Early Use of Mammography for Cancer Detection and Benefits of Screening Mammography
Screening mammography is the practice of using radiologic imaging to evaluate breast tissue in otherwise asymptomatic patients in an effort to find clinically occult cancers. With regular screening exams, cancers may be detected at an earlier stage. This reduces the risk of advanced regional and metastatic disease while increasing the overall survival and cure rate for the patient. Routine screening also enables patients to have both improved surgical and oncological outcomes with the added benefit of potentially improved cosmetic outcomes if findings are small.
Radiography of the breast started in the early 20th century. One of the first published articles was from Stafford Warren, a radiologist, in 1930 using an in vivo stereoscopic mammographic technique prior to breast cancer surgery. Of the 119 patients he imaged, interpretive errors based on his mammographic techniques (both those benign and malignant) were made in only 8 patients. In Warren’s report, he found “in many of the cases, there was no unanimity of opinion in the pre-operative clinical diagnosis … the opinion from the mammogram, on the other hand, was very often definitive and most frequently correct.”
Later in the 1960s, several landmark studies were performed solidifying mammography’s influence and impact on breast cancer screening. One of them was an early randomized controlled study headed by Philip Strax, Sam Shapiro, and Louis Venet from 1963 to 1967. In its initial year evaluating screening mammography, Strax studied 20,211 women aged 40 to 64 from the Health Insurance Plan of Greater New York (HIP). During that trial, preliminary results established mammography found 21 breast cancers in clinically normal women. Meanwhile, 24 cancers were found only by physical exam, with 20 of those cases not visualized on mammography. Ten of the 55 detected cancers were visualized by both mammography and clinical exam. Meanwhile, the control group of 29,694 developed 46 cancers during the first year of observation. An earlier study from 1966, by Griesbach and Eads, had similar results without a control. A third study from Wolfe found 16 carcinomas in 3891 clinically normal women with routine mammograms in 1965. These early trials demonstrated that mammography had the potential to find as many if not more cancers than generalized medical care at the time.
The researchers (Strax, Shapiro, and Venet) evaluating patients from the HIP had the benefit of following patients for several years, sometimes up to a decade, if not longer. A follow-up report published in 1982 using data from the HIP demonstrated a 38.1% reduction in mortality from breast cancer in the first 5 years after starting at, minimum, annual screening mammography. At 10 years after initially beginning screening, the reduction in mortality was found to be 24%. However, after the first 3 to 4 years, annual screening mammography was not required for the study, but rather at the discretion of the patient. Because of this, the study authors felt that if screening mammography was performed at a rate similar to what was performed during the first 5 years of the study, during years 6 to 10, the overall decreased mortality rate would be higher. The study authors subsequently estimated the 10-year mortality rate to be 32.4%. The HIP study also revealed that those patients who were in the study group had a higher rate of no axillary involvement (56.4%) versus those in the control group (46.3%). Screening only detected cancers had an even higher proportion without nodal involvement at 70.5%.
More recently, using data from 550,000 women in nine Swedish counties from the Swedish Cancer Register, Duffy et al. in 2020 calculated a decrease in the 10-year breast cancer–related mortality rate by 41% when screening mammography was utilized. Additionally, among those diagnosed with breast cancer, those diagnosed through screening practices were 25% less likely to be of an advanced stage. Earlier, in 2019, Tabár et al. published similar results from a study of one Swedish county, which demonstrated a 60% decrease in 10 years in the breast cancer death rate and a 47% decrease at 20 years for women diagnosed through screening mammography compared to those who did not participate in a standard screening program.
Screening Mammography Risks
Despite demonstrating significant benefits with mammography, there are some associated downsides. Often, the biggest concern from both patients and ordering practitioners is the overall radiation dose from the exam itself. The estimated dose of radiation for a standard two-view screening mammogram is approximately 3 mGy (milligray) in total. Comparatively, this is equivalent to approximately 6 weeks of natural background radiation. With the often-standard incorporation of digital breast tomosynthesis (often referred to as DBT or 3D imaging) as part of the annual screening exam, the glandular tissue is potentially exposed up to double this amount. Recent developments in technology have enabled manufacturers to reconstruct standard 2D images from tomosynthesis acquisitions, often referred to as “synthesized views.” This technology was approved by the Federal Drug Administration (FDA) in 2013 and helps reduce the overall radiation dose for the patient. Synthesized views preserve the added benefit of DBT while maintaining high image quality.
