risk of probable involvement. Left-sided DCIS is managed with cardiac-sparing technique, as previously shown. In elderly patients over 65–70 years of age with low-grade DCIS with ER-positive receptors, observation with tamoxifen or aromatase inhibitors is a viable option and is typically presented to the patient along with postoperative radiation options. In some instances, microinvasive carcinoma is found at the time of pathology postoperatively, requiring reconsideration of treatment including sentinel lymph node assessment. A multidisciplinary discussion is thus required with this pathological upstaging and should be completed prior to any initiation of radiation planning.
Lobular carcinoma in situ has a very different biological behavior than DCIS and, absent any microinvasive findings, is not treated with radiation.
Invasive Breast Cancer, Breast-Conserving Adjuvant Radiation Treatment
The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) published a meta-analysis of 17 randomized trials and determined that radiation therapy following BCS reduced the 10-year recurrence risk of ipsilateral breast cancer by 15.7% and reduced the risk of breast cancer death by 3.8% at 15 years. The meta-analysis, however, included node-positive and node-negative patients with different risk features and surgery. In this analysis, the benefit of adjuvant radiation therapy varied according to stratification, prompting additional trial-based studies. These studies provide the framework for the recommendation of adjuvant radiation therapy for breast cancer and is referred to in the consent process following diagnosis or when breast conservation versus mastectomy is being considered.
The PRIME-II study, published in The Lancet in 2015, randomized women aged 65 years and older with T1 or T2 invasive breast cancer (maximum 3 cm) with hormone receptor–positive, axillary node–negative, surgical margin–negative breast cancer to receive endocrine therapy only versus adjuvant radiation with endocrine therapy. At 5 years the radiated breast tumor recurrence was 1.3% versus 4.1% in the endocrine-only arm, but there was no difference in the development of distant metastasis, contralateral breast cancer, or survival. The authors concluded that omission of radiotherapy may be considered for some patients.
National Comprehensive Cancer Network (NCCN) Guidelines 4.2023 currently recommend the hypofractionated course of 4256 cGy in 16 fractions whole-breast treatment, with boost when there is an expected increased risk of recurrence in the tumor bed.
A more hypofractionated course of five fractions has been advocated for select cases. The FAST Forward study from the United Kingdom was published in 2020 and randomized these patients to various treatment arms including 26 Gy in five fractions, 27 Gy in five fractions, or 40 Gy in 15 fractions. Eligibility included patients at least 18 years of age with invasive carcinoma of the breast (pT1–3, pN0–1, M0). The study was not blinded because of the nature of the delivery, but the five-fraction course delivered in 1 week was noninferior to 40 Gy in 15 fractions for local control and normal tissue toxicity at 5 years.
NCCN guidelines also note 28.5 Gy in five fractions as a hypofractionated option; however additional updates in hypofractionated clinical studies will be needed to clarify the optimal treatment prescription.
In addition, select patients may be candidates for accelerated partial breast irradiation (APBI), which is also delivered in just five fractions. Instead of targeting the whole breast, the radiation dose is limited to the tumor bed plus a margin for microscopic disease, acknowledging that the index quadrant is the most common location for local recurrence after lumpectomy. The ABPI-IMRT-Florence Trial randomized patients to conventional whole-breast irradiation (WBI) versus APBI delivered in five fractions totaling 30 Gy versus 25 fractions totaling 50 Gy plus boost. At 10 years, the cumulative incidence of ipsilateral breast tumor recurrence (IBTR) was not statistically different between the arms; however the APBI arm had less acute and late toxicity, as well as better patient-reported cosmetic outcomes.
Adjuvant radiation therapy follows a multidisciplinary process, delivered following surgical clearance; medical oncology assessment of adjuvant chemotherapy benefits; presentation to the patient involving radiation treatment side effects and benefits, i.e., treatment versus observation; and patient input regarding treatment location and duration. Ideally, the radiation oncologist would consult the patient prior to the initial breast-conservation surgery to discuss these issues.
Post-Mastectomy Radiation Therapy
Total breast mastectomy is potentially indicated for any number of circumstances including large tumor size, small breast size with expected unsatisfactory conservation cosmesis, multicentric disease, or patient preference. For large and higher-risk disease, NACT may be administered prior to mastectomy.
Radiation therapy may typically be omitted in post-mastectomy patients with negative nodes and clear margins from a T1 or T2 primary breast cancer.
Indications for post-mastectomy radiation include positive or very close (<1 mm) margins and/or tumor size typically greater than 5 cm. Treatment of regional lymph nodes is indicated for positive lymph node spread. The chest wall is normally treated to 45–50.4 Gy in 25–28 fractions and the relevant regional lymph nodes to the same dose. Internal mammary nodes are often considered based on risk features such as inner quadrant primary tumor location or when involved in a staging workup (CT scan or PET scan; Fig. 8.7 ). Skin bolus, which is essentially artificial tissue placed over the mastectomy incision or chest wall, will increase the radiation dose to that superficial tissue and is employed when there is concern for increased local recurrence. A mastectomy scar boost dose may be delivered with superficial electron therapy in typically five fractions, bringing the total dose to that site to approximate 60 Gy. In patients undergoing post-mastectomy reconstruction we rely upon the surgical team to give clearance before proceeding with radiation.