Radiation Oncology in the Developing World
One of the greatest challenges facing the international radiation oncology community and all other domains of cancer control is the burden of cancer in low- and middle-income countries. Low-income countries had a 2010 per capita gross national income of US $1,005 or less, and for middle-income countries the range was US $1,006 to $12,275.1 Nations falling into these income groups are often described as developing countries. This chapter will describe the unique characteristics of cancer in this setting and the challenges to health service provision. It will also provide reasons for hope that the global challenge of cancer can be met.
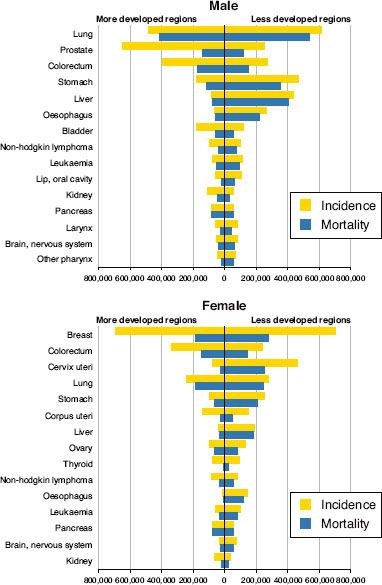
FIGURE 28.1. Estimated number of new cancer cases (incidence) and deaths (mortality) worldwide in 2008. Data are shown for more developed and less developed countries by cancer site and sex, ranked by global cancer incidence. (From Ferlay J, Shin HR, Bray F, et al. GLOBOCAN 2008 v1.2, cancer incidence and mortality worldwide. IARC CancerBase No. 10 [Internet]. Lyon, France: International Agency for Research on Cancer, 2010. Available at: http://globocan.iarc.fr, with permission.)
 GLOBAL BURDEN OF DISEASE
GLOBAL BURDEN OF DISEASE
Based on projections for 2008, an estimated 14% of all deaths worldwide are due to cancer.2 In comparison to the worldwide burden of cancer, cardiovascular disease and other chronic conditions are responsible for, respectively, 30% and 19% of deaths globally. In total, 63% of mortality worldwide is the result of noncommunicable disease. Eighty percent of these deaths occur in low- and middle-income countries (LMCs).2 In low-income countries, where deaths from communicable disease and other related causes are common, chronic disease was the cause of almost as many deaths in 2008 and is expected to surpass them by 2015 or earlier.2
In 2008, 56% of cancers worldwide occurred in less developed countries, as did 64% of all cancer deaths.3 The burden of cancer in developing countries relates to increasing life expectancy in developing countries, population growth patterns, and rising incidence of risk factors for chronic diseases in developing countries.4,5 For example, an estimated 65% of people ≥60 years of age lived in less developed countries in 2010, and this is expected to rise to 79% by 2050.4 The growing burden of cancer and other noncommunicable diseases in LMCs represents a significant epidemiologic transition and a dual challenge for disease control efforts.
 EPIDEMIOLOGY OF CANCER WORLDWIDE
EPIDEMIOLOGY OF CANCER WORLDWIDE
Worldwide, there were 12.7 million new cases of cancer and 7.6 million cancer deaths in 2008.3 Lung cancer is the most common cause of cancer worldwide (1.61 million new cases in 2008), followed by breast cancer (1.38 million) and colorectal cancer (1.24 million) (Fig. 28.1).3 Lung cancer is also the most common cause of cancer death, with 1.38 million deaths in 2008.3 Gastric cancer (0.74 million) and liver cancer (0.70 million) are the second and third most common causes of cancer death, respectively. Age-standardized incidence rates of cancer in developed countries are nearly double the rates in developing countries, though mortality rates are far more similar. These findings reflect variation in prevalence and distribution of major risk factors and limitations in early detection and treatment resources in developing countries.6
Although cervix cancer is the 10th most commonly diagnosed cancer among women in developed countries, it is second only to breast cancer in developing nations.3 This reflects a lack of sufficient prevention of cervical cancer in many LMCs. Cervix cancer is an extremely common cancer in Latin America, Sub-Saharan Africa, and parts of Asia such as India (Fig. 28.2).3 Gastric cancer and hepatocellular carcinoma are also common in many LMCs, with 47% of all cases of gastric cancer in the world occurring in China alone (Fig. 28.2).3 Kaposi’s sarcoma is a common cancer in Sub-Saharan Africa because of the AIDS epidemic,7 and esophageal cancer has the highest incidence rates worldwide in regions of Asia and Africa.3 Oral cancer has a high incidence in South Asia, particularly among men.3
Among nine common modifiable risk factors for cancer, tobacco smoking is associated with the largest proportion of attributable risk.8 In the developing world, an estimated 49% of men and 8% of women were current smokers in 1995.9 With large populations and high tobacco use in China and India, tobacco is an extremely important risk factor for cancer in Asia.10 In general, many LMCs have demonstrated increased tobacco use during the past three decades. National consumption continues to rise in many countries, and in others, a peak occurred in the 1980s and 1990s.11 History has shown a 30- to 40-year delay between the peak in smoking rates in a population and the peak in tobacco-related mortality.12 Thus, an increasing rate of tobacco-related malignancies is expected in LMCs during the next half century.13,14
Over 26% of cancers in developing countries are attributed to infectious causes.15 Hepatitis B is a major risk factor for hepatocellular carcinoma in developing countries. Other factors are hepatitis C16 and aflatoxin produced from Aspergillus in certain poorly preserved foods.17 Human papillomavirus (HPV) and Helicobacter pylori are important etiologic agents, and there are numerous other infectious agents relevant to cancer in the developing world. These include Epstein-Barr virus, HIV, schistosomiasis, human T-cell leukemia virus type 1 (HTLV-1), and human herpesvirus 8 (HHV-8).15,18 The prevalence of infectious causes is notable given the preventability of many of these causes through public health measures (e.g., hepatitis B, HPV vaccines).
There are a number of other factors that are relevant to patterns of global cancer incidence. Diet19 and obesity are risk factors for some cancers.20 This is notable given increasing trends in unhealthy diet and sedentary lifestyle among developing countries.21 The impact of genetic polymorphisms on patterns of global cancer incidence has not been fully elucidated, but there is some suggestion of their relevance.22,23 Similarly, the role of occupational and environmental exposures to cancer in developing countries requires continued exploration.24
In many developing countries, cancer often presents in advanced stages, due to factors such as lack of comprehensive screening and poor access to effective treatments.25 As a result, case fatality rates are much higher in developing countries, with rates for breast and cervical cancer in low-income countries being more than double rates in high-income countries.26 With cervical cancer being so common in developing countries and the high frequency of advanced cancer presentations requiring local therapy, radiation therapy has an extremely important role in developing countries. The following sections will describe radiation oncology in developing countries in terms of access, quality, and economics.
FIGURE 28.2. Global variation in estimated age-standardized cancer incidence per 105 in 2008 for specific cancers based on International Agency for Research on Cancer statistics. Incidence is grouped by country quintile with higher incidence indicated by darker color. Rates for both sexes are shown for lung and stomach cancer. A: Cervix cancer rates are highest in Latin America, Sub-Saharan Africa, and parts of Asia including India. B: Breast cancer rates are high among high-income countries. Among low- and middle-income countries, rates are high in parts of Latin America and lower in parts of Africa and Asia. C: Lung cancer rates are high in both developed and developing parts of the world, including China, Southeast Asian countries, and parts of South America. D: Rates of stomach cancer are highest in East Asia. High rates are found in Latin America, other parts of Asia, and Eastern Europe. (From Ferlay J, Shin HR, Bray F, et al. GLOBOCAN 2008 v1.2, cancer incidence and mortality worldwide. IARC CancerBase No. 10 [Internet]. Lyon, France: International Agency for Research on Cancer, 2010. Available at: http://globocan.iarc.fr, with permission.)
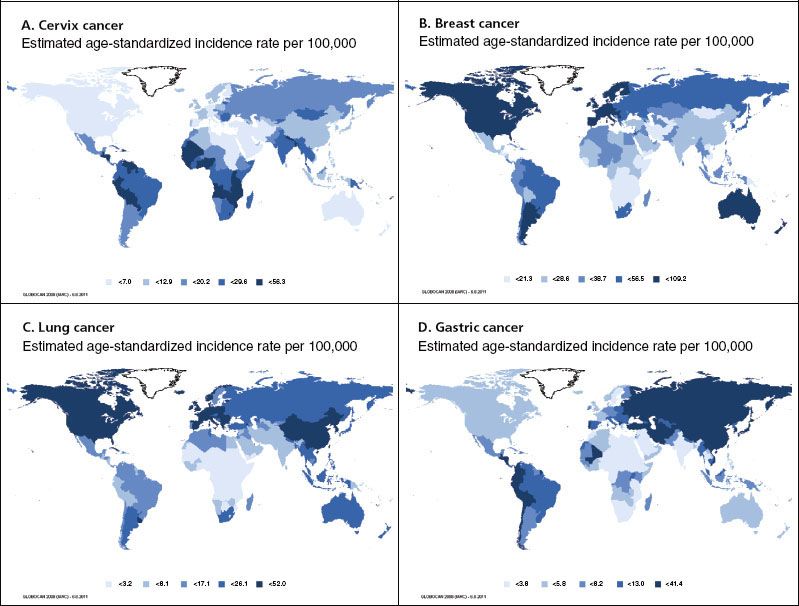
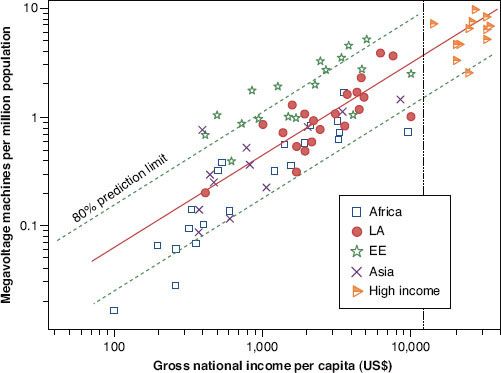
FIGURE 28.3. Megavoltage machines per million population versus gross national income per capita on a log-log scale. Countries are divided by income more than US $12,000 (high income) and then by region: Africa, Latin America (LA), Eastern Europe (EE), and Asia. The solid line is a linear regression line and dotted lines are the 80% confidence limit. The vertical dotted line represents the cutoff for high income. (From Levin V, Tatsuzaki H. Radiotherapy services in countries in transition: gross national income per capita as a significant factor. Radiother Oncol 2002;63:147–150, with permission from Elsevier.)
 GLOBAL STATUS OF ACCESS TO RADIATION THERAPY
GLOBAL STATUS OF ACCESS TO RADIATION THERAPY
Access to radiation therapy is a multifactorial issue. Availability of machines and personnel for treatment is a key part of access to care. Other considerations include spatial accessibility, acceptability, affordability, accommodation, and awareness.27,28 The most pertinent elements of access to radiation therapy in developing countries are discussed here.
Availability of equipment and personnel for radiation therapy are common limiting factors in developing countries. Less than 5% of global medical spending on cancer is in developing countries.29 This is despite developing countries containing over 80% of the world’s population4 and almost 80% of the world’s premature death, disability, and ill health from cancer.2 Insufficient medical training programs make it difficult to address the lack of key personnel for radiation oncology.30–33,34 International Atomic Energy Agency (IAEA) data suggest that developing countries only have about a third of the world’s 12,206 megavoltage radiation therapy units despite an estimated need for between double and triple the current number.35 There are currently 23 countries with populations over 1 million with no known machines, mostly in Africa.35
The greatest limitations in machine supply are strongly associated with low national economic status (Fig. 28.3).36 Given the expected rise in cancer incidence in LMCs, these large mismatches between need and availability will only increase if current machine supply is not improved. In addition to machine availability, one must also consider the need for other physical resources. These include clinical space, bunkers, other equipment (brachytherapy, simulation, immobilization, treatment planning, beam modification, dosimetry, quality assurance), a reliable power supply for linear accelerators, and the availability of parts (and technical support) for machine maintenance and repair.37
The state of radiation therapy resources varies between developing countries and regions.31,32,36,38 Some selected examples are provided for illustration. In 1999, Levin et al.38 documented the availability and distribution of radiation therapy equipment in Africa. Only 22 of 56 countries in Africa were confidently known to have megavoltage radiation therapy facilities. In total, more than 400 million Africans had effectively no access to radiation therapy. Although machine supply has since modestly increased, there are still dramatic shortfalls in machine supply in Sub-Saharan Africa.35
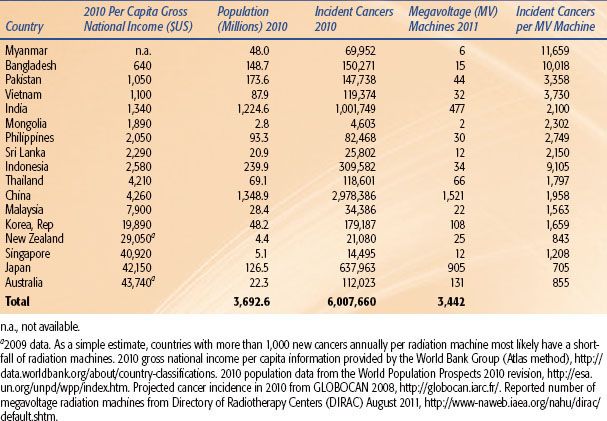
In the Asia and Pacific Region, Tatsuzaki and Levin31 found an 82-fold variation in the number of megavoltage machines per million population for 1999. China’s and India’s machine supply has increased in recent years, though capacity is still well below what is needed to treat all patients (Table 28.1).3,35,39 Workforce resources vary considerably between countries, and most countries have less than two radiation oncologists per 1,000 incident cancers annually.3,33,35 More physicists are required if radiotherapy capacity is to expand in the Asia Pacific region.33
In the era of multidisciplinary cancer therapy, for instance, for head and neck squamous cell carcinoma, availability of other elements of diagnosis and therapy such as surgical oncology, medical oncology, oncology nursing, pathology, radiology, rehabilitation, supportive care, and palliative care are all important to effective cancer management.40 Without adequate pathology and radiology, it is not possible to effectively diagnose cancer and distinguish curative from palliative cases. The need for surgical capacity is especially noted, given its central role in curative treatment of the most common cancers globally, especially in their early stages. Access to palliative care, including pain control for moderate to severe pain, is also a major issue. The World Health Organization (WHO) estimates 5 billion people live in countries with limited or no access to narcotic analgesics and other controlled substances, with an estimated 5.5 million patients with terminal cancer dying each year without adequate treatment.41
Spatial accessibility refers to the geographic accessibility of medical treatments. Available information suggests spatial accessibility is a major issue in LMCs.35,42 With radiation therapy centers often in large cities, rural populations may face substantial financial challenges when traveling into cities for the duration of radiation treatment.
Acceptability of available options can impact an individual’s willingness to take advantage of services and adhere to recommended therapies. For example, a small study from Cameroon found beliefs, fears, cultural factors, and awareness were among explanations for delay in seeking medical attention for cancer.43 Values surrounding effects of pelvic radiation treatment on fertility, loss of hair with some chemotherapy, and anatomic changes associated with surgery such as mastectomy are some potential factors in need of further description and quantification in developing countries. Culturally appropriate cancer control plans sensitive to a region’s social and political concerns are needed, including initiatives to overcome stigma and improve awareness.44
Affordability of radiation therapy and other forms of cancer therapy are a major concern in LMCs.45 Households often have limited or no health insurance coverage, especially in low-income and lower middle-income countries and more often among the poor in LMCs.46,47 This is notable as cancer-related public health care may be inadequate or nonexistent. The cost of travel to the nearest cancer center can itself be another major financial obstacle, and costs of staying for the length of radiation treatment in another location can mean lost income and more cost to the patient and family.45 A family may lose additional income due to caregiver absence from work.45,48
Awareness of the basic cancer principles and the value of cancer screening and early detection may limit timely access to cancer services for the public in LMCs. A large Union for International Cancer Control (UICC) survey of multiple LMCs found substantial lack of awareness of common preventable causes of cancer and found that a quarter or more of respondents in Asia and Africa did not think cancer could be cured.49 Limited awareness of principles of cancer diagnosis and appropriate referral among nonspecialist health care workers may be further limiting factors for access to cancer treatment. Health care worker training in oncologic principles may be extremely basic or insufficient in some cases.50
TABLE 28.1 MEGAVOLTAGE MACHINE SUPPLY AND CANCER BURDEN IN 17 ASIA PACIFIC COUNTRIES
 QUALITY OF RADIATION ONCOLOGY IN DEVELOPING COUNTRIES
QUALITY OF RADIATION ONCOLOGY IN DEVELOPING COUNTRIES
Key dimensions of quality are described by the Institute of Medicine as safety, effectiveness, patient-centeredness, timeliness, efficiency, and equity.51 Quality can be assessed through consideration of a health system’s structure, process, and outcomes.52 Elements of structure are physical resources, human resources, and organizational structure. Limitations in physical and human resources in LMCs have already been described. The access issues that relate to late presentation and failure to receive indicated treatment arguably have the greatest impact on outcomes and quality of radiation therapy in developing countries. Quality may be further degraded by the inequitable access of the few available resources between country and city, rich and poor.
The organizational structure of health care in developing countries has historically revolved around communicable disease, nutritional deficiencies, and child and maternal health. The additional burden of noncommunicable disease in developing countries, commonly cancer, cardiovascular disease, chronic lung disease, and mental illness, impose a major strain on current resources and health care models. Challenges to the structure of cancer control and radiation therapy in limited-resource settings may also include insufficient priority of cancer control among some governments and donor agencies with many competing priorities. Other issues may include political or social instability, conflict, corruption, and fragmented service provision.
The process of health care refers to what occurs while care is provided. For radiation oncology, this includes technical elements of quality assurance, treatment prescription, treatment planning, and treatment delivery. It also includes the integration of multidisciplinary services needed alongside radiation oncology for effective cancer management. A major process issue in some countries is system-related delay in diagnosis.53,54 This probably contributes to high rates of advanced disease at presentation. System-related diagnostic delay can relate to weak or nonexistent referral systems or limited resources for diagnosis. It is compounded by patient-related delay in seeking medical attention due to previously described access issues.55 The additional impact on delay due to waiting times for radiation following radiation oncology consultation requires further characterization in LMCs.
The technical process of radiotherapy is a vital element of quality. For this reason, the IAEA and WHO have maintained a postal dose audit program using thermoluminescent devices (TLDs). A report focusing on measurements from developing countries found acceptable results, with most machines calibrated within the ± 5% dose acceptance limit. Sixteen percent of machines registered measurements outside this range in the first round of testing, with 93% measuring dose within 5% of the standard after the second round.56 Notably, a dosimetric audit in Latin America and the Caribbean suggested an association between on-duty medical physics support and acceptable TLD results.57 This emphasizes the importance of adequate staffing to a radiation department’s quality assurance process.
Current reports are too limited to comment on the quality of general patterns of the radiation oncology clinical process in regions of the developing world. There are most certainly specific opportunities for gains. Taking advantage of hypofractionation to increase throughput where there is supportive evidence has not always occurred, as one survey on patterns of palliative radiation for bone metastasis in Africa suggests.58 Implementation of multidisciplinary decision making among oncologists in LMCs is important but not always present.59,60 Treatment refusal or nonadherence by patients can be a major problem in some cases and is an important area for quality improvement where it exists.42,48,61 Audits of the clinical decision-making and the treatment-planning process may provide a useful means of ensuring patient safety, improving processes, and creating opportunities for continuing education.62,63 This is particularly important with the introduction of technology at new locations. For example, initial experience with this approach in a new radiation therapy center in an Asian developing country found suboptimal management in 52% of cases.62
Adverse event rates in developing countries treating with radiation are largely unknown. A report examining the risk profile of radiation therapy for the WHO could not identify any detailed reports of adverse events from Africa or Asia.64 It is important to highlight the need for adverse event recording and reporting for the purpose of patient safety and quality improvement for all countries utilizing radiation therapy.
Finally, quality of radiation oncology in developing countries relates to outcomes. Of all cancer outcomes, there is the most information on survival. Generally, overall survival for cancer patients is lower, and sometimes dramatically so, for populations in developing countries. In a large multinational series from the International Agency for Research on Cancer (IARC), 5-year age-standardized relative survival for cervix cancer was 79% in Seoul, South Korea, but 46% in Mumbai, India; 22% in The Gambia; and only 13% in Kampala, Uganda.65 Similarly, for breast cancer, survival rates ranged from 90% in Hong Kong SAR to 13% in The Gambia. When absolute survival was stratified by extent of disease, in many cases, treatment outcomes were still inferior in regions with less developed health services compared to regions with more developed services (e.g., local and regional extent breast cancer and larynx cancer). This may reflect access and quality issues in diagnosis, treatment, and follow-up and/or limitations of the available data.
 ECONOMICS OF RADIATION THERAPY IN DEVELOPING COUNTRIES
ECONOMICS OF RADIATION THERAPY IN DEVELOPING COUNTRIES
Radiation therapy has been shown to be cost-effective in numerous developed world settings.66,67–68 A study by the Breast Health Global Initiative (BHGI) suggests that a comprehensive breast cancer program involving early detection and treatment, including radiotherapy, can be cost-effective in developing world settings.69 Notably, the BHGI study found that it was more cost-effective to invest in early detection in addition to comprehensive cancer therapy resources for breast cancer than in cancer therapy resources alone. This finding reflects the ability of early detection to increase the chances of cure due to earlier stage presentation and to some degree the lower cost of treating earlier stage versus locally advanced disease. In other situations, preventing cancer reduces the number of patients needing treatment, which can also impact on overall cost of therapy for a population.
In addition to cost-effectiveness, the actual cost of delivering interventions must be taken into account when planning. Though per-patient costs of radiation can be quite low compared to other modalities given the long usage cycle of megavoltage radiotherapy units, large up-front costs can serve as a major deterrent to establishing services. Unfortunately, there is little context-specific information on the economics of cancer therapies in developing countries. One exception is an IAEA-supported study demonstrating wide variation in the cost of delivering a fraction of radiation between a sample of units in developing and developed countries.70 For the costs considered, the median cost per fraction delivered by a cobalt machine was less than half that for linear accelerators. Cost variation was most associated with radiation machine cost and machine usage for linear accelerators, and for cobalt machines, machine cost, usage, and personnel cost.
Undoubtedly, applications of various radiation therapy techniques and modern equipment will yield opportunities to maximize the cost–benefit ratio of radiation treatment in developing countries. Hypofractionation yields opportunities to treat more patients with the same supply of equipment.71 Hypofractionation for cervical cancer and lung cancer are examples of identified areas for research.72–74 Investigation of brachytherapy or intraoperative radiation therapy (IORT) may provide means of delivering adjuvant treatments rapidly. High–dose-rate (HDR) brachytherapy markedly increases patient throughput (e.g., for cervical cancer) compared to low–dose-rate (LDR) brachytherapy per machine.75 An IAEA study of accelerated radiation therapy for head and neck cancer in developing countries suggests an opportunity for increasing effectiveness of treatment without increasing departmental resources, though with increased, but tolerable, acute toxicity.76
There has been some debate about the relative merits of cobalt-60 versus linear accelerator technology for limited resource settings. The ideal mix of machines will change depending on site-specific considerations and future market dynamics. Regarding the latter, development of lower-cost entry-level linear accelerators and, on the other hand, increases in costs of new and more sophisticated cobalt equipment would affect decision making.77 It is useful to remember that quality assurance costs, maintenance costs, and associated personnel requirements of cobalt machines are estimated to be substantially less than for linear accelerators and reliability is generally higher.70,77
 TRANSLATING KNOWLEDGE INTO ACTION
TRANSLATING KNOWLEDGE INTO ACTION
Recognized priorities for action fall into six categories: (a) advocacy, (b) investment, (c) planning, (d) capacity building, (e) quality, and (f) research.47,77–82 The varying resources, priorities, and disease burden seen in countries at different stages of development mean that there is no single solution that will apply in all cases.81 In low-income countries with extreme resource limitations, a strategy focusing on cost-effective prevention, raising awareness of cancer within the population, monitoring of process and outcomes, good palliative care, and focused early detection and treatment goals would be a reasonable starting point.83
Advocacy. An international coalition to support cancer control and cancer care in developing countries is emerging. The UICC plays an important role as an umbrella organization for advocacy. Other groups range from international agencies (e.g., IAEA, WHO, IARC), to national organizations (e.g., U.S. National Cancer Institute), to professional groups (e.g., American Society for Radiation Oncology [ASTRO], European Society for Radiotherapy and Oncology [ESTRO], American Society of Clinical Oncology [ASCO], International Organization for Medical Physics [IOMP]), to nongovernmental organizations (NGOs) (e.g., Lance Armstrong Foundation, International Network for Cancer Treatment and Research [INCTR], Axios International, AfrOx, American Cancer Society), to academic institutions and hospitals (e.g., the Global Task Force on Expanded Access to Cancer Care and Control in Developing Countries [GTF.CCC] convened by Harvard, St. Jude Children’s Research Hospital). Through the advocacy of the UICC and many other partners (e.g., NCD Alliance), the Political Declaration84 of the United Nations High-Level Meeting on the Prevention and Control of Non-communicable Diseases (September 19–20, 2011) was an important acknowledgment by governments of the global problem of cancer and other noncommunicable diseases. It was also a substantial step toward specific and concerted action by the international community.
Investment. The advocacy and work of the many cancer control groups range from local to global, and from grassroots to high-level agencies. All approaches are extremely important for generating the political will to invest in cancer control. Particularly in middle-income countries, incorporation of cancer care into public health insurance for those living in poverty is an important, and challenging, goal to meet.47,85 Given the shortage of national funding for cancer care in poorer countries, international private and public donor support and advocacy for tiered pricing will be notably important in improving access to cancer therapy.
Planning. Development of radiation therapy capacity cannot occur in isolation. Radiation therapy resources must be integrated into a broader context of multidisciplinary cancer care and cancer control and into a functional health system capable of tackling the double burden of communicable and noncommunicable diseases afflicting developing countries.86 A national cancer control plan and collection of cancer registry and health data are central in organizing resources in an equitable and appropriate fashion.87 Notably, the GTF.CCC has published an important resource for planning, advocacy, and priority setting entitled Closing the Cancer Divide: A Blueprint to Expand Access in Low and Middle Income Countries.88
Prevention (e.g., tobacco control, hepatitis B and HPV vaccination) and early detection are crucial in reducing the burden of advanced cancers in developing countries. When early detection and prevention are combined with timely access to effective cancer therapy, there is great potential for dramatically reducing deaths from cancer in developing countries as well as minimizing national costs of cancer therapy.47 Numerous relevant resources on cancer control and other noncommunicable diseases have been published online by the WHO, including a series of modules on cancer control planning,89 and the Framework Convention on Tobacco Control.
The IAEA plays a prominent role in quality assurance, safety standards, and dose calibration of radiation therapy equipment internationally. It has also been involved in numerous technical cooperation projects and radiation therapy clinical trials in developing countries. In 2004, the IAEA launched the Program of Action for Cancer Treatment (PACT) to widen the scope of its work in radiation therapy planning and capacity building. Its wide-ranging plan started with the development of sustainable demonstration radiation treatment sites in six countries throughout the developing world (Albania, Nicaragua, Sri Lanka, Tanzania, Vietnam, and Yemen). The PACT program situates the delivery of radiation therapy within a comprehensive framework including prevention, early detection, treatment, and palliation. Plans sensitive to the target country’s social and political situation are developed through local and international partnerships. Other IAEA initiatives for developing countries include strengthening pediatric radiation oncology and quality audits.
At a global level, breast cancer guidelines stratified by availability of resources have been developed through the Breast Health Global Initiative.90 This is a useful paradigm for developing resource-appropriate and stepwise, scalable goals and guidelines for cancer care that is being adopted for other cancers.91 A related approach has been used by the IAEA to describe additional resource requirements, benefits, and risks for specific approaches in lung cancer treatment, including curative and palliative radiation.92
Developing innovative means of organizing and funding cancer services is needed. The IAEA’s PACT program offers opportunities to identify successful models of service delivery and planning. A model of radiotherapy service provision utilizing geographically dispersed telemedicine-linked sites with varying levels of capacity has also been proposed by an Indian group to maximize available resources.93 Another concept that is being explored is utilizing community health workers and primary care to expand cancer-related service provision. Proposed activities are cancer prevention, early detection, some treatment (e.g., systemic therapy), palliation, and follow-up.47 A social business model is one potential solution to financing radiation therapy services.94 This is being explored as part of a Bangladesh initiative.
Capacity Building. The importance of improving human resources for radiation oncology and oncology in general cannot be overstated given the global workforce shortage of trained health care professionals. Some initial efforts have been made in developing curricula and educational approaches specific to the discipline of radiation medicine.95,96 The IAEA has been notably involved in these efforts. The issue of loss of trained staff from developing to developed countries is especially important to consider in developing educational programs. Urban regions in LMCs may have high-level expertise that can be utilized in developing national training programs. A complementary approach is online training. This is the approach of the Virtual University for Cancer Control and Regional Training Network (VUCCnet) initiative in Africa. A growing number cancer centers, regional groups, and specific nations have been involved in twinning projects building capacity for cancer therapy in limited-resource countries. These initiatives have been particularly strong in pediatric oncology, with demonstrated success.97,98 They provide an appealing means for broad participation in improving cancer control in developing countries.
Numerous oncology societies in developed and developing countries support initiatives to build capacity in developing countries. The African Organization for Research and Training in Cancer (AORTIC) is an Africa-based collaboration with an advocacy, research, and training focus in cancer control for Africa. ESTRO, ASTRO, and a number of other radiation oncology societies support initiatives for education and capacity building in developing countries. ASCO has developed a number of ongoing initiatives in training, mentoring, and continuing education of oncologists in developing countries, including translation by local editors of their flagship journal into 12 languages.99 The U.S. National Cancer Institute Radiation Research Program is partnering with other oncology groups to develop a capacity-building Cancer Expert Corps.
Regional and national initiatives are very important for improving the capacity to treat cancer in developing countries. For example, the Forum for Nuclear Cooperation in Asia (FNCA) organizes radiation therapy protocols in the Asian region for the common problems of cervical cancer and nasopharyngeal cancer.100 The FNCA involves Asian countries at a wide range of economic levels. At a national level, the Association of Radiation Oncologists of India (AROI) publishes a scientific journal and supports various educational and professional activities.101
Quality. Embedded within the themes of investment, planning, and capacity building is the implicit theme of structure-related quality improvement. Process-related initiatives in quality are another very important part of ensuring optimal outcomes. These are often referred to indirectly (e.g., safety, effectiveness, patient-centeredness, timeliness, efficiency, equity).51 Quality improvement relating to process and organizational structure is important as it holds the potential for improving some outcomes more rapidly than other drivers of health, such as economic growth.102 One important element of quality for radiation oncology in developing countries is safety, given the potential for unsafe treatment to negate any benefit of available treatment.103 Safety includes the clinical process, the various elements of technical quality assurance, maintenance, worker safety, public safety, and source security.104,105 Safety requires investment in appropriate dosimetry equipment, sufficiently trained human resources, and time for quality assurance activities.105 Internal and external audits, peer review, regulation, accreditation, certification, checklists, adverse event reporting, common protocols, quality improvement, and independent checking are examples of interventions ensuring safety and quality assurance in radiation oncology.64,106,107
Research. There are many fundamental questions that remain unanswered specific to cancer in developing countries. For instance, it cannot be assumed that approaches to treating cancer from developed settings will produce the same results when applied in other countries. Considerations include potential differences in disease bulk, malnutrition, rates of chronic infections such as HIV/tuberculosis/hepatitis B, and genetic polymorphisms affecting disease biology and treatment response.108–112 Resources for supportive care and quality assurance are also considerations. There are many unknowns in cancer epidemiology and basic science, and, as mentioned, more health services research is emphatically needed into areas such as access, quality, and economics.87
Supporting research on cancer by investigators in the developing world is important as it can build local research capacity and provide a means of adapting scientific knowledge to local circumstances to meet national health priorities.113 The U.S. National Cancer Institute has also been involved with numerous international collaborations. Protocol-driven clinical research can also strengthen local treatment capacity. The INCTR has been involved in designing clinical trials relevant to developing world situations, as has the IAEA.
International research partnerships are essential in the interconnected and interdependent world we live in.113 Many developing countries have quite advanced resources to sustain research activities; for instance, a number of clinical trials for cervical cancer radiotherapy have occurred in India (e.g., HDR vs. LDR brachytherapy, radiation vs. chemoradiation).114,115 India is also home of the Advanced Center for Treatment, Research and Education in Cancer (ACTREC), part of the Tata Memorial Center. Regional research collaborations are developing, for example, the FNCA. In addition, of note, a number of research/teaching twinning partnerships between developed and developing countries have been formed.97,98,116
Undoubtedly, industry and development will play an important role in improving access to quality radiation therapy equipment. Equipment that is affordable, safe, and technically suitable for developing country conditions is needed.77 The IAEA has taken leadership in advocacy for such equipment, and there is hope that new solutions will proliferate. Creative public–private partnerships will be important, as will be innovative equipment design. This last point has been exemplified by a group in Canada that has pioneered cobalt-60 tomotherapy.117
 CONCLUSION
CONCLUSION
Cancer in the developing world is an urgent problem, reaching critical proportions. Almost 60% of all cancer cases occur in the developing world. Vast numbers of people in developing countries have either limited access or no access to radiation therapy. At a time when new gains in oncology outcomes in the developed world are incremental, oncologists have the chance to help make some of the largest survival gains in history in the developing world. In addition, the potential for health care gains through cancer prevention and early detection, and the relief of suffering through palliative care are enormous. The poor deserve fair access to quality cancer care. The challenge will now be to deliver this in a thoughtful and contextually appropriate way.
 REFERENCES
REFERENCES
1. World Bank. World Bank country classifications. 2010. Available at: http://data.worldbank.org/about/country-classifications. Accessed August 13, 2011.
2. World Health Organization. Projections of mortality and burden of disease, 2004-2030. 2008. Available at: http://www.who.int/healthinfo/global_burden_disease/projections/en/index.html. Accessed August 13, 2011.
3. Ferlay J, Shin HR, Bray F, et al. GLOBOCAN 2008 v.1.2, Cancer incidence and mortality worldwide. IARC CancerBase No. 10 [Internet]. 2010. Available at: http://www-dep.iarc.fr. Accessed August 14, 2011.
4. Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat. World population prospects: the 2010 revision. 2011. Available at: http://esa.un.org/unpd/wpp/index.htm. Accessed August 6, 2011.
5. Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349(9063):1436–1442.
6. Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61(2):69–90.
7. Wabinga HR, Parkin DM, Wabwire-Mangen F, et al. Cancer in Kampala, Uganda, in 1989-91: changes in incidence in the era of AIDS. Int J Cancer 1993;54(1):26–36.
8. Danaei G, Vander Hoorn S, Lopez AD, et al. Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet 2005;366(9499):1784–1793.
9. Jha P, Ranson MK, Nguyen SN, et al. Estimates of global and regional smoking prevalence in 1995, by age and sex. Am J Public Health 2002;92(6):1002–1006.
10. World Health Organization. WHO report on the global tobacco epidemic, 2011: warning about the dangers of tobacco. Appendix V: country profiles. 2011. Available at: http://www.who.int/tobacco/global_report/2011/en_tfi_global_report_2011_appendix_V_table_1.pdf. Accessed August 28, 2011.
11. World Health Organization. WHO report on the global tobacco epidemic, 2008: the MPOWER package. Geneva: World Health Organization, 2008.
12. Lopez AD, Collishaw NE, Piha T. A descriptive model of the cigarette epidemic in developed countries. Tobacco Control 1994;3(3):242–247.
13. Niu SR, Yang GH, Chen ZM, et al. Emerging tobacco hazards in China: 2. Early mortality results from a prospective study. BMJ 1998;317(7170):1423–1424.
14. World Health Organization and Centers for Disease Control. Tobacco or health: a global status report. 1997. Available at: http://www.cdc.gov/tobacco/WHO/index.htm. Accessed December 2, 2006.
15. Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer 2006;118(12):3030–3044.
16. Perz JF, Armstrong GL, Farrington LA, et al. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol 2006;45(4):529–538.
17. IARC. IARC monographs on the evaluation of carcinogenic risks to humans: some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins. Vol. 56. United Kingdom: World Health Organization, 1993.
18. IARC. IARC monographs on the evaluation of carcinogenic risks to humans: schistosomes, liver flukes and Helicobacter pylori. United Kingdom: World Health Organization, 1994.
19. Key TJ, Schatzkin A, Willett WC, et al. Diet, nutrition and the prevention of cancer. Public Health Nutr 2004;7(1A):187–200.
20. Chow WH, Gridley G, Fraumeni JF Jr, et al. Obesity, hypertension, and the risk of kidney cancer in men. N Engl J Med 2000;343(18):1305–1311.
21. Popkin BM. The nutrition transition and obesity in the developing world. J Nutr 2001;131(3):871S–873S.
22. Liede A, Narod SA. Hereditary breast and ovarian cancer in Asia: genetic epidemiology of BRCA1 and BRCA2. Hum Mutat 2002;20(6):413–424.
23. Bono AV. The global state of prostate cancer: epidemiology and screening in the second millennium. BJU Int 2004;94(Suppl 3):1–2.
24. Vineis P, Xun W. The emerging epidemic of environmental cancers in developing countries. Ann Oncol 2009;20(2):205–212.
25. Kanavos P. The rising burden of cancer in the developing world. Ann Oncol 2006;17(Suppl 8):viii15–viii23.
26. Beaulieu N, Bloom DE, Reddy Bloom L, et al. Breakaway: the global burden of cancer-challenges and opportunities. New York: Economist Intelligence Unit: The Economist, 2009.
27. Penchansky R, Thomas JW. The concept of access: definition and relationship to consumer satisfaction. Med Care 1981;19(2):127–140.
28. Mackillop WJ. Health services research in radiation oncology: towards achieving the achievable for patients with cancer. In: Gunderson LL, Tepper JE, eds. Clinical radiation oncology, 2nd ed. New York: Churchill Livingstone, 2006.
29. Farmer P, Frenk J, Knaul FM, et al. Expansion of cancer care and control in countries of low and middle income: a call to action. Supplementary Webbapendix. Lancet 2010;376(9747):1186–1193.
30. Frenk J, Chen L, Bhutta ZA, et al. Health professionals for a new century: transforming education to strengthen health systems in an interdependent world. Lancet 2010;376(9756):1923–1958.
31. Tatsuzaki H, Levin CV. Quantitative status of resources for radiation therapy in Asia and Pacific region. Radiother Oncol. 2001;60(1):81–89.
32. Zubizarreta EH, Poitevin A, Levin CV. Overview of radiotherapy resources in Latin America: a survey by the International Atomic Energy Agency (IAEA). Radiother Oncol 2004;73(1):97–100.
33. Kron T, Cheung K, Dai J, et al. Medical physics aspects of cancer care in the Asia Pacific region. Biomed Imaging Interv J 2008;4(3):e33.
34. Zaidi H. Medical physics in developing countries: looking for a better world. Biomed Imaging Interv J 2008;4(1):e29.
35. International Atomic Energy Agency. Directory of radiotherapy centers (DIRAC). Available at: http://www-naweb.iaea.org/nahu/dirac/default.shtm. Accessed August 7, 2011.
36. Levin V, Tatsuzaki H. Radiotherapy services in countries in transition: gross national income per capita as a significant factor. Radiother Oncol 2002;63(2):147–150.
37. Bese NS, Munshi A, Budrukkar A, et al. Breast radiation therapy guideline implementation in low- and middle-income countries. Cancer 2008;113(8 Suppl):2305–2314.
38. Levin CV, El Gueddari B, Meghzifene A. Radiation therapy in Africa: distribution and equipment. Radiother Oncol 1999;52(1):79–84.
39. Barton MB, Frommer M, Shafiq J. Role of radiotherapy in cancer control in low-income and middle-income countries. Lancet Oncol 2006;7(7):584–595.
40. Sanabria A, Domenge C, D’Cruz A, et al. Organ preservation protocols in developing countries. Curr Opin Otolaryngol Head Neck Surg 2010;18(2):83–88.
41. Medicines Access and Rational Use, Department of Essential Medicines and Pharmaceutical Policies, Health Systems and Services. World Health Organization briefing note – February 2009. Access to controlled medications programme. Improving access to medications controlled under international drug conventions. Geneva: World Health Organization, 2009.
42. Anyanwu SN, Egwuonwu OA, Ihekwoaba EC. Acceptance and adherence to treatment among breast cancer patients in Eastern Nigeria. Breast 2011;20(Suppl 2):S51–S53.
43. Ekortarl A, Ndom P, Sacks A. A study of patients who appear with far advanced cancer at Yaounde General Hospital, Cameroon, Africa. Psychooncology 2007;16(3):255–257.
44. Leong BD, Chuah JA, Kumar VM, et al. Trends of breast cancer treatment in Sabah, Malaysia: a problem with lack of awareness. Singapore Med J 2009;50(8):772–776.
45. Obi SN, Ozumba BC. Cervical cancer: socioeconomic implications of management in a developing nation. J Obstet Gynaecol 2008;28(5):526–528.
46. Wagner AK, Graves AJ, Reiss SK, et al. Access to care and medicines, burden of health care expenditures, and risk protection: results from the World Health Survey. Health Policy 2011;100(2–3):151–158.
47. Farmer P, Frenk J, Knaul FM, et al. Expansion of cancer care and control in countries of low and middle income: a call to action. Lancet 2010;376(9747):1186–1193.
48. Arrossi S, Matos E, Zengarini N, et al. The socio-economic impact of cervical cancer on patients and their families in Argentina, and its influence on radiotherapy compliance. Results from a cross-sectional study. Gynecol Oncol 2007;105(2):335–340.
49. Machlin A, Wakefield M, Spittal M, et al. Cancer-related beliefs and behaviours in eight geographic regions. Geneva: Union for International Cancer Control, 2009.
50. Fles R, Wildeman MA, Sulistiono B, et al. Knowledge of general practitioners about nasopharyngeal cancer at the Puskesmas in Yogyakarta, Indonesia. BMC Med Educ 2010;10:81.
51. Institute of Medicine Committee on Quality of Health Care in America. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academy Press, 2001.
52. Donabedian A. The quality of care. How can it be assessed? JAMA 1988;260(12):1743–1748.
53. Bright K, Barghash M, Donach M, et al. The role of health system factors in delaying final diagnosis and treatment of breast cancer in Mexico City, Mexico. Breast 2011;20(Suppl 2):S54–S59.
54. Khoo SP, Shanmuhasuntharam P, Mahadzir WM, et al. Factors involved in the diagnosis of oral squamous cell carcinoma in Malaysia. Asia Pac J Public Health 1998;10(1):49–51.
55. Dye TD, Bogale S, Hobden C, et al. Complex care systems in developing countries: breast cancer patient navigation in Ethiopia. Cancer 2010;116(3):577–585.
56. Izewska J, Andreo P, Vatnitsky S, et al. The IAEA/WHO TLD postal dose quality audits for radiotherapy: a perspective of dosimetry practices at hospitals in developing countries. Radiother Oncol 2003;69(1):91–97.
57. Izewska J, Vatnitsky S, Shortt KR. Postal dose audits for radiotherapy centers in Latin America and the Caribbean: trends in 1969–2003. Rev Panam Salud Publica 2006;20(2–3):161–172.
58. Sharma V, Gaye PM, Wahab SA, et al. Patterns of practice of palliative radiotherapy in Africa, part 1: bone and brain metastases. Int J Radiat Oncol Biol Phys 2008;70(4):1195–1201.
59. Cazap E, Buzaid AC, Garbino C, et al. Breast cancer in Latin America: results of the Latin American and Caribbean Society of Medical Oncology/Breast Cancer Research Foundation expert survey. Cancer 2008;113(8 Suppl):2359–2365.
60. El Saghir NS, El-Asmar N, Hajj C, et al. Survey of utilization of multidisciplinary management tumor boards in Arab countries. Breast 2011;20(Suppl 2):S70–S74.
61. Mohanti BK, Nachiappan P, Pandey RM, et al. Analysis of 2167 head and neck cancer patients’ management, treatment compliance and outcomes from a regional cancer centre, Delhi, India. J Laryngol Otol 2007;121(1):49–56.
62. Shakespeare TP, Back MF, Lu JJ, et al. External audit of clinical practice and medical decision making in a new Asian oncology center: results and implications for both developing and developed nations. Int J Radiat Oncol Biol Phys 2006;64(3):941–947.
63. Mohanti BK. Introducing radiotherapy to Oman. Eur J Cancer 2008;44(3):333.
64. Barton M, Shafiq J, eds. Radiotherapy risk profile: technical manual. Geneva: World Health Organization, Radiotherapy Safety Team within the World Alliance for Patient Safety, 2008.
65. Sankaranarayanan R, Swaminathan R, eds. Cancer survival in Africa, Asia, the Caribbean and Central America. IARC Scientific Publications No. 162. Lyon, France: International Agency for Research on Cancer, 2011.
66. Barton MB, Gebski V, Manderson C, et al. Radiation therapy: are we getting value for money? Clin Oncol (R Coll Radiol) 1995;7(5):287–292.
67. Barton MB, Jacob SA, Gebski V. Utility-adjusted analysis of the cost of palliative radiotherapy for bone metastases. Australas Radiol 2003;47(3):274–278.
68. Marks LB, Hardenbergh PH, Winer ET, et al. Assessing the cost-effectiveness of postmastectomy radiation therapy. Int J Radiat Oncol Biol Phys 1999;44(1):91–98.
69. Groot MT, Baltussen R, Uyl-de Groot CA, et al. Costs and health effects of breast cancer interventions in epidemiologically different regions of Africa, North America, and Asia. Breast J 2006;12(Suppl 1):S81–S90.
70. Van Der Giessen PH, Alert J, Badri C, et al. Multinational assessment of some operational costs of teletherapy. Radiother Oncol 2004;71(3):347–355.
71. Vikram B. Radiation therapy for the developing countries. J Cancer Res Ther 2005;1(1):7–8.
72. Kitchener HC, Hoskins W, Small W, Jr, et al. The development of priority cervical cancer trials: a Gynecologic Cancer InterGroup report. Int J Gynecol Cancer 2010;20(6):1092–1100.
73. van Lonkhuijzen L, Thomas G. Palliative radiotherapy for cervical carcinoma, a systematic review. Radiother Oncol 2011;98(3):287–291.
74. Kepka L, Casas F, Perin B, et al. Radiochemotherapy for lung cancer in developing countries. Clin Oncol (R Coll Radiol) 2009;21(7):536–542.
75. Nag S, Dally M, de la Torre M, et al. Recommendations for implementation of high dose rate 192Ir brachytherapy in developing countries by the Advisory Group of International Atomic Energy Agency. Radiother Oncol 2002;64(3):297–308.
76. Overgaard J, Mohanti BK, Begum N, et al. Five versus six fractions of radiotherapy per week for squamous-cell carcinoma of the head and neck (IAEA-ACC study): a randomised, multicentre trial. Lancet Oncol 2010;11(6):553–560.
77. Salminen EK, Kiel K, Ibbott GS, et al. International Conference on Advances in Radiation Oncology (ICARO): outcomes of an IAEA meeting. Radiat Oncol 2011;6:11.
78. AfrOx. London declaration on cancer control in Africa. London: AfrOx 2007.
79. Anderson BO, Cazap E, El Saghir NS, et al. Optimisation of breast cancer management in low-resource and middle-resource countries: executive summary of the Breast Health Global Initiative consensus, 2010. Lancet Oncol 2011;12(4):387–398.
80. Anderson BO, Ballieu M, Bradley C, et al. Access to cancer treatment in low- and middle-income countries-an essential part of global cancer control. A CanTreat Position Paper. 2010. Available at: http://axios-group.com/index.php/download_file/view/85/151/. Accessed July 6, 2011.
81. Sloan FA, Gelband H, eds. Cancer control opportunities in low- and middle-income countries. Washington, DC: National Academy Press, 2007.
82. Union for International Cancer Control. The world cancer declaration 2008. 2011. Available at: http://www.uicc.org/declaration/download-declaration. Accessed July 31, 2011.
83. World Health Organization. National cancer control programmes: policies and managerial guidelines. Geneva: World Health Organization, 2002.
84. United Nations General Assembly 66th Session. Political declaration of the high-level meeting of the General Assembly on the Prevention and Control of Non-communicable Diseases. A/66/L.1. Sept. 16, 2011. New York: United Nations, 2011.
85. Harris J. Cancer: the new challenge for health care in the developing world. HemOnc Today 2009. Available at: http://www.healio.com/hematology-oncology. Accessed November 10, 2012.
86. Samb B, Desai N, Nishtar S, et al. Prevention and management of chronic disease: a litmus test for health-systems strengthening in low-income and middle-income countries. Lancet 2010;376(9754):1785–1797.
87. Hanna TP, Kangolle AC. Cancer control in developing countries: using health data and health services research to measure and improve access, quality and efficiency. BMC Int Health Hum Rights 2010;10:24.
88. Knaul FM, Frenk J, Shulman LN, for the Global Task Force on Expanded Access to Cancer Care and Control in Developing Countries. Closing the cancer divide: a blueprint to expand access in low and middle income countries. Boston, MA: Harvard Global Equity Initiative, 2011.
89. World Health Organization. Cancer control: knowledge into action: WHO guide for effective programs; module 4. Diagnosis and treatment. Geneva: World Health Organization, 2008.
90. Anderson BO, Shyyan R, Eniu A, et al. Breast cancer in limited-resource countries: an overview of the Breast Health Global Initiative 2005 guidelines. Breast J 2006;12(Suppl 1):S3–S15.
91. Collingridge D. Delivering consensus from the Asian Oncology Summit 2009. Lancet Oncol 2009;10(11):1029–1030.
92. Macbeth FR, Abratt RP, Cho KH, et al. Lung cancer management in limited resource settings: guidelines for appropriate good care. Radiother Oncol 2007;82:123–131.
93. Datta NR, Rajasekar D. Improvement of radiotherapy facilities in developing countries: a three-tier system with a teleradiotherapy network. Lancet Oncol 2004;5(11):695–698.
94. Winstead ER. In Bangladesh, developing models of cancer care. NCI Cancer Bull 2010;7(23).
95. Coffey M, Engel-Hills P, El-Gantiry M, et al. A core curriculum for RTTs (radiation therapists/radiotherapy radiographers) designed for developing countries under the auspices of the international atomic energy agency (IAEA). Radiother Oncol 2006;81:324–325.
96. Podgorsak EB. Radiation oncology physics: a handbook for teachers and students. Vienna: IAEA, 2005.
97. Ribeiro RC, Pui CH. Saving the children–improving childhood cancer treatment in developing countries. N Engl J Med 2005;352(21):2158–2160.
98. Masera G, Baez F, Biondi A, et al. North-South twinning in paediatric haemato-oncology: the La Mascota programme, Nicaragua. Lancet 1998;352(9144):1923–1926.
99. Patel JD, Galsky MD, Chagpar AB, et al. Role of American Society of Clinical Oncology in low- and middle-income countries. J Clin Oncol 2011;29(22):3097–3102.
100. Ohno T, Nakano T, Kato S, et al. Accelerated hyperfractionated radiotherapy for cervical cancer: multi-institutional prospective study of forum for nuclear cooperation in Asia among eight Asian countries. Int J Radiat Oncol Biol Phys 2008;70(5):1522–1529.
101. Tiwari M. Interview with outgoing president of AROI. J Cancer Res Ther 2009;5(1):62.
102. Peabody JW, Taguiwalo MM, Robalino DA, et al. Improving the quality of care in developing countries. In: Jamison DT, Breman JG, Measham AR, et al., eds. Disease control priorities in developing countries. Washington, DC: World Bank and Oxford University Press, 2006.
103. Borras C. Overexposure of radiation therapy patients in Panama: problem recognition and follow-up measures. Rev Panam Salud Publica 2006;20(2–3):173–187.
104. Barton MB. Improving access to education and cancer care in developing countries. 14th World Conference on Lung Cancer. Amsterdam: International Association for the Study of Lung Cancer, 2011.
105. Kutcher GJ, Coia L, Gillin M, et al. Comprehensive QA for radiation oncology: report of AAPM Radiation Therapy Committee Task Group 40. 1994/04/01 ed. College Park, MD: American Institute of Physics, 1994.
106. Mytton OT, Velazquez A, Banken R, et al. Introducing new technology safely. Qual Saf Health Care 2010;19(Suppl 2):i9–i14.
107. Newton RC, Mytton OT, Aggarwal R, et al. Making existing technology safer in healthcare. Qual Saf Health Care 2010;19(Suppl 2):i15–i24.
108. Negi RR, Gupta M, Kumar M, et al. Concurrent chemoradiation in locally advanced carcinoma cervix patients. J Cancer Res Ther 2010;6(2):159–166.
109. McArdle O, Kigula-Mugambe JB. Contraindications to cisplatin based chemoradiotherapy in the treatment of cervical cancer in Sub-Saharan Africa. Radiother Oncol 2007;83(1):94–96.
110. Carles J, Monzo M, Amat M, et al. Single-nucleotide polymorphisms in base excision repair, nucleotide excision repair, and double strand break genes as markers for response to radiotherapy in patients with stage I and II head-and-neck cancer. Int J Radiat Oncol Biol Phys 2006;66(4):1022–1030.
111. Grau C, Prakash Agarwal J, Jabeen K, et al. Radiotherapy with or without mitomycin c in the treatment of locally advanced head and neck cancer: results of the IAEA multicentre randomised trial. Radiother Oncol 2003;67(1):17–26.
112. Wu X, Gu J, Wu TT, et al. Genetic variations in radiation and chemotherapy drug action pathways predict clinical outcomes in esophageal cancer. J Clin Oncol 2006;24(23):3789–3798.
113. Frenk J, Chen L. Overcoming gaps to advance global health equity: a symposium on new directions for research. Health Res Policy Syst 2011;9(1):11.
114. Patel FD, Sharma SC, Negi PS, et al. Low dose rate vs. high dose rate brachytherapy in the treatment of carcinoma of the uterine cervix: a clinical trial. Int J Radiat Oncol Biol Phys 1994;28(2):335–341.
115. Singh TT, Singh IY, Sharma DT, et al. Role of chemoradiation in advanced cervical cancer. Indian J Cancer 2003;40(3):101–107.
116. Veerman AJ, Sutaryo, Sumadiono. Twinning: a rewarding scenario for development of oncology services in transitional countries. Pediatr Blood Cancer 2005;45(2):103–106.
117. Schreiner LJ, Kerr A, Salomons G, et al. The potential for image guided radiation therapy with cobalt-60 tomotherapy. In: Ellis RE, Peters TM, eds. Lecture notes in computer science: Proc. 6th Annual International Conference on Medical Image Computing and Computer Assisted Intervention (MICCAI). Vol 8. Heidelberg: Springer-Verlag, 2003:449–456.
< div class='tao-gold-member'>