Not only may patients sometimes worry about the radiation dose from mammography, but there may also be psychological effects. Patients may dread the actual physical compression of the breast tissue during image acquisition. This, combined with the fear of possibly getting called back for further imaging, can create anxiety for the patient. The potential for psychological effects associated with anxiety surrounding screening mammograms is a patient risk that should be considered and discussed with patients as needed. There is no evidence to suggest less frequent examination would reduce patient anxiety. In a survey published by Radiological Society of North America (RSNA), over 70% of women showed preference for annual screening exams compared to biennial evaluation. Additionally, only a minority (17%) of women believed biennial screening would reduce exam-related anxiety. Not every woman will perceive the same risks and benefits. Therefore, it is important to have individualized clinical discussions with each patient.
Another possible downside to screening mammography is the potential cost of the exam itself and the potential added expenses for follow-up testing. Specifically, for breast imaging, many advancements have been made to reduce or eliminate out of pocket costs for screening mammography. Despite these changes, some private insurance patients are still charged for their examinations. In a study performed by Tran et al., approximately 5% of patients included in their analysis paid an out-of-pocket expense for their baseline screening mammogram. Those charged for a screening mammogram were found to have delayed subsequent screening examinations compared to those who were not charged by their insurance provider. Although not specifically addressed in this analysis, these possible delays due to cost concerns, risk potential future delays in cancer diagnoses. This highlights the importance for all to have adequate medical coverage for annual screening exams if desired.
Standardization of Technique and Patient Positioning
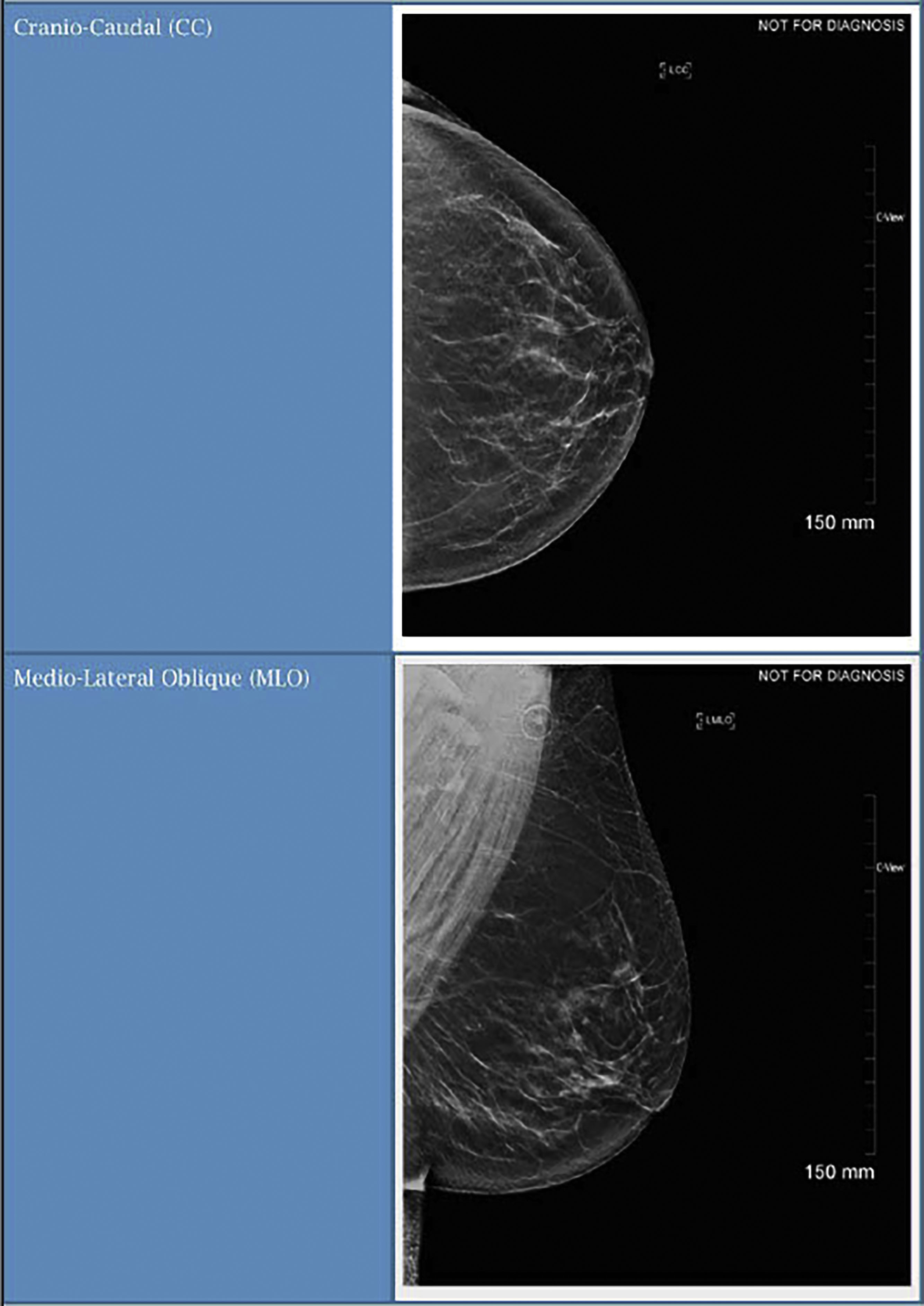
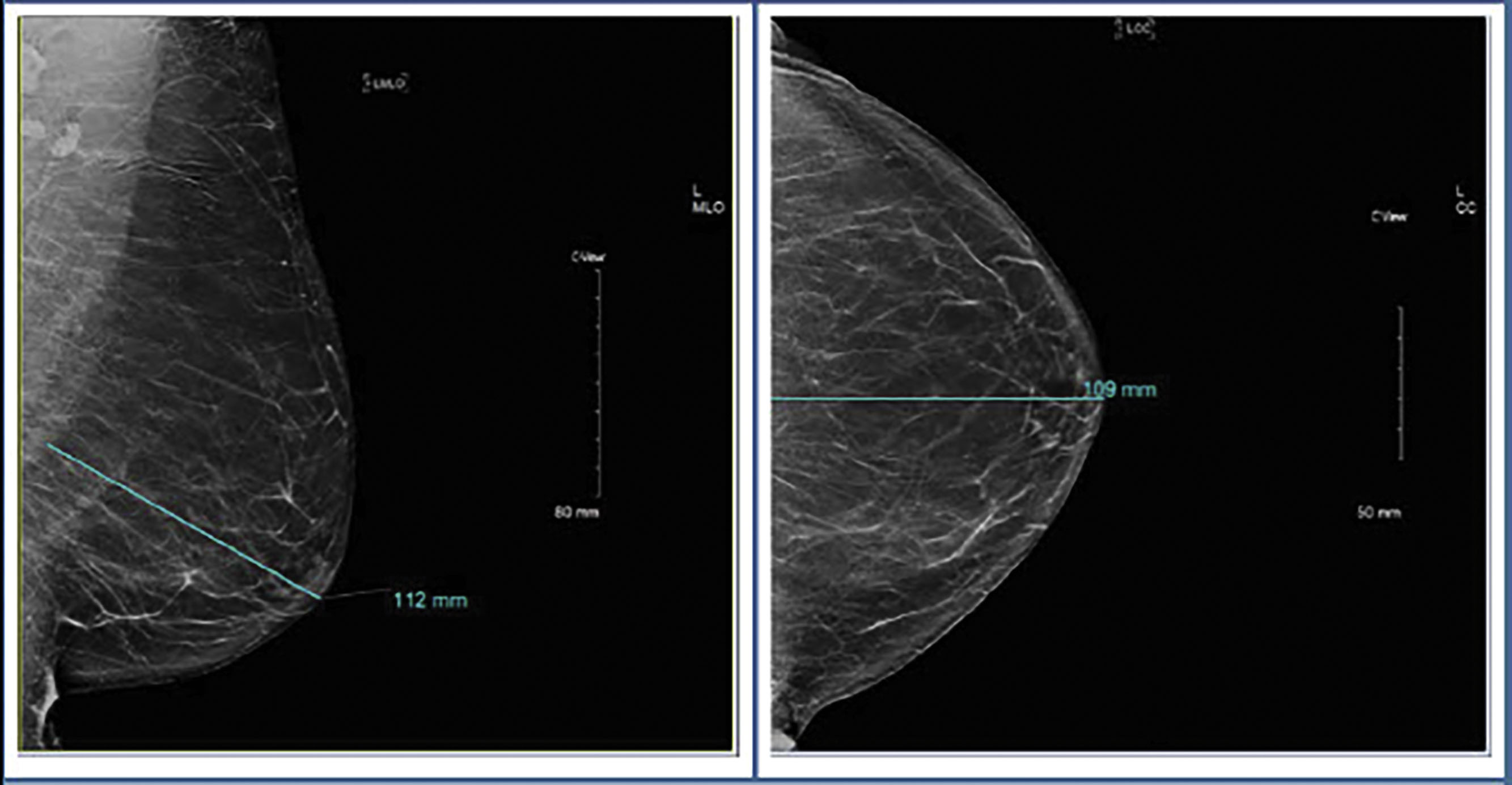
Screening mammography consists typically of two images, the cranio-caudal (CC) and the medial-lateral oblique (MLO) views ( Fig. 4.1 ). Ideally, the nipple should be in profile on both views, but is only required to be in profile on a single view. To ensure that enough tissue is included on both images, radiologists often assess the posterior nipple line. A line is drawn posteriorly and perpendicularly from the nipple toward the pectoralis muscle on the MLO view. A similar line is drawn on the CC view, extending posteriorly from the nipple toward the central posterior portion of the CC film. The two measurements should be within 1 cm of each other to make sure that enough tissue is included ( Fig. 4.2 ).
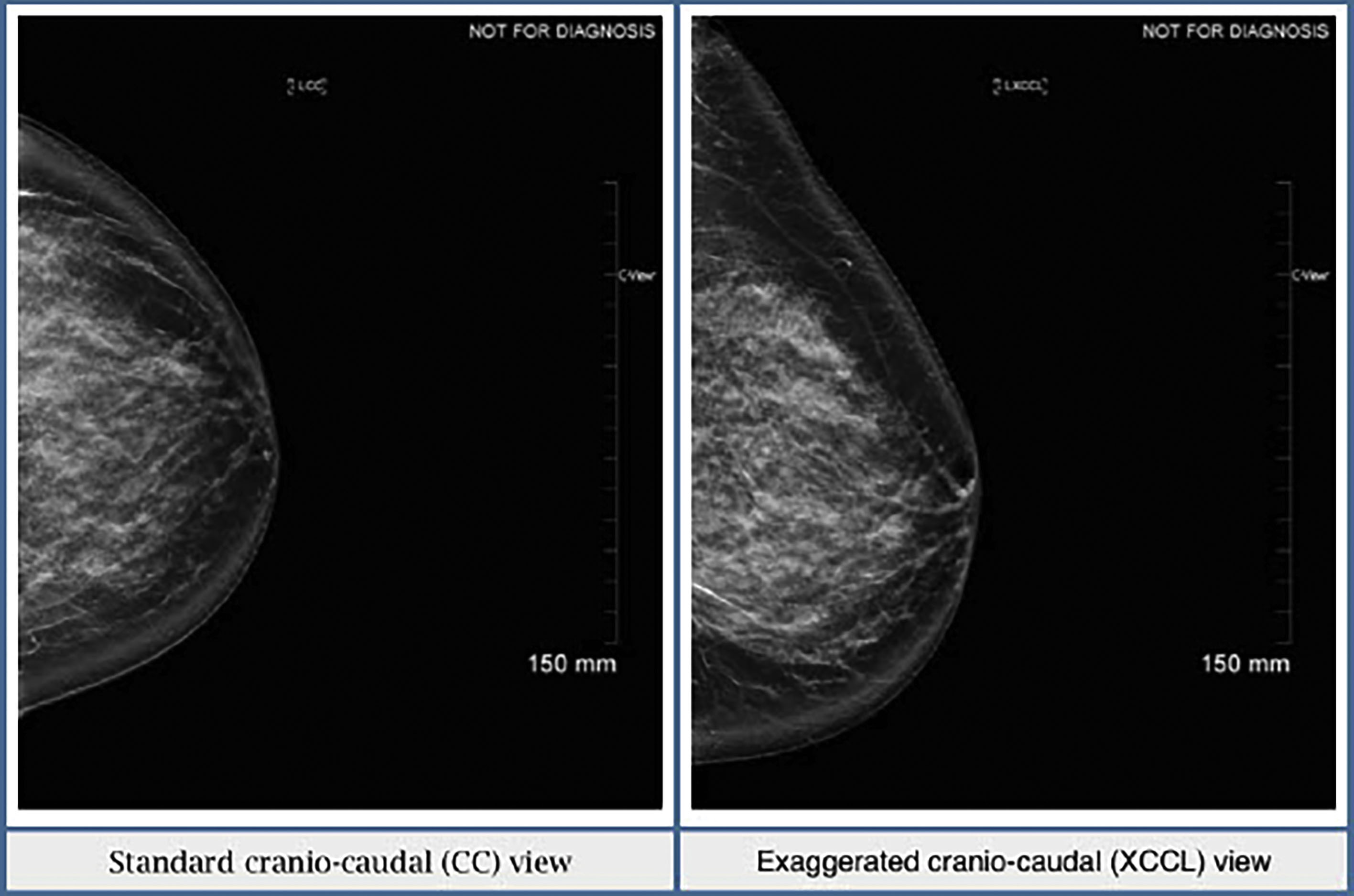
Sometimes there is not enough tissue included laterally on the CC view. If this is the case, an exaggerated cranio-caudal (XCCL) view should then be obtained. In this view, the breast is positioned similarly to a CC view but, as the name suggests, the technologist exaggerates and includes more lateral breast tissue ( Fig. 4.3 ). Ideally, the XCCL should be obtained in less than 10% of all screening exams. It should not be part of the standard mammogram unless it is a baseline exam and prominent tissue is missing on the regular CC view, or an area of concern is seen in the lateral posterior breast tissue.
Characterizing Breast Tissue Density
In response to the increased utilization of mammography and the need for standardization of reporting and management recommendations, the American College of Radiology (ACR), along with support from various other medical groups, drafted the first version of the Breast Imaging-Reporting and Data System (BI-RADS) atlas with the most recent BI-RADS atlas published in 2013. This updated atlas provides radiologists with a specific lexicon for each imaging modality and with guidelines regarding report format and patient management. This creates a foundation for all interpreting radiologists to essentially speak the same language and establish standards of care for mammography.
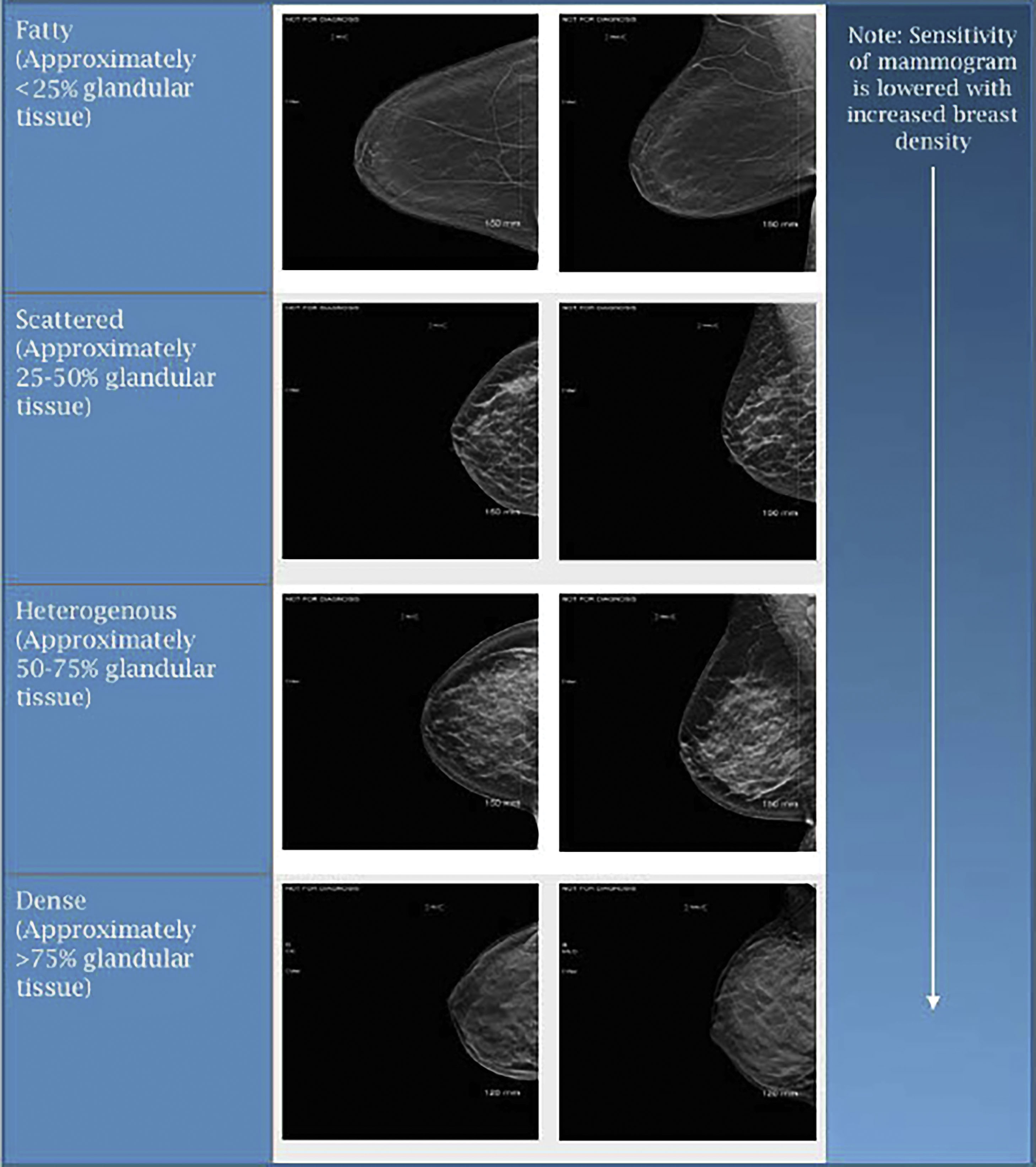
When reviewing mammograms, tissue density is characterized into one of four categories: predominantly fatty, scattered areas of glandular tissue, heterogeneously dense, and extremely dense ( Fig. 4.4 ). On mammography, glandular tissue composed of breast stroma appears “white” and fat appears “dark.” Previous BI-RADS guidelines had quantified the different classifications of breast tissue density as follows: Non-dense breasts such as predominantly fatty breasts have less than 25% of glandular tissue with scattered areas of glandular tissue containing roughly 25% to 50% of glandular tissue. Meanwhile dense breasts such as those that are heterogeneously dense have approximately 50% to 75% of glandular tissue, and those with extremely dense breasts have greater than 75% of glandular tissue. These quantifiers were established to help more evenly divide women amongst each of the tissue densities. However, more recent literature suggests that these quantifiers are not needed, and they have been removed from current published reporting standard guidelines. Regardless of whether or now the quantifiers were used, breast tissue density remained unevenly distributed among screening mammograms, with approximately 10% of mammograms having fatty tissue, 40% scattered, 40% heterogeneous, and 10% dense. It is important to acknowledge that a woman’s breast tissue density is not stagnant and may be variable over their lifetime. Often younger women have dense breasts, with more fatty changes in tissue density noted in postmenopausal patients. Besides aging, tissue density changes have been noticed with hormonal usage and weight fluctuations, among other etiologies. Technical variances in compression during image acquisition have also been shown to have an impact on breast tissue density. With dense breast tissue, mammography becomes less sensitive and there is increased potential for obscuring areas of interest including small masses. Those women with dense breasts have been reported to have a 1.2- to 2.1-fold higher risk of breast cancer. The interval cancer rate for dense breasts may be as much as 17-fold higher as compared to women with fatty breasts.
Mammography Limitations/Incorporation of Tomosynthesis
One of the biggest limitations of standard 2D mammography is the possibility for overlapping tissue. If the breast tissue is dense, or there are denser islands of tissue, possible areas of interest may be inadvertently obscured on 2D mammography and create a false-negative assessment for the patient. Alternatively, areas of normal tissue may also overlap, creating a false area of interest (often referred to as summation artifact). This may lead to increased patient callbacks and higher patient anxiety. DBT, approved by the FDA for use in 2011, addresses some of those limitations. While each tomosynthesis manufacturer has a slightly different technique, the overall concept is similar throughout. The breasts are positioned, compressed, and held stationary, exactly the same as a standard 2D mammogram. Images are obtained most often in CC and MLO views. The x-ray tube moves in an arc taking several low-dose images, which are then reconstructed into thin sections parallel to the detector. Images are then reconstructed into a 3D plane for the radiologist to “scroll through.” The ability to “scroll through” dense islands of tissue helps the interpreting radiologist better assess for possible underlying areas of interest that may otherwise be missed on standard 2D mammography ( Table 4.1 ). Since its approval, tomosynthesis has shown to reduce recall rates and improved cancer detection rates, up to 40%. Given its profound impact and integral usage on mammography, tomosynthesis manufacturers have recently developed a “synthesized” 2D image reducing the need for “standard 2D” imaging. This technology was approved by the FDA in 2013 and helps reduce the overall radiation dose for the patient while maintaining high image quality.
Screening Guidelines for Average-Risk Women
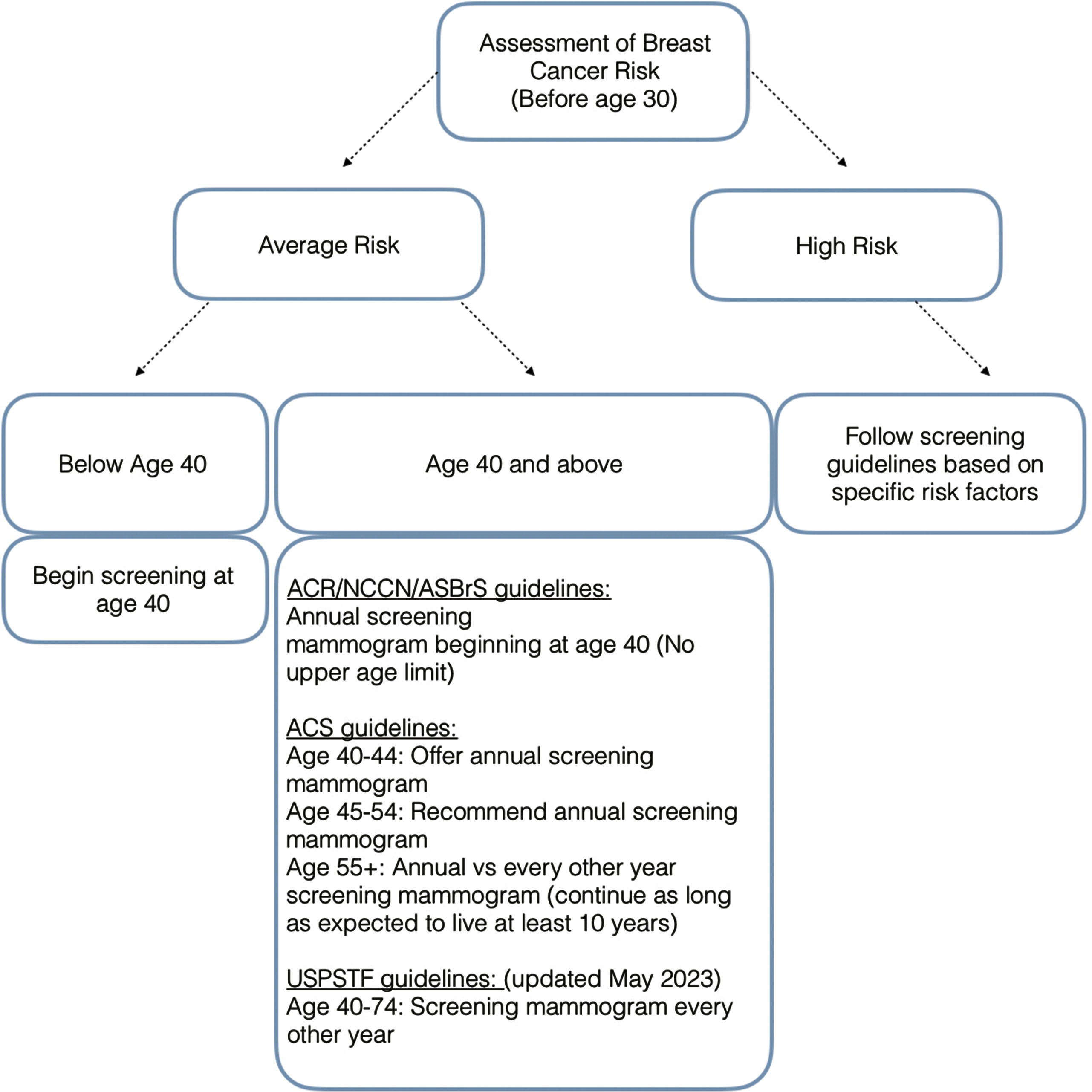
The American College of Radiology (ACR), in conjunction with the National Comprehensive Cancer Network (NCCN) and the American Society of Breast Surgeons (ASBrS), recommends women of an average risk for breast cancer be screened using mammography annually beginning at the age of 40 ( Fig. 4.5 ). These guidelines recommending that screening begin at the age of 40 are further supported in populations of minority ethnicities where there are higher rates of cancer detection and lower survival rates below the age of 50. With screening beginning annually at the younger age of 40, cancers can be detected at earlier stages and treated with improved therapy response and survival ( Table 4.2 ).
| Annual screening mammography at ages 40-84 | Annual Screening Mammography at ages 45-54, followed by biennial screening at ages 55-79 | Biennial screening at ages 50-74 | |
|---|---|---|---|
| Mortality Reduction (Percentage) | 39.6 | 30.8 | 23.2 |
| Breast Cancer Deaths Averted (Per 1,000 women) | 11.9 | 9.25 | 6.95 |
| Life Years Gained (Per 1,000 women screened) | 189 | 149 | 110 |
| Number Needed to Screen (per death averted) | 84 | 108 | 144 |
| Number Needed to Screen (per life year gained) | 5.3 | 6.7 | 9.1 |
Biennial screening is discouraged by the ACR, NCCN, and ASBrS due to more advanced cancers at the time of diagnosis and increased mortality for the patient. Based on 2009 CISNET models, mortality reduction is as low as 23.2% for biennial screening (compared to approximately 40% for annual screening; Table 4.2 ). Within the same analysis, 189 life-years are gained per 1000 women screened annually, while only 110 life-years are gained in women screened biannually ( Table 4.2 ). More recent CISNET models in 2017 continue to affirm that annual screening mammography offers the largest reduction in mortality from breast cancer at approximately 40%.
The ACR, NCCN, and ASBrS do not recommend terminating screening at a particular age in the absence of significantly reduced life expectancy from severe preexisting comorbidities. The importance of early detection and opportunity for treatment remains in older patient populations, as approximately 20% of breast cancers are diagnosed in patients 75 or older. This is also confirmed with data from the National Cancer Institute (NCI) demonstrating that of all recorded breast cancers from 2015-2019, approximately 18.9% were patients aged 75 and above. Using data from the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) database, Sanderson demonstrated that women aged 69-84 years had lower 10- year breast cancer mortality if screened annually than those who were screened biannually or sporadically, reaffirming the continued need for annual screening in this age group.
Contrary to the recommended screening guidelines for average-risk women set forth by NCCN, ACR, and ASBrS, the American Cancer Society (ACS) has established its own guidelines regarding screening mammography for average-risk women. Per the ACS Guidelines, patient’s between ages of 40 and 44 have the option to start or delay annual screening mammography. Women between the ages of 45 and 54 are recommended to have annual screening exams, while beginning at the age of 55, women can either switch to a mammogram every other year or continue to have annual screening mammography ( Fig. 4.5 ). Similar to the ACR guidelines, the ACS does not recommend terminating screening at a particular age. The ACS guidelines state that screening should continue as long as a woman is in good health and is expected to live at least 10 years or more.
Differences between recommendations set forth by the NCCN/ACR/ASBrS and the ACS stem from each group’s interpretation of evidence pertaining to screening mammography, risks versus benefits assessed, and the value of those risks to the interpreting group. In its 2015 update, the ACS acknowledges that with annual screening examinations, tumors are detected at smaller sizes and survival is improved among those diagnosed with cancer, particularly in patients below age 50. The ACS, however, emphasizes that annual examinations lead to higher false positives, and by screening every other year in certain age groups the mortality benefits of annual screening mammography can be maintained at a rate of approximately 81%; meanwhile, false-positive results would also be reduced by half. Together, these findings led the American Cancer Society (ACS) to establish a “hybrid” system of breast cancer screening recommendations based on age and screening intervals.
These recommendations, however, do not consider subsequent additional risks to the patient following a breast cancer diagnosis, including chemotherapy side effects, possible chemotherapy toxicity, as well as the potential need for more aggressive surgical interventions or resections if a tumor is diagnosed at a larger size or higher stage. While there have unquestionably been advances in breast cancer treatment, these treatments cannot always overcome the disadvantages of being diagnosed with advanced-stage disease. Also, undergoing annual mammography prior to a breast cancer diagnosis is a predictor of overall increased patient health and survival. It has been documented that women who had missed any annual mammograms in a prior 5-year period had a 2.3-fold increase in all-cause mortality compared with those who did not miss any.
The United States Preventative Task Force (USPTF) recently modified their 2016 guidelines, which originally stated that women aged 50-74 years should get biennial screening mammograms and that those ages 40-49 should make individual decisions on their need for screening. The USPTF specifically stated in their 2016 guidelines that “women who place a higher value on the potential benefit than the potential harms may choose to begin biennial screening between the ages of 40-49 years”. In their prior 2016 guidelines, the USPTF also poignantly stated that “beginning mammography screening at a younger age and screening more frequently may increase the risk for overdiagnosis and subsequent overtreatment.”
In the USPTF’s May 2023 draft update, the Task Force now recommends that all women from the ages of 40 to 74 get biennial screening mammography. They state that this change offers the potential for up to “19 percent more lives being saved” and address particular concerns regarding Black women due to earlier disease onset and the probability of a more aggressive cancer. While discussing the draft update, Task Force member Dr. John Wong acknowledges that younger women are increasingly getting breast cancer “with the number of newly diagnosed women increasing about two percent each year”.
Despite differences in recommendations, all of the discussed groups agree that annual mammography starting at age 40 for average risk women saves the most lives and that women should be allowed to begin screening mammography at age 40 if they desire. Additionally, all groups recommend that patients should have individualized discussions with their physician to help women make an informed decision weighing the benefits and risks of screening mammography.
Higher-Than-Average Risk Populations
Beginning in 2018, recommendations were suggested to screen women over the age of 25 to identify those who are high risk for breast cancer to enable initiation of more assertive screening practices (see Fig. 4.5 , Table 4.2 ). High-risk patients are defined as those having a risk equivalent to or higher than the average risk at age 40 for breast cancer. Sensitivity for cancer detection may be reduced by mammography for those who are at high risk. Subsequently this may require supplemental screening, most commonly with contrast-enhanced breast magnetic resonance imaging (MRI). Most recent guidelines published by the ACR suggest that all patients be evaluated by age 30 to identify those at a higher risk.
Personal History
There is an elevated risk of subsequent breast cancers in breast cancer survivors. In the decade immediately following a breast cancer diagnosis, there is an annual 0.5% to 1% risk of a contralateral cancer diagnosis. The overall lifetime recurrence rate is much higher at greater than 19%.
Lobular neoplasia, including atypical lobular hyperplasia or lobular carcinoma in situ, is an independent risk factor for a higher lifetime risk of breast cancer, with rates reaching 10% to 20%. The rate is higher for lobular carcinoma in situ than that for atypical lobular hyperplasia. These patients are therefore considered high risk, and annual screening with mammography is recommended to begin at the time of diagnosis but not earlier than age 25. Additionally, according to NCCN guidelines, contrast-enhanced MRI for screening purposes can be considered and begun at age 25 ( Table 4.3 ).