Chapter Outline
Standardisation 533
Reference preparations and control materials 535
Quality assurance procedures 536
Internal quality control procedures 537
Control charts 537
Duplicate tests on patients’ specimens 538
Use of patient data for internal quality control 538
Correlation check 539
External quality assessment procedures 539
Analysis of external quality assessment data 540
Target values 540
Preparation of extended-life material for use in quality assessment 543
Preparation of preserved whole blood 543
Preparation of haemolysate 543
Preparation of stabilised whole blood control material 544
Preparation of stable control material for external quality assessment 544
Preparation of quality control material for platelet count 545
Quality assurance (QA) in the haematology laboratory is intended to ensure reliable diagnostic test results with the necessary degree of accuracy and precision. There are some key definitions in QA that are fundamental to an understanding of its practice ( Table 25-1 ).
| Specificity | Measures only the analyte of interest |
| Accuracy | The closeness of agreement between the true value and the observed value |
| Precision | The closeness of agreement among a series of measurements of a single sample. A test can be precise without being accurate. |
| Linearity | The ability of a test to obtain results that are directly proportional to the analyte concentration |
| Limits | The upper and lower limits of detection of the assay |
| Range | The interval between the upper and lower limits of detection |
| Robustness | A measure of how much a test or assay is affected by small variations in methodology |
In any test, inaccuracy, imprecision or both can occur as a result of using unreliable standards, controls or reagents, incorrect instrument calibration or poor laboratory technique. Precision can be controlled by replicate tests and by repeated tests on previously measured specimens. Accuracy can, as a rule, be checked only by the use of reference materials that have been assayed by reference methods and against standards of known concentration e.g. World Health Organisation (WHO) reference plasma.
Quality assurance must ensure adequate control of the pre-analytical and postanalytical phases (i.e. from test selection and specimen collection to the timely despatch of an informative report) as well as the analytical phase, as the majority of diagnostic errors occur as a result of untoward events in these stages of the diagnostic cycle. There are essentially two parts to QA:
- 1.
Internal quality control (IQC)
- 2.
External quality assessment (EQA) – often termed proficiency testing
This chapter describes the use of reference preparations and the principles and procedures of QA. The blood count is used as the illustrative model.
Standardisation
Standardisation of medical laboratory practice is essential for the quality of patient care, especially when patients may be served by multiple pathology providers. It encompasses the materials and methods used and the harmonisation of terminology, units and reference ranges. A variety of standards, reference preparations and methods may be used to facilitate standardisation of laboratory outcomes but these are not available for all analytes or tests.
- 1.
Material standards or reference preparations are used to calibrate analysers and to assign values to calibrators. Where possible, they are traceable to defined physical or chemical measurement based on the metrological units of length (metre), mass (kilogram), amount of substance (mole) and time (seconds).
- 2.
A reference method is a precisely defined technique which, in association with a reference preparation, provides sufficient precise and accurate data for scientific purposes and for assessing the validity of other methods.
- 3.
A selected method is one that is directly comparable with and traceable to the international reference method. It serves as an alternative to the reference method when an international reference material is not available; it should be used for evaluation and validation of a proposed routine (working) method.
- 4.
A working (or recommended) method is intended for use in routine practice, taking account of economy of labour and materials and ease of performance and having been shown by a validation study with a reference method to be sufficiently reliable for its intended purpose.
Standardisation in haematology is the concern of the International Council for Standardisation in Haematology (ICSH) and other international and national organisations, whose recommendations are published in haematology journals or are available from the organisations’ websites (see Table 25-2 ). The International Organisation of Standardisation (ISO) and the Comité Européen de Normalisation (CEN) have established standards for medical laboratory practice, the use of in vitro medical devices (IVDs) and the provision of EQA. At a national level, the British Committee for Standards in Haematology (BCSH) publishes guidelines in books, on websites or as journal articles, and in the USA, a wide range of practice guidelines have been published by the Clinical and Laboratory Standards Institute (CLSI) (formerly the National Committee for Clinical Laboratory Standards, NCCLS). Table 25-3 lists international standards and guides applicable to laboratory medicine.
| Abbreviation | Organisation | Website |
|---|---|---|
| AENOR | Asociación Española de Normalización y Certificación | www.aenor.es |
| AFNOR | Association Française de Normalization | www.afnor.org |
| AMREF | African Medical and Research Foundation, Nairobi, Kenya | www.amref.org |
| ANCLSH | Asian Network for Clinical Laboratory Standardisation and Harmonization | www.ancls.org |
| ASQ | American Society of Quality | www.asq.org |
| BCSH | British Committee for Standards in Haematology | www.bcshguidelines.com |
| CAP | College of American Pathologists | www.cap.org |
| CEN | The European Committee for Standardisation | www.cen.eu |
| CLSI | Clinical and Laboratory Standards Institute | www.clsi.org |
| EQALM | European Organisation for External Quality Assurance Providers in Laboratory Medicine | www.eqalm.org |
| ICSH | International Council for Standardisation in Haematology | www.icsh.org |
| IFCC | International Federation of Clinical Chemistry and Laboratory Medicine | www.ifcc.org |
| INSTAND | Gesellschaft zur Förderung der Qualitätssicherung in medizinischen Laboratorien | www.instandev.de |
| IRMM | Institute of Reference Materials and Measurements | Irmm.jrc.ec.europa.eu |
| ISO | International Organisation for Standardisation | www.iso.org |
| JCAHO | Joint Commission for the Accreditation of Healthcare Organisations | www.jointcommission.org |
| PPTC | Pacific Paramedical Training Centre New Zealand | www.pptc.org.nz |
| RCPA | Royal College of Pathologists Australia | www.rcpaqap.com.au |
| UK NEQAS | United Kingdom National External Quality Assessment Service | www.ukneqas.org.uk |
| WHO | World Health Organisation EQAS for Haematology | www.who.int/diagnostics_laboratory/quality/haematology/en/index.html |
| Standard | Title |
|---|---|
| BS EN ISO 9001 | Quality management systems – requirements |
| BS EN 14136 | Use of external quality assessment schemes in the assessment of the performance of in vitro diagnostic examination procedures |
| BS EN ISO 15189 | Medical laboratories – particular requirements for quality and competence |
| BS EN ISO 22870 | Point-of-care testing (POCT) – requirements for quality and competence |
| BS EN ISO 9000 | Quality management systems – fundamentals and vocabulary |
| BS EN ISO/IEC 17025 | General requirements for the competence of testing and calibration laboratories |
| BS EN ISO/IEC 17043 | Conformity assessment – general requirements for proficiency testing |
| BS ISO 13528 | Statistical methods for use in proficiency testing by interlaboratory comparisons |
Accreditation (see p. 525) is a process by which an authorised national body or organisation gives formal recognition that a laboratory is considered competent to carry out specific tasks, as measured against published quality standards. It is a procedure that ensures the correct conditions for the provision of a quality service, providing a measure of confidence for the service user, although it does not measure the quality of the laboratory’s output. Accreditation requires the laboratory to demonstrate a process of continuous quality improvement and audit of its quality management system and internal processes.
In the European Union (EU), the production and supply of in vitro devices, including instruments, kits, calibrators and reagents used in the laboratory, is regulated by the EU In Vitro Diagnostic Medical Devices directive. This requires that manufacturers use the Conformité Européenne (CE) mark on their products to certify compliance with their claims. The CE marking process is supervised nationally by the appropriate authority in each EU member state and is applicable to ‘in-house’ test methods where these are used to test samples from patients from other organisations. EQA schemes have a role in identifying unsatisfactory performance by in vitro diagnostic devices.
Reference preparations and control materials
The main international authority concerned with reference preparations or reference reagents for laboratory medicine is the WHO. In the European Union, the Institute for Reference Materials and Measurements (IRMM) has established a number of ‘certified reference materials’ for haematology and clinical chemistry. International standards are not intended for routine use but serve as stable standards for assigning values to commercial (or laboratory produced) ‘ secondary standards ’ or calibrators. International reference materials relevant to haematology ( Table 25-4 ) are held at designated institutions; the relevant websites should be checked for availability of any particular one.
| Organisation | Materials of interest | Source |
|---|---|---|
| WHO | Blood products, including haemoglobin A 2 and haemoglobin F | www.who.int/bloodproducts/catalogue Most products are kept at the National Institute for Biological Standards and Controls ( www.nibsc.org ). The catalogue lists other custodian laboratories where appropriate. |
| Blood safety | ||
| Coagulation factors | ||
| Fibrinolytic agents, protease inhibitors, anticoagulants | ||
| Immunoglobulins and human sera | ||
| Platelet-specific proteins | ||
| Haematinics | ||
| IRMM | Clinical chemistry | https://ec.europa.eu/jrc/en/reference-materials |
| Haemoglobincyanide | ||
| Human serum proteins | ||
| Leukaemia monitoring | ||
| Thromboplastin (bovine and rabbit) | ||
| ICSH | Haemoglobin reference standard | www.eurotrol.com |
Methods used for assigning values to reference materials must be as accurate and precise as is practical. Standardised reference methods have been described for haemoglobin concentration (Hb), red blood cell count (RBC), white blood cell count (WBC) and packed cell volume/haematocrit (PCV/Hct) (see Chapter 3 ).
Haemoglobin reference preparations
The accessibility of an international reference standard of haemiglobincyanide (HiCN), first developed by the ICSH, has improved the accuracy of haemoglobin measurement. In some countries, preparations that conform to the international standard are certified by the appropriate national authorities. An important feature of this material is that it is stable for at least several years. A limited quantity of the international standard can be obtained from the WHO; a comparable certified reference material is available from IRMM, and the ICSH has produced a new preparation with similar specifications (see Table 25-4 ). Where the use of the cyanide reagent for routine haemoglobinometry is prohibited, the haemiglobincyanide standard can still be used to assign a haemoglobin value to a lysate or a whole blood preparation, which is then used as the local secondary standard after appropriate dilution. Undiluted lysate is usually stable for up to 6 months, if frozen for several years. Whole blood is stable for about 3 weeks, but for only a few days after dilution. Both whole blood and lysates are useful for QA of haemoglobinometry; reference samples should be introduced into batches of blood samples with all the samples being assayed together. This applies to both automated and manual methods. Many laboratories are unable to make use of this reference preparation as it is unsuitable for direct use in automated haematology analysers; however, the analyser manufacturer will trace the instrument’s calibration to the reference preparation.
Quality control preparations
Controls are preparations which are used for either IQC or EQA. Some control preparations have assigned values but they should not be used as standards as the assigned values are usually only approximations and the preparations may be stable for a limited time only.
Control materials are available commercially and they can also be made locally, although there are difficulties in the preparation and validation of such ‘homemade’ materials. For example, stored plasma may become turbid; chemical or serological analysis may be affected by instability of enzymes; added preservatives may interfere with immunological reactions. With the blood count, there are especially difficult problems due to the instability of blood cells and the need to ensure homogeneity in aliquot samples, while procedures that enhance the stability of blood samples may also affect the behaviour of the cells. The preparation of extended life materials for QA is described at the end of this chapter.
Quality assurance procedures
The procedures that should be included in a QA programme vary with the tests undertaken, the instruments used, the size of the laboratory, the numbers of specimens handled, the computer facilities available and the amount of time that can be devoted to QA. At least some form of IQC must be undertaken and there must be participation in EQA for any test that a laboratory offers. Where no EQA programme is available the laboratory should establish some other means of assessing interlaboratory comparability, e.g. sample exchange with another laboratory. Some control procedures should be performed daily and other performance checks should be done at appropriate intervals. A review of performance is particularly important when there is a change in staff and after maintenance service or repair has been carried out on equipment. An example of a QA protocol is summarised in Table 25-5 .
| Calibration with reference standards | |
| Instruments: | 6-month intervals or more frequently if control chart or EQA indicates bias or fluctuation in results and after any repair/service |
| Pipettes, balances: | 6–12 month intervals |
| Diluting systems: | Initially and at 1–2-week intervals |
| Control Chart with Control Material | |
| Daily, with each batch of specimens or at regular intervals in a continuous process Duplicate tests on two or three patients’ samples: if control chart or delta check shows discrepancies | |
| Analysis of Patients’ results | |
| Daily to check constancy of mean MCV, MCH, MCHC | |
| Correlation assessment of test report | |
| Cumulative results: following previous tests and if changes in clinical state Blood film examination if unusual test results and/or instrument flags appear | |
All laboratory staff require training in QA and how it is implemented in the laboratory. A useful training manual from WHO describes the principles and methods, together with practical exercises to illustrate these. Another invaluable teaching source is the Westgard QC website ( www.westgard.com ). Similarly, EQA providers will include guides on their programmes and may supply educational information as part of, or in addition to, their performance reports.
Internal quality control procedures
Internal quality control monitors the performance of the test procedures in the laboratory on daily or a batch-to-batch basis. It includes measurements on specially prepared control materials, repeated measurements on patients’ specimens and statistical analysis of patients’ test data. This ensures continual validation of the reliability of the results produced by the laboratory, before reports are released.
Control charts
These were first applied in clinical chemistry by Levey and Jennings as a means to monitor laboratory performance using control materials and are now widely used in haematology for both automated and manual procedures. Control material is included in every batch of patients’ specimens or at specified intervals during the shift, and the result is logged on a control chart. To check precision, it is not necessary to know the exact value of the control specimen; however, if its value has been determined reliably by a reference method, the same material can also be used to check accuracy or to calibrate an instrument. If possible, controls with high, normal and low values should be used to monitor the method across the range of its linearity. It is advisable to use at least one control sample per batch, even if the batch is small, or at set intervals during a large run or a continuous process. Because the controls are intended to simulate random sampling, they must be treated in the same way as the patients’ specimens as far as possible. The results obtained with the control samples can be plotted manually on a Levey–Jennings style chart, as described below, or can be plotted automatically by the instrument, which may also provide an alert if the analysis falls outside the preset, acceptable limits of performance.
When plotting a control chart manually, the mean value and standard deviation (SD) of the control specimen is first established in the laboratory. Using arithmetic graph paper, draw a horizontal line to represent the mean (as a base) and, on an appropriate scale of quantity and unit, draw lines representing + 2SD and − 2SD above and below the mean. Plot the results of successive control sample measurements. If the test performance is satisfactory, sequential results oscillate about the mean value and less than 5% of the results fall outside ± 2SD.
Figure 25-1 illustrates a control chart from an automated system; a similar principle can be used for simple methods where the data are plotted manually ( Fig. 25-2 ).
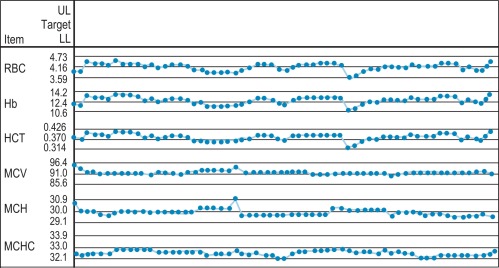
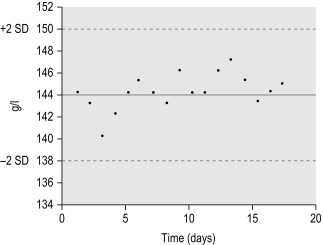
Any of the following indicates a fault in technique or in the instrument or reagent:
- 1.
One deviant result outside ± 3SD may indicate a gross error or ‘blunder’.
- 2.
One or two results on or beyond ± 2SD may indicate a random error in the system.
- 3.
Several consecutive similar results on one side of the mean indicate a consistent bias, possibly due to a calibration fault or a systematic error.
- 4.
Consecutive fluctuating values, rising and falling by ± 2SD, indicate imprecision.
- 5.
A trend of sequentially increasing or decreasing values with the repeated measurements is indicative of drift.
The fault may be in the reagents or the laboratory-ware, caused by incorrect adjustment/calibration of the instrument or other equipment (e.g. pipettes), technical error or even clerical error in transcribing the results. Before an intensive investigation is carried out, the test should be repeated with another control sample, and the possibility that the inconsistency is the result of deterioration of the batch of control material or insufficient mixing of the sample should be excluded. This control process is unlikely to detect an error in an individual specimen, which can only be detected by correlation checks. For haemoglobinometry, it may be useful to use both whole blood and lysate in a quality control check because differences in results obtained with these two preparations help to identify errors resulting from incorrect dilution, inadequate mixing or failure of a reagent to bring about complete lysis. If the control specimen is included with each batch of tests or is repeated during the course of a day, the measurements should not differ by more than the established coefficient of variation (CV) (see p. 566).
Duplicate tests on patients’ specimens
Duplicate tests on patients’ specimens provide another way of checking the precision of routine work. To start the process, test 10 consecutive specimens in duplicate under careful conditions. Calculate the differences between the pairs of results and derive the SD (p. 566). Subsequent duplicate measurements on any specimen in the same batch of tests should not differ from each other by more than ± 2SD. This method will detect random errors but not incorrect calibration. It is also insensitive to gradual drift which may, however, be detected if duplicate measurement is carried out in a later batch, provided that the specimen has not altered on standing. If the test is always badly done or has an inherent fault, the SD will be wide and the procedure may be ineffective.
This procedure is suitable for both manual and automated methods; it is impractical for routine blood counts in a busy laboratory, where three or four specimens in a batch can be tested in duplicate from time to time as a rough check of consistency.
Use of patient data for internal quality control
Where a minimum of 100 test requests are processed each day, there should be no significant day-to-day variability in the means of the red cell indices obtained by an automated blood counter, provided that the population of patients remains stable and that samples from a particular clinical source (e.g. renal or oncology patients) are not processed all in the same set, disproportionately influencing the mean. Assuming that the sample population is stable, any significant change in the means of the red cell indices will indicate a change in instrument calibration or a drift owing to a fault in its function. The procedure was developed by Bull , using a computerised algorithm to estimate the daily patient means of absolute values for mean cell volume (MCV), mean cell haemoglobin (MCH) and mean cell haemoglobin concentration (MCHC). In laboratories using manual methods, a simple adaptation of the same principle can be applied, confined to MCHC and excluding results from any specific clinic that are likely to be biased. From the daily means for all measurements on 10 consecutive working days, an overall daily mean and SD are established. The mean MCHC is then calculated at the end of each day. If the test does not vary by more than ± 2SD, it is considered to be satisfactory, but may be misleading if there is a simultaneous error in the same direction in both Hb and Hct. The results may be displayed graphically as illustrated in Figure 25-3 .
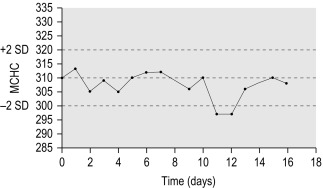
To start this programme for an automated laboratory, it is first necessary to assay samples from at least 300 to 500 patients using an automated blood counter over several days and to establish the means of the MCV, MCH and MCHC. Then, using the algorithm, it is possible to analyse the means in successive batches of 20 specimens. Plotting these means will allow any drift of the three indices to be recognised and used to identify instrument faults; an increased SD will signify loss of precision. To ensure that each batch is representative, the samples should be randomised before analysis and, if possible, within any batch of 20, no more than seven should come from one clinical source or have the same clinical condition. The method is now incorporated in automated blood counters ( Fig. 25-4 ).
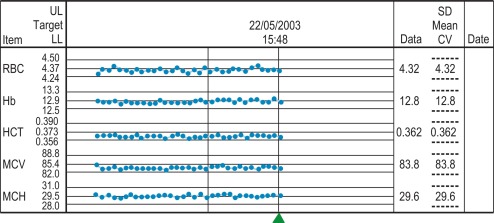
A similar instrument-specific procedure is used by some manufacturers who gather the data submitted through network links by users of their instruments. This enables them to maintain a constant check of performance of these instruments overall and to detect any that require recalibration or investigation of faults.
Correlation check
A correlation check implies that any unexpected patient’s result must be checked to see whether it can be explained on clinical grounds or whether it correlates with other tests. For example, an unexpectedly higher or lower Hb might be explained by blood transfusion or haemorrhage, respectively. A low MCHC may be confirmed by demonstrating hypochromic red cells on a stained blood film; a high MCV must correlate with macrocytosis. Similarly, the laboratory should have a policy on the examination of a blood film to confirm marked leucocytosis or leucopenia, thrombocytosis or thrombocytopenia, to distinguish between platelets and red cell fragments or conversely between giant platelets and normal-sized red cells and to check features flagged by the automated analyser. The examination of a stained blood film as a correlation check will only be effective if the film is correctly made, stained and examined systematically by an experienced member of staff.
Recording or reviewing cumulative laboratory data on an individual patient is good clinical practice and provides an inbuilt quality control system. This is especially useful in detecting pre-analytical phase errors (e.g. patient and sample identification errors, incorrect sample collection, inadequate suspension of the blood before testing, partial clotting or deterioration on storage).
A formal way of detecting aberrant results is the ‘delta check’. The blood count on any patient should not differ from counts obtained in the previous 2–3 weeks by more than an amount that takes into account both the test CV and physiological variation, providing that the patient’s clinical condition has not altered significantly. A discrepant result without apparent clinical reason must be suspect until confirmed by a repeat test on a fresh specimen. The occurrence of a contrasting discrepancy in two different specimens on the same day would suggest that two specimens have been transposed. The test can, of course, also be carried out on the blood of healthy individuals whose blood count remains virtually constant on a day-to-day basis, subject only to physiological change (see p. 13).
External quality assessment procedures
External quality assessment (EQA) is the evaluation by an outside agency of the performance of a number of laboratories using specially supplied samples of known but undisclosed content. The objective is to achieve between laboratory and between method comparability, but this does not necessarily guarantee accuracy unless the content of the specimen distributed for testing is traceable to a reference material.
EQA complements IQC, providing a long-term, retrospective assessment of participant performance against that of other laboratories and is a requirement for medical laboratories for accreditation to ISO15189. Even when all precautions are taken to achieve accuracy and precision in the laboratory, errors arise that are only detectable by objective EQA of performance on material that has been supplied specially for the purpose. Such schemes may be organised internationally, nationally or regionally and are accredited against the ISO17043 standard. The operation of EQA schemes is based on the circulation of survey material to a number of participating laboratories or individuals who return their results to the EQA organiser for analysis. Performance of each participant is evaluated against the target value for the material and out of consensus results identified. The participant is encouraged to review performance and take appropriate action in response to unsatisfactory performance. Action may be taken by the EQA organiser or appropriate professional body to improve performance if the problem becomes persistent.
The term EQA and proficiency testing are frequently used interchangeably, although proficiency testing may be defined as laboratory performance evaluation for regulatory purposes.
EQA has a number of complementary functions in addition to assessment of laboratory performance:
- 1.
Collecting information on the reliability of particular methods, materials and equipment
- 2.
Identifying problems with any device that require reaction from the manufacturer and/or reporting to the responsible national authority as described in EN 14136.
- 3.
Providing information on performance required for the purpose of licensing or accreditation
- 4.
Identifying laboratories whose performance provides a benchmarking standard
- 5.
Improving performance through education and the sharing of best practice
- 6.
Demonstrating competence to third parties and service users
- 7.
Promoting harmonisation of medical laboratory procedures
There are many EQA schemes available to laboratories. Some are officially promoted or sponsored by national governments or local health authorities. Unless participation in a particular scheme is mandatory, the laboratory must understand how EQA supports quality and how to select an appropriate EQA scheme. ICSH has prepared guidelines for the organisation and management of EQA schemes which are intended to help maintain a meaningful standard in the organisation of such schemes and to harmonise the way in which they function. The following principles and technical criteria are important in the provision of an effective EQA scheme, although some elements will depend upon the availability of survey material:
- 1.
Surveys should be sufficiently frequent to make sequential performance records meaningful and to identify participants who are persistently unsatisfactory as soon as possible.
- 2.
Specimens for blood count and other high throughput tests should be distributed at least monthly, other tests every 2 to 3 months, depending on their clinical importance and reliability of analytical methods.
- 3.
There should be at least two specimens for every test, with values at diagnostically critical levels.
- 4.
To ensure that EQA relates to practice, survey samples should simulate natural specimens as closely as feasible and participants should be obligated to handle them in the same way as they handle routine specimens.
- 5.
The material used in surveys should be stable, at least until the closing date of the survey.
- 6.
The survey specimens must test negative for antibodies to human immunodeficiency virus (HIV) and hepatitis B and C antigens and must be labelled in accordance with national regulations for packaging and transport of biological material.
- 7.
Data processing must be as rapid as possible, with prompt reports to participants.
- 8.
Organiser/participant confidentiality must be maintained. Provision of information on an individual participant’s results to a third party (e.g. a licensing authority) is the responsibility of the participant and not the EQA organiser, unless this is explicitly required by legislation.
- 9.
EQA provider organisations must be professionally led and should function independently of government health authorities.
- 10.
EQA schemes must be financially and operationally independent of commercial pressures, although industry may provide a useful service by organising interlaboratory comparison services for their users.
Analysis of external quality assessment data
The analysis of participants’ results and assessment of performance are different for quantitative, semiquantitative (ordinal) and qualitative (categorical or nominal) EQA data. Although the procedure for data analysis may vary according to the scheme, the purpose should be to allow an objective assessment of the participating laboratory’s result against an assigned or target value. All schemes should monitor nonparticipation, as EQA is only effective if results are submitted regularly.
Target values
The absence of metrological standards in haematology (with the exception of Hb) makes it difficult to establish the ‘true’ value for a test in haematology EQA. The EQA target value for a quantitative haematology assay can usually be assumed to be the result obtained by best performance of selected participants in the survey, by experts using reference methods or the consensus mean or median of participants’ results after trimming to remove outliers. In practice, the consensus of participants’ results is the usual method for determination of the target value in automated cell counting EQA. Consensus values for quantitative tests should only be calculated when the number of participants’ results available is sufficient to allow statistically meaningful analysis of results, and this generally will require a minimum of 20 participants per analysis group. Where a consensus all methods mean is used, it may be skewed by the instrument or method that has the greatest number in the data set; therefore, it is desirable to provide both all methods and method or submethod statistical analysis to elucidate performance problems and ensure fair treatment of participants. The use of stabilised EQA survey material (e.g. in automated counting) may also require analysis by matrix, instrument or method group as the performance of the stabilised material may not be analogous to fresh blood.
Before the consensus summary statistics (mean or median, SD and CV) are calculated, it is necessary to remove outliers using appropriate statistical methods. Major blunders (e.g. resulting from transposition of the EQA test specimens or reporting in the incorrect units) should be removed, either by the removal of any result that is more than ± 3SD from the mean or by the exclusion of the top and bottom 5% of participants’ results. The resulting ‘trimmed’ consensus mean and SD are then recalculated. The median is used when there is a non-Gaussian distribution of data with a wide scatter of results. If the median is used the estimated SD is calculated as:
Estimated SD = Central 50 % spread of data interquartile range ÷ 1.349
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree