Chapter Outline
Erythrocytes 439
Red cell antigens 439
Clinical significance of red cell alloantibodies 447
Mechanisms of immune destruction of red cells 448
Antigen–antibody reactions 450
General points of serological technique 450
Platelet and neutrophils 457
Platelet and neutrophil alloantigen systems 457
Isoantibodies 459
Autoantibodies 460
Demonstration of platelet and neutrophil antibodies 460
Methods of demonstrating antibodies 462
Molecular genotyping of platelet alloantigens 466
Erythrocytes
Red cell antigens
Since Landsteiner’s discovery in 1901, that human blood groups existed, a vast body of serological, genetic and biochemical data on red cell (blood group) antigens has been accumulated. More recently, the biological functions of some of these antigens have been appreciated.
A total of 30 blood group systems have been described ( Table 21-1 ). Each system is a series of red cell antigens, determined either by a single genetic locus or by very closely linked loci. In addition to the blood group systems, there are six ‘collections’ of antigens (e.g. Cost), which bring together other genetically, biochemically or serologically related sets of antigens and a separate series of low-frequency (e.g. Rd) and high-frequency (e.g. Vel) antigens, which do not fit into any system or collection. A numerical catalogue of red cell antigens is being maintained by an International Society of Blood Transfusion (ISBT) Working Party.
| ISBT No. | System Name | System Symbol | Epitope | Chromosome |
|---|---|---|---|---|
| 001 | ABO | ABO | Carbohydrate ( N -acetyl-D-galactosamine, galactose). A, B and H antigens mainly elicit IgM antibody reactions, although anti-H is very rare, see the Hh antigen system (Bombay phenotype, ISBT 018) | 9 |
| 002 | MNS | MNS | GPA/GPB (glycophorins A and B). Main antigens M, N, S, s | 4 |
| 003 | P | P l | Glycolipid. Antigen P1 | 22 |
| 004 | Rh | RH | Protein. C, c, D, E, e antigens (there is no ‘d’ antigen; lowercase ‘d’ indicates the absence of D) | 1 |
| 005 | Lutheran | LU | Protein (member of the immunoglobulin superfamily). Set of 21 antigens | 19 |
| 006 | Kell | KEL | Glycoprotein. Antibodies to K 1 can cause haemolytic disease of the newborn (anti-Kell), which can be severe | 7 |
| 007 | Lewis | LE | Carbohydrate (fucose residue). Main antigens Le a and Le b – associated with tissue ABH antigen secretion | 19 |
| 008 | Duffy | FY | Protein (chemokine receptor). Main antigens Fy a and Fy b . Individuals lacking Duffy antigens altogether are immune to malaria caused by Plasmodium vivax and Plasmodium knowlesi | 1 |
| 009 | Kidd | JK | Protein (urea transporter). Main antigens Jk a and Jk b | 18 |
| 010 | Diego | DI | Glycoprotein (band 3, AE1 or anion exchange). Positive blood is found only among North-Central and East Asians and Native Americans | 17 |
| 011 | Yt or Cartwright | YT | Protein (acetylcholinesterase) | 7 |
| 012 | XG | XG | Glycoprotein | X |
| 013 | Scianna | SC | Glycoprotein | 1 |
| 014 | Dombrock | DO | Glycoprotein (fixed to cell membrane by GPI) | 12 |
| 015 | Colton | CO | Aquaporin 1. Main antigens Co(a) and Co(b) | 7 |
| 016 | Landsteiner-Wiener | LW | Protein (member of the immunoglobulin superfamily) | 19 |
| 017 | Chido/Rogers | CH/RG | C4a C4b (complement fractions) | 6 |
| 018 | Hh/Bombay | H | Carbohydrate (fucose residue) | 19 |
| 019 | Kx | XK | Glycoprotein | X |
| 020 | Gerbich | GE | GPC/GPD (glycophorins C and D) | 2 |
| 021 | Cromer | CROM | Glycoprotein (DAF, decay accelerating factor or CD55, regulates complement fractions C3 and C5, attached to the membrane by GPI) | 1 |
| 022 | Knops | KN | Glycoprotein (CR1 or CD35, immune complex receptor) | 1 |
| 023 | Indian | IN | Glycoprotein (CD44 adhesion function) | 11 |
| 024 | Ok | OK | Glycoprotein (CD147) | 19 |
| 025 | Raph | MER2 | Transmembrane glycoprotein | 11 |
| 026 | JMH | JMH | Protein (fixed to cell membrane by GPI) | 6 |
| 027 | Ii | I | Branched (I)/unbranched (i) polysaccharide | 6 |
| 028 | Globoside | GLOB | Glycolipid. Antigen P | 3 |
| 029 | GIL | GIL | Aquaporin 3 | 9 |
| 030 | Rh-associated glycoprotein | RHAG | Rh-associated glycoprotein | 6 |
Apart from those of the ABO system, most of these antigens were detected by antibodies stimulated by transfusion or pregnancy.
Alternative forms of a gene coding for red cell antigens at a particular locus are called alleles and individuals may inherit identical or non-identical alleles. Most blood group genes have been assigned to specific chromosomes (e.g. ABO system on chromosome 9, Rh system on chromosome 1). The term genotype is used for the sum of the inherited alleles of a particular gene (e.g. AA, AO) and most red cell genes are expressed as codominant antigens (i.e. both alleles are expressed in the heterozygote). The phenotype refers to the recognisable product of the alleles and there are many racial differences in the frequencies of red cell phenotypes, as shown in Table 21-2 .
| System | Phenotype | US Black Population (%) | US White Population (%) |
|---|---|---|---|
| ABO | O | 49 | 43.7 |
| A | 26 | 41.7 | |
| B | 20.5 | 10.6 | |
| AB | 4.5 | 4 | |
| Lewis | Le (a − b −) | 28.5 | 6 |
| Rh | Dce | 47.8 | 2.1 |
| DCcEe | 4.2 | 13.4 | |
| dce | 5.6 | 14.6 | |
| DCe | 2.6 | 18.9 | |
| MNSs | S − s + | 68.1 | 45 |
| S + s + | 24.5 | 44 | |
| S + s − | 5.9 | 11 | |
| S − s − | 1.5 | Rare | |
| Duffy | Fy (a − b −) | 63.7 | Rare |
| Fy (a − b +) | 18.8 | 34 | |
| Fy (a + b +) | 2 | 44 | |
| Fy (a + b −) | 15.5 | 17 | |
| Kidd | Jk (a + b −) | 50 | 27.5 |
| Jk (a + b +) | 41.4 | 49.4 | |
| Jk (a − b +) | 8.6 | 23.1 |
Red cell antigens are determined either by carbohydrate structures or protein structures. Carbohydrate-defined antigens are indirect gene products (e.g. ABO, Lewis, P). The genes code for an intermediate product, usually an enzyme that creates the antigenic specificity by transferring sugar molecules onto a protein or lipid. Protein-defined antigens are direct gene products and the specificity is determined by the inherited amino acid sequence and/or the conformation of the protein. Proteins carrying red cell antigens are inserted into the membrane in one of three ways: single pass, multipass or linked to glycosyl phosphatidylinositol (GPI-linked). Only a few red cell antigens are erythroid-specific (Rh, LW, Kell and MNSs), the remainder being expressed in many other tissues. The structure and functions of the membrane proteins and glycoproteins carrying blood group antigens have been reviewed by Daniels. An illustration of the putative functions of molecules containing blood group antigens is provided in Table 21-3 .
| Class | Blood Group System | Structure | Function |
|---|---|---|---|
| Transporter/channel | Kidd | Multipass GP | Urea transporter |
| Colton | Aquaporin 1 | ||
| Multipass GP | Water channel | ||
| Diego | Band 3, multipass GP | Anion exchanger | |
| Receptors | Duffy | DARC, multipass GP | Chemokine ( Plasmodium vivax receptor) |
| Indian | Single-pass GP | Hyaluronate receptor | |
| Complement pathway | Chido/Rogers | Complement absorbed | Complement component onto red cells |
| Cromer | DAF | Complement regulator | |
| Knops | Complement receptor 1 | Complement regulator | |
| Adhesion | LW | IgSF | Binds CD11/CD18 |
| Integrins | |||
| Molecule | Lutheran | IgSF | Laminin receptor |
| Enzyme | Yt | GPI-linked GP | Unknown on red cells |
| Acetylcholinesterase | |||
| Kell | Single-pass GP | Endopeptidase | |
| Structural protein | Gerbich | Glycophorins C and D Single-pass GP | Attachment to membrane skeleton |
However, the main clinical importance of a blood group system depends on the capacity of alloantibodies (directed against the antigens not possessed by the individual) to cause destruction of transfused red cells or to cross the placenta and give rise to haemolytic disease in the fetus or newborn. This in turn depends on the frequency of the antigens and the alloantibodies and the characteristics of the latter: thermal range, immunoglobulin class and ability to fix complement. On these criteria, the ABO and Rh systems are of major clinical importance. Anti-A and anti-B are naturally occurring and are capable of causing severe intravascular haemolysis after an incompatible transfusion. The RhD antigen is the most immunogenic red cell antigen after A and B, being capable of stimulating anti-D production after transfusion or pregnancy in the majority of RhD-negative individuals.
ABO system
Discovery of the ABO system by Landsteiner marked the beginning of safe blood transfusion. The ABO antigens, although most important in relation to transfusion, are also expressed on most endothelial and epithelial membranes and are important histocompatibility antigens. Transplantation of ABO-incompatible solid organs increases the potential for hyperacute graft rejection, although ABO-incompatible renal transplantation can be successfully carried out with plasmapheresis in addition to immunosuppression of the recipient. Major ABO-incompatible stem cell transplants (e.g. group A stem cells into a group O recipient) will provoke haemolysis, unless the donation is depleted of red cells.
ABO antigens and encoding genes.
There are four main blood groups: A, B, AB and O ( Table 21-4 ). In the British Caucasian population, the frequency of group A is 42%, B 9%, AB 3% and O 46%, but there is racial variation in these frequencies. The epitopes of ABO antigens are determined by carbohydrates (sugars), which are linked either to polypeptides (forming glycoproteins) or to lipids (glycolipids).
| Blood Group | Subgroup | Antigens on Red Cells | Antibodies in Plasma |
|---|---|---|---|
| A | A 1 | A + A 1 | Anti-B |
| A 2 | A | (Anti-A 1 ) * | |
| B | − | B | Anti-A, Anti-A 1 |
| AB | A 1 B | A + A 1 + B | None |
| A 2 B | A + B | (Anti-A 1 ) * | |
| O | − | (H) † | Anti-A |
| Anti-A 1 | |||
| Anti-B | |||
| Anti-A,B ‡ |
* Anti-A 1 found in 1–2% of A 2 subjects and 25–30% of A 2 B subjects.
† The amount of H antigen is influenced by the ABO group; O cells contain most H and A 1 B cells least. Anti-H may be found in occasional A 1 and A,B subjects (see text).
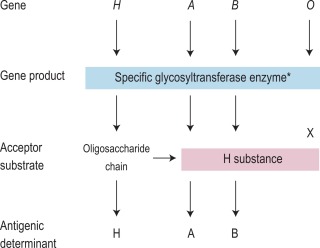
The expression of ABO antigens is controlled by three separate genetic loci: ABO located on chromosome 9 and FUT1 ( H ) and FUT2 ( Se ), both of which are located on chromosome 19. The genes from each locus are inherited in pairs as Mendelian codominants. Each gene codes for a different enzyme (glycosyltransferase), which attaches specific monosaccharides onto precursor disaccharide chains ( Table 21-5 ). There are four types of disaccharide chains known to occur on red cells, on other tissues and in secretions. The Type 1 disaccharide chain is found in plasma and secretions and is the substrate for the FUT2 ( Se ) gene, whereas Types 2, 3 and 4 chains are only found on red cells and are the substrate for the FUT1 ( H ) gene. It is likely that the O and B genes arose by mutation of the A gene. The O gene does not encode a functional enzyme; group O individuals commonly have a deletion at nucleotide 261 (the O1 allele), which results in a frame-shift and premature termination of the translated polypeptide and the production of an enzyme with no catalytic activity. The B gene differs from A by seven nucleotide substitutions, four of which lead to a different amino acid being encoded. The expression of A and B antigens is dependent on the H and Se genes, which both give rise to glycosyltransferases that add L-fucose, producing the H antigen. The presence of an A or B gene (or both) results in the production of further glycosyltransferases, which convert H substance into A and B antigens by the terminal addition of N -acetyl-D-galactosamine and D-galactose, respectively ( Fig. 21-1 ). Because the O gene produces an inactive transferase, H substance persists unchanged on group O cells. In the extremely rare Oh Bombay phenotype, the individual is homozygous for the inactive h allele of FUT1 and hence cannot form the H precursor of the A and B antigen. Their red cells type as group O, but their plasma contains anti-H in addition to anti-A, anti-B and anti-A,B, which are all active at 37 °C. As a consequence, individuals with an Oh Bombay phenotype can only be safely transfused with other Oh red cells.
| Gene | Allele | Transferase |
|---|---|---|
| FUT1 | H | α-2-L-fucosyltransferase |
| h | None | |
| ABO | A | α-3- N -acetyl-D-galactosaminyltransferase |
| B | α-3-D-galactosyltransferase | |
| O | None | |
| FUT2 | Se | α-2-L-fucosyltransferase |
| se | None | |
| FUT3 | Le | α-3/4-L-fucosyltransferase |
| le | None |
Serologists have defined two common subgroups of the A antigen. Approximately 20% of group A and group AB individuals belong to group A 2 and group A 2 B, respectively, the remainder belonging to group A 1 and group A 1 B. These subgroups arise as a result of inheritance of either the A 1 or A 2 alleles. The A 2 transferase is less efficient in transferring N -acetyl-D-galactosamine to available H antigen sites and cannot utilise Types 3 and 4 disaccharide chains. As a consequence, A 2 red cells have fewer A antigen sites than A 1 cells and the plasma of group A 2 and group A 2 B individuals may also contain anti-A 1 . The distinction between these subgroups can be made using the lectin Dolichos biflorus, which only reacts with A 1 cells. The H antigen content of red cells depends on the ABO group and, when assessed by agglutination reactions with anti-H, the strength of reaction tends to be graded O > A 2 > A 2 B > B > A 1 > A 1 B. Other subgroups of A are occasionally found (e.g. A 3 , A x ), resulting from mutant forms of the glycosyltransferases produced by the A gene that are less efficient at transferring N -acetyl-D-galactosamine onto H substance.
The A, B and H antigens are detectable early in fetal life but are not fully developed on the red cells at birth. The number of antigen sites reaches ‘adult’ level at around 1 year of age and remains constant until old age, when a slight reduction may occur.
Secretors and non-secretors.
The ability to secrete A, B and H substances in a water-soluble form is controlled by FUT2 (dominant allele Se ). In a Caucasian population, about 80% are secretors (genotype SeSe or Sese ) and 20% are nonsecretors (genotype sese ) ( Table 21-6 ). Secretors have H substance in the saliva and other body fluids together with A substances, B substances or both, depending on their blood group. Only traces of these substances are present in the secretions of nonsecretors, although the antigens are expressed normally on their red cells and other tissues.
| Genes | Blood Group of Red Cells | ABH Substance Present in Saliva | Incidence (%) |
|---|---|---|---|
| Secretor | |||
| SeSe or Sese ( FUT2 gene) | A B AB O | A + H B + H A + B + H H | 80 |
| Nonsecretors | |||
| sese ( FUT2 gene) | A, B, AB or O | None | 20 |
An individual’s secretor status can be determined by testing for ABH substance in saliva (see p. 457).
ABO antigens and disease.
Rarely group A individuals acquire a B antigen from a bacterial infection that results in the release of a deacetylase enzyme. This converts N -acetyl-D-galactosamine into α-galactosamine, which is similar to galactose, the immunodominant sugar of group B, thereby sometimes causing the red cells to appear to be group AB. In the original reported cases, five out of seven of the patients had carcinoma of the gastrointestinal tract. Case reports attest to the danger of individuals with an acquired B antigen being transfused with AB red cells, resulting in a fatal haemolytic transfusion reaction following the production of hyperimmune anti-B.
The inheritance of ABH antigens is also known to be weakly associated with predisposition to certain diseases. Group A individuals have 1.2 times the risk of developing carcinoma of the stomach than group O or B; group O individuals have 1.4 times more risk of developing peptic ulcer than non-group O individuals; and nonsecretors of ABH have 1.5 times the risk of developing peptic ulcer than secretors. The ABO group also affects plasma von Willebrand factor (VWF) and factor VIII levels; group O healthy individuals have levels around 25% lower than those of other ABO groups. ABO blood group appears to mediate its effect by accelerating clearance of VWF but the mechanism is not yet clear. ABH antigens are also frequently more weakly expressed on the red cells of persons with leukaemia.
ABO antibodies
Anti-A and Anti-B.
ABO antibodies, in the absence of the corresponding antigens, appear during the first few months after birth, probably as a result of exposure to ABH antigen-like substances in the diet or the environment (i.e. they are ‘naturally occurring’) ( Table 21-4 ). This allows for reverse (serum/plasma) grouping as a means of confirming the red cell phenotype. The antibodies are a potential cause of dangerous haemolytic transfusion reactions if transfusions are given without regard to ABO compatibility. Anti-A and anti-B are always, to some extent, immunoglobulin (Ig) M. Although they react best at low temperatures, they are nevertheless potentially lytic at 37 °C. Hyperimmune anti-A and anti-B occur less frequently, usually in response to transfusion or pregnancy, but they may also be formed following the injection of some toxoids and vaccines. They are predominantly of IgG class and are usually produced by group O and sometimes by group A 2 individuals. Hyperimmune IgG anti-A and/or anti-B from group O or group A 2 mothers may cross the placenta and cause haemolytic disease of the newborn (HDN). These antibodies react over a wide thermal range and are more effective haemolysins than the naturally occurring antibodies. Group O donors should always be screened for high-titre anti-A and anti-B antibodies, which may cause haemolysis when group O platelets or plasma are transfused to recipients with A and B phenotypes.
Plasma-containing blood components from such high-titre universal donors should be reserved for group O recipients.
Anti-A 1 and anti-H.
Anti-A 1 reacts only with A 1 and A 1 B cells and is occasionally found in the serum of group A 2 individuals (1–8%) and not uncommonly in the serum of group A 2 B subjects (25–50%). However, anti-A 1 normally acts as a cold agglutinin and is very rarely reactive at 37 °C, when it is only capable of limited red cell destruction. There have been a few reports of red cell haemolysis ascribed to anti-A 1 , which some authors have questioned because, although the antibodies reacted only with A 1 red cells, no attempts were made to absorb them with A 2 cells, which would have revealed their anti-A specificity.
Anti-H reacts most strongly with group O and A 2 red cells and also normally acts as a cold agglutinin (auto- antibody). A notable, but rare, exception is the anti-H allo-antibody that occurs in the Oh Bombay phenotype, which is an IgM antibody and causes lysis at 37 °C ( Table 21-4 ) so that Oh Bombay phenotype blood is required for transfusion.
Lewis system
Lewis antigens and encoding genes.
The Lewis antigens (Le a and Le b ) are located on soluble glycosphingolipids found in saliva and plasma and are secondarily absorbed into the red cell membranes from the plasma.
The Le gene at the FUT3 ( LE ) locus is located on chromosome 19 and codes for a fucosyltransferase, which acts on an adjacent sugar molecule to that acted on by the Se gene. Where Se and Le are present, the Le b antigen is produced; where Le but not Se is present, Le a is produced; and where Le is not present, neither Le a nor Le b is produced. After transfusion of red cells, donor red cells convert to the Lewis type of the recipient owing to the continuous exchange of glycosphingolipids between the plasma and red cell membrane.
Neonates have the phenotype Le(a − b −) because low levels of the fucosyltransferase are produced in the first 2 months of life.
Lewis antibodies.
Lewis antibodies are naturally occurring and are usually IgM and complement binding. In vitro, their reactivity is enhanced with the use of enzyme-treated red cells, when lysis may occur. However, only rare examples of anti-Le a that are strictly reactive at 37 °C have given rise to haemolytic transfusion reactions and there is no good evidence that anti-Le b has ever caused a haemolytic episode. Explanations for the relative lack of clinical significance include their thermal range, neutralisation by Lewis antigens in the plasma of transfused blood and the gradual elution of Lewis antigens from the donor red cells. Consequently, it is acceptable to provide red cells for transfusion that have not been typed as negative for the relevant Lewis antigen but are compatible with the recipient plasma when the compatibility test is performed strictly at 37 °C.
Lewis antibodies have not been implicated in haemolytic disease of the fetus or newborn. The role of Lewis in influencing the outcome of renal transplants is unclear.
The P system and globoside collection
Antigens.
The P 1 antigen of the P1PK system and the P and P k antigens of the globoside (P) collection are related but nonallelic. The relevant genes are at 3q26.1 for P ( B3GALT3 ) and at 22q13.2 ( A4GALT ) for P 1 and P k . All antigens are derived from the precursor, lactosyl ceramide dihexoside. Carbohydrate products related to the P system are widely distributed in nature.
Expression of P 1 varies considerably between individuals. One in 100 000 individuals is p (negative for P 1 and P 2 ) and is resistant to parvovirus B19 infection.
Antibodies.
Anti-P 1 is a common naturally occurring antibody of no clinical significance and in its presence P 1 -positive red cells for transfusion can be provided that are crossmatch compatible at 37 °C. Allo-anti-P is also a naturally occurring antibody found in individuals with the rare P k phenotype. Auto-anti-P is the specificity attributed to the Donath–Landsteiner antibody; it is a potent biphasic haemolysin, responsible for paroxysmal cold haemoglobinuria.
Anti-PP 1 P k is a naturally occurring high-titre IgM or IgG antibody and it is found only in individuals with the rare p phenotype. It is reactive at 37 °C and is capable of causing intravascular haemolysis and HDN. It is also associated with spontaneous miscarriage in early pregnancy.
Rh system
The Rh system, formerly known as the Rhesus system, was so named because the original antibody that was raised by injecting red cells of rhesus monkeys into rabbits and guinea pigs reacted with most human red cells. Although the original antibody (now called anti-LW) was subsequently shown to be different from anti-D, the Rh terminology has been retained for the human blood group system. The clinical importance of this system is that individuals who are D negative are often stimulated to make anti-D if transfused with D-positive blood or, in the case of pregnant women, if exposed to D-positive fetal red cells that have crossed the placenta.
Rh antigens and encoding genes.
This is a very complex system. At its simplest, it is convenient to classify individuals as D positive or D negative, depending on the presence of the D antigen. This is largely a preventive measure, to avoid transfusing a D-negative recipient with the cells expressing the D antigen, which is the most immunogenic red cell antigen after A and B. At a more comprehensive level, it is convenient to consider the Rh system as a gene complex that gives rise to various combinations of three alternative antigens – C or c, D or d and E or e – as originally suggested by Fisher. The d gene was thought to be amorphic without any corresponding antigen on the red cell. Subsequently it was confirmed that the RH locus is on chromosome 1 and comprises two highly homologous, very closely linked genes, RHD and RHCE, each with 10 exons. Each gene codes for a separate transmembrane protein with 417 residues and 12 putative transmembrane domains. The D and CE proteins differ at 35 residues. The RHCE gene has four main alleles; CE, Ce, ce and cE. Positions 103 and 226 on the CE polypeptide, situated in the external loops, determine the C/c (serine/proline) and E/e (proline/alanine) polymorphisms, respectively. This concept of D and CcEe genes linked closely and transmitted together is consistent with the Fisher nomenclature.
In Caucasian D-negative individuals, the RHD gene is deleted, whereas in black and other populations, single- point mutations, partial deletions or recombinations have been described. In individuals with a weak D antigen (D u ), there is a quantitative reduction in D antigen sites, believed to arise from an uncharacterised transcriptional defect. These individuals do not make anti-D antibodies following a D antigen challenge. Partial D individuals lack one or more epitopes of the D antigen, defined using panels of monoclonal reagents. D VI is perhaps the most important partial D phenotype because such individuals not infrequently make anti-D. Partial D phenotypes arise from DNA exchanges between RHD and RHCE genes and from other rearrangements. RhD variants types 1–3 may be distinguished serologically, but red cell genotyping may better distinguish RhD variants of types 4 onwards. Comprehensive reviews of this system have been provided by Avent and Reid and Daniels et al .
The Rh haplotypes are named either by the component antigens (e.g. DCe, dce) or by a single shorthand symbol (e.g. R 1 = DCe, r = dce). Thus a person may inherit DCe ( R 1 ) from one parent and dce ( r ) from the other and have the genotype DCe/dce ( R 1 r ). The haplotypes in order of frequency and the corresponding shorthand notation are given in Table 21-7 . Although two other nomenclatures are also used to describe the Rh system, namely, Wiener’s Rh-Hr terminology and Rosenfield’s numeric notation, the CDE nomenclature, derived from Fisher’s original theory, is recommended by a World Health Organisation Expert Committee in the interest of simplicity and uniformity. The Rh antigens are defined by corresponding antisera, with the exception of ‘anti-d’, which does not exist. Consequently, the distinction between homozygous DD and the heterozygous Dd cannot be made by direct serological testing but may be resolved by informative family studies. It is still routine practice to predict the genotype from the phenotype on the basis of probability tables for the various Rh genotypes in the population ( Table 21-7 ). However, in women with immune anti-D and a history of an infant affected by HDN, RH DNA typing is used in prenatal testing for the fetal D status to decide on the clinical management of the pregnancy, e.g. the need for monitoring for fetal anaemia using middle cerebral artery Doppler ultrasound. Suitable sources include amniotic fluid (amniocytes) and trophoblastic cells (chorionic villi) or after 15 weeks’ gestation, maternal blood can be used because it contains fetal DNA. , In practice, multiplex polymerase chain reaction (PCR) is used, with more than two primer sets, to detect the different molecular bases for D-negative phenotypes in non-Caucasians. RH DNA typing also has applications in paternity testing and forensic medicine. There are racial differences in the distribution of Rh antigens, e.g. D negativity is more common in Caucasians (approximately 15%), whereas R o ( Dce ) is found in approximately 48% of black Americans but is uncommon (approximately 2%) in Caucasians. The Rh antigens are present only on red cells and are a structural part of the cell membrane. Complete absence of Rh antigens (Rh-null phenotype) may be associated with a congenital haemolytic anaemia with spherocytes and stomatocytes in the blood film, increased osmotic fragility and increased cation transport. This phenotype arises either as a result of homozygosity for silent alleles at the RH locus (the amorph type) or more commonly by homozygosity for an autosomal suppressor gene (X), genetically independent of the RH locus (the regulator type). Rh antigens are well-developed before birth and can be demonstrated on the red cells of very early fetuses.
| Haplotype | Approximate Frequencies | |
|---|---|---|
| English | Nigerian | |
| DCe R | 0.421 | 0.060 |
| dce r | 0.389 | 0.203 |
| DcE R | 0.141 | 0.115 |
| Dce R 0 | 0.026 | 0.591 |
| dcE r″ | 0.012 | 0 |
| dCe r′ | 0.010 | 0.031 |
Antibodies.
Fisher’s nomenclature is convenient when applied to Rh antibodies, and antibodies directed against all Rh antigens, except d, have been described: anti-D, anti-C, anti-c, anti-E and anti-e. Rh antigens are restricted to red cells and Rh antibodies result from previous alloimmunisation by previous pregnancy or transfusion, except for some naturally occurring forms of anti-E and anti-C W . Immune Rh antibodies are predominantly IgG (IgG 1 and/or IgG 3 ), but may have an IgM component. They react optimally at 37 °C, they do not bind complement and their detection is often enhanced by the use of enzyme-treated red cells. Haemolysis, when it occurs, is therefore extravascular and predominantly in the spleen.
Anti-D is clinically the most important antibody; it may cause haemolytic transfusion reactions and was a common cause of fetal death resulting from haemolytic disease of the newborn before the introduction of anti-D prophylaxis. Anti-D is accompanied by anti-C in 30% of cases and anti-E in 2% cases. Primary immunisation following a transfusion of D-positive cells becomes apparent within 2–5 months, but it may not be detectable following exposure to a small dose of D-positive cells in pregnancy. However, a second exposure to D-positive cells in a subsequent pregnancy will provoke a prompt anamnestic or secondary immune response.
Of the non-D Rh antibodies, anti-c is most commonly found and can also give rise to severe haemolytic disease of the fetus and newborn. Anti-E is less common, whereas anti-C is rare in the absence of anti-D.
Kell and Kx systems
Antigens and encoding genes.
A total of 34 antigens have been identified (K1–K34), but three very closely linked sets of alleles are clinically important: K (KEL1) and k (KEL2); Kp a (KEL3), Kp b (KEL4) and Kp c (KEL21); and Js a (KEL6) and Js b (KEL7). These antigens are encoded by alleles at the KEL locus on chromosome 7, but their production also depends on genes at the KX locus on the X chromosome. The K antigen is present in 9% of the English population. The Kp b antigen has a high frequency in Caucasians; the Js b antigen is universal in Caucasians and almost universal in black populations.
The Kell protein is a single-pass glycoprotein and is believed to be complexed by a disulphide bridge to the Kx protein, which is multipass with 10 putative transmembrane domains. It has considerable sequence homology to other neutral endopeptidases.
In the McLeod phenotype, red cells lack Kx and there is a marked decrease in all Kell antigens, an acanthocytic morphology and compensated haemolysis. The McLeod syndrome is X-linked with slow progression of cardiomyopathy, skeletal muscle wasting and neurological defects.
Kell antibodies.
Immune anti-K is the most common antibody found outside the ABO and Rh systems. It is commonly IgG 1 and occasionally complement binding. Other immune antibodies directed against Kell antigens are less common. The presence of some of these antibodies, such as anti-k, anti-Kp b and anti-Js b , may cause considerable difficulties in the selection of antigen-negative units for transfusion.
Duffy system
Duffy antigens and encoding genes.
The Duffy (Fy) locus is on chromosome 1 ( ACKR1 gene) and encodes a multipass protein with seven or nine putative transmembrane domains.
The locus has the following alleles: Fy a , Fy b , which code for the codominant Fy a and Fy b antigens, respectively; Fy x , which is responsible for a weak Fy b antigen; and Fy, which is responsible when homozygous for the Fy(a − b −) phenotype in black populations. This Fy gene is identical to the Fy b gene in its structural region but has a mutation in the promoter region, resulting in the lack of production of red cell Duffy glycoprotein.
The Fy glycoprotein (also known as Duffy antigen receptor for chemokines, DARC) is a receptor for the CC and CXC classes of proinflammatory chemokines and is expressed on vascular endothelial cells and Purkinje cells in the cerebellum, but its precise role as a potential scavenger of excess chemokines is unknown. The Fy glycoprotein is also a receptor for Plasmodium vivax.
Duffy antibodies
Anti-Fy a is much more common than anti-Fy b and all other Duffy antibodies are rare apart from anti-Fy 3 (to both Fy a and Fy b ), which occurs in some African/Afro-Caribbean patients, in whom Fy(a − b −) antigen status is common. They are predominantly IgG 1 and are sometimes complement binding.
Kidd (JK) system
Kidd antigens and encoding genes.
Genes at the HUT 11 ( JK ) locus on chromosome 18 ( SLC14A1 gene) encode a multipass protein, which carries the Kidd antigens and functions as the human erythroid urea transporter. The codominant alleles, Jk a and Jk b , represent a polymorphism of SLC14A1, which differs by a single amino acid substitution at position 280 (Asp/Asn).
The Jk(a − b −) phenotype is very rare and is caused by homozygous inheritance of the silent allele, Jk, at the JK ( SLC14A1 ) locus or by inheritance of the dominant inhibitor gene In ( Jk ) unlinked to the JK locus. These Jk(a − b −) cells are resistant to lysis by solutions of urea and have a selective defect in urea transport.
Kidd antibodies.
Anti-Jk a is more common than anti-Jk b ; both are usually IgG. Kidd antibodies are usually complement binding, which is thought to be because most of them contain an IgG 3 fraction. Anti-Jk3 is produced by individuals of the rare Jk(a − b −) phenotype.
Kidd antibodies can be difficult to detect because they often show dosage (may only react with cells showing homozygous expressions of Jk a or Jk b ), they fall to undetectable levels in plasma and they are often present in mixtures of alloantibodies. A previous history of antibodies is therefore important, to avoid a post-transfusion haemolytic reaction, due to an anamnestic response by an antibody that was below the level of detection before transfusion.
MNSs system
MNSs antigens and encoding genes.
GYPA and GYPB are closely linked genes on chromosome 4 and encode glycophorin A (GPA) and glycophorin B (GPB), respectively. Both GPA and GPB are single-pass membrane sialoglycoproteins. M and N are alleles of GYPA (encoding the M and N antigens on GPA) and S and s are alleles of GPYB (encoding the S and s antigens on GPB). Many rare variants have been described owing to gene deletions, mutations and segmental exchanges.
The U antigen is found on the red cells of Caucasians and 99% of black populations. U-negative individuals are, with rare exceptions, S − s − and lack GPB or have an altered form of GPB.
MNSs antibodies.
Anti-M is a relatively common antibody that may be IgM or IgG. Rare examples are reactive at 37 °C when they can give rise to haemolytic transfusion reactions. Anti-M very rarely gives rise to HDN.
Anti-N is uncommon and of no clinical significance.
Anti-S and anti-s are usually IgG; both have, rarely, been implicated in haemolytic transfusion reactions and HDN.
Anti-U is a rare immune antibody, usually containing an IgG 1 component. It has been known to cause fatal haemolytic transfusion reactions and occasionally severe HDN.
Other blood group systems
Lutheran system.
The antigens in the Lutheran system are not well-developed at birth and as a consequence there are no documented cases of clinically significant haemolytic disease of the newborn owing to Lutheran antibodies.
Anti-Lu a is uncommon and rarely of clinical significance. Anti-Lu b has caused extravascular haemolysis.
Yt (Cartwright) system.
The antigens Yt a and Yt b are found on GPI-linked acetylcholinesterase. Some examples of anti-Yt a have caused accelerated red cell destruction.
Colton system.
The antigens in the Colton system, Co a and Co b , are carried on the water-transport protein, channel- forming integral protein (CHIP-1). Anti-Co a and the rarer anti-Co b are both sometimes clinically significant.
Dombrock system.
The antigens in the Dombrock system include Do a and Do b and also include the high-incidence antigens Gy a , Hy and Jo a . Antibodies of this system are usually weak, but all should be considered as potentially significant.
Clinical significance of red cell alloantibodies
The significance of the alloantibodies described, with respect to the nature of the haemolytic transfusion reaction they produce, is provided in Table 21-8 . The majority of haemolytic transfusion reactions, however, are the result of ABO incompatibility.
| Blood Group System | Intravascular Haemolysis | Extravascular Haemolysis |
|---|---|---|
| ABO,H | A, B, H | |
| Rh | All | |
| Kell | K | K, k, Kp a , Kp b , Js a , Js b |
| Kidd | Jk a | Jk a , Jk b , Jk 3 |
| Duffy | Fy a , Fy b | |
| MNS | M, S, s, U | |
| Lutheran | Lu b | |
| Lewis | Le a | |
| Cartwright | Yt a | |
| Colton | Co a , Co b | |
| Dombrock | Do a , Do b |
Mollison et al . analysed the significance of blood group antigens other than those of the ABO system and D by looking at the prevalence of transfusion-induced red cell alloantibodies, excluding anti-D, -CD and -DE ( Table 21-9 ). Rh antibodies, mainly anti-c or anti-E, accounted for 53% of the total and anti-K and anti-Fy a accounted for a further 38%, leaving only about 9% for all other specificities. A similar distribution of the different red cell antibodies was found in a smaller group of patients who experienced immediate haemolytic transfusion reactions (HTR). However, the figures for delayed HTR showed a striking increase in the relative frequency of Jk antibodies, which reflects the outlined characteristics of Jk antibodies.
| Patient Group | No. Studied | Blood Group Alloantibodies (% of Total) | ||||
|---|---|---|---|---|---|---|
| Rh † | K | Fy | Jk | Other | ||
| Transfused (some pregnant) | 5228 | 53.1 | 28.1 | 10.2 | 4.0 | 4.7 |
| Immediate HTR ‡ | 142 | 42.2 | 30.3 | 18.3 | 8.5 | 0.7 |
| Delayed HTR ‡ | 82 | 34.2 | 14.6 | 15.9 | 32.9 | 2.4 |
* Excluding antibodies of ABO, Lewis, P systems and anti-M and anti-N.
† Excluding anti-D (or -CD or -DE); almost all were anti-c or anti-E.
Haemolytic disease of the fetus and newborn has not been associated with antibodies directed against Lewis antigens and only very mild disease is produced by anti-Lu a and anti-Lu b . With these exceptions, all other IgG antibodies directed against antigens in the systems mentioned should be considered capable of causing haemolysis in this setting.
The significance of the many other blood group antigens not referred to in the text is summarised in Table 21-10 . However, it should be noted that the antibodies listed are usually wholly or predominantly IgG and would be detectable in routine pretransfusion testing using the indirect antiglobulin test (IAT).
| Antigen | Antigen Frequency (%) Caucasians | Associated HTR | Associated HDN | Comments |
|---|---|---|---|---|
| Di a | 0 | Yes | Yes | Part of DI system. Di a |
| Di b | 100 | Yes | Yes | More common in American Indians and North-Central and East Asians |
| Wr a | < 0.1 | Yes | Yes | |
| Xg a | 65 (males); 88 (females) | Rarely | Rarely | Xg a only antigen in system |
| Sc1 | > 99.9 | No | No | 3 antigens in SC system |
| Sc2 | < 0.1 | No | Mild | |
| Ge2 | 100 | Some | No | 7 antigens in GE system |
| Ge3 | > 99.9 | Some | No | |
| Cr a | 100 | Some | No | 10 antigens in CR system |
| Ch1 | 96 | No | No | 9 antigens in CH/RG systems, reside on chromosome 4 |
| Rg1 | 98 | No | No | Reside on C4 |
| Kn a | 98 | No | No | Belong to KN system of 5 antigens |
| McC a | 98 | No | No | |
| Yk a | 92 | No | No | |
| In a | 0.1 | Yes | No | In a has incidence of 4% in Asian Indians |
| In b | 99 | Yes | No | Asian Indians |
| LW a | 100 | Some | Mild | – |
| JMH | > 99.9 | No | No | One of 901 series * of high-incidence antigens |
| Vel | > 99.9 | Yes | No | One of 901 series * ; complement binding |
| Bg a | approx. 15 | No | No | Corresponds to HLA-B7, detectable on red cells |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree