Introduction
■Cancer immunotherapy has come a long way since its inception centuries ago when, in 1777, the surgeon to the Duke of Kent injected himself with malignant cells in an effort to develop a cancer vaccine. The ability to harness one’s own immune system to combat malignancy has gained widespread clinical utility in the management of a broad spectrum of malignancies such as melanoma, non–small-cell lung cancer (NSCLC), renal cell carcinoma, and urothelial carcinoma, to name a few. In addition, chimeric antigen receptor CAR T-cell therapy is now approved for use in the second line setting for B-cell acute lymphoblastic leukemia and diffuse large B-cell lymphoma. Multiple ongoing clinical trials are exploring the clinical utility of immunotherapy both as monotherapy and in combination with other agents or treatment modalities such as radiation. Therefore, in the foreseeable future, it is likely that the clinical applications of these therapies will continue to expand.
■The majority of immuno-oncology agents used in clinical practice at this point target immune checkpoint pathways such as cytotoxic T-lymphocyte–associated protein 4 (CTLA-4)-B7-1/B7-2 and programmed cell death 1 transmembrane protein (PD-1)-PD-L1/PD-L2. As these are key pathways in promoting immune tolerance, monoclonal antibodies that interfere with these pathways predictably lead to toxicities which resemble autoimmune phenomenon in their clinical manifestations. Pneumonitis is among the spectrum of immune-related adverse events (irAEs) noted with these agents. Although the overall reported incidence of pneumonitis is low at 2.7% with PD-1 inhibition and less than 1% in patients receiving CTLA-4 inhibitors, it is one of the most feared complications because it has the potential to cause significant morbidity and, potentially, mortality. In this chapter, we describe the various clinical manifestations, pathogenesis, and management of pulmonary toxicities with immunotherapeutic agents.
Immunotherapy-related Pneumonitis
- ■
Epidemiology and risk factors: Pneumonitis is the most common manifestation of pulmonary toxicity of immune checkpoint inhibitors. As mentioned above, the overall incidence of pneumonitis with the use of immune checkpoint inhibitors is less than 5% with high grade (≥grade 3) toxicities occurring in 1% to 2% of patients with CTLA-4 inhibitors, 1.1% of patients treated with PD-1 inhibitors, and in 0.4% of patients treated with PD-L1 inhibitors. , A combination of monoclonal antibodies targeting both the CTLA-4 as well as the PD-1-PD-L1/PD-L2 axes is not only associated with a higher incidence of pneumonitis (10%) versus those who received monotherapy with either agent (3%) but is also more likely to be severe and resistant to therapy. ,
The primary site of the tumor appears to be associated with the risk of developing pneumonitis. In a meta-analysis of 26 clinical trials of PD-1 inhibitors, the incidence of all-grade pneumonitis was significantly higher in patients with NSCLC or renal cell carcinoma than those with melanoma. However, other studies have shown similar incidences of high-grade pneumonitis irrespective of the primary malignancy, although the incidence of pneumonitis-related mortality appears to be higher in patients with NSCLC. In addition, in a retrospective study of patients with pneumonitis, patients with NSCLC had an earlier onset of pneumonitis in comparison to patients with melanoma. Patients with NSCLC developed pneumonitis at a median time of 2.1 months (range 0.2–27.4 months) versus 5.2 months in patients with melanoma (range 0.2–18.1 months). Another meta-analysis of clinical trials using PD-1 and PD-L1 inhibitors in patients with NSCLC also revealed a significantly higher rate of pneumonitis in treatment-naïve patients in comparison to those who had received prior systemic therapy. A retrospective study of 164 patients with lung cancer who were treated with a PD-1/PD-L1 inhibitor did not show any difference in the incidence of subsequent pneumonitis among patients who received thoracic radiation and those who did not. However, some authors have reported radiation recall pneumonitis after the use of immune checkpoint inhibitors; therefore larger studies are needed to determine the role of radiation and subsequent risk of pneumonitis with immune checkpoint inhibitors. Pneumonitis has been observed in both smokers and nonsmokers; therefore it is unclear whether there is an association between the two. Current smokers and those with underlying lung conditions, however, did appear to be at an increased risk for worse clinical outcomes. Many immunotherapy trials excluded patients with preexisting autoimmune conditions due to concern for exacerbation of the underlying autoimmune process. However, a retrospective study looking at outcomes in 52 patients with preexisting autoimmune disorders treated with PD-1 inhibitors showed that immunotherapy use in this patient population led to a flare of their underlying disease in 38% of the time; however, most flares were mild and did not require discontinuation of therapy. In addition, 29% of these patients developed irAEs; 10% were grade 3 and 8% were grade 4. Therefore, although further clinical experience is needed in this population to evaluate the safety of immunotherapy as treatment appears to be relatively well tolerated.
- ■
Clinical features: The median time to onset of pneumonitis reported in the literature is 2 to 24 months after initiation of therapy. An earlier onset may be seen in patients with NSCLC, as mentioned above, and those receiving combination therapy. Due to the long half-life of immune checkpoint inhibitors, immune-related pneumonitis should be suspected in all patients presenting with new respiratory symptoms despite discontinuation of immunotherapy. Patients often present with dyspnea, cough, decreased exercise tolerance, worsening hypoxia, and occasionally, low-grade fever or chest pain. However, in one series, up to one-third of the patients were asymptomatic. It is unclear if incidentally detected evidence of pneumonitis on radiography has a different clinical course or outcome than symptomatic pneumonitis. Clinically, patterns of lung involvement include organizing pneumonia, nonspecific interstitial pneumonitis, hypersensitivity pneumonitis, acute lung injury, and acute respiratory distress syndrome. Other less common clinical manifestations include sarcoidosis-like granulomatous disease , and radiation recall pneumonitis. As the intrathoracic adenopathy seen with sarcoid-like reactions may be mistaken for metastatic/progressive malignancy, clinicians should be aware of the potential for this adverse effect.
- ■
Pathogenesis: Immune checkpoint pathways, including the PD-1 and CTLA-4 pathways, function as naturally occurring brakes in the process of T cell activation. These are of vital importance in maintaining immune homeostasis in the body and allowing for negative regulation of autoreactive T cells. This inhibitory signal to T cells is mediated by the binding of PD-1 to its ligands PD-L1 and PD-L2 and CTLA-4 to CD80 and CD86 in the presence of T cell activation. The importance of these pathways in preventing autoimmunity is further supported by the development of severe and fatal autoimmune organ failure in CTLA-4 and PD-1 knockout mice.
- ■
These immune checkpoint pathways can be utilized by malignant cells to downregulate the adaptive immune response to tumors, therefore escaping immunosurveillance. Monoclonal antibodies that interfere with the binding of PD-1 or CTLA-4 with their ligands lead to the expansion of cytotoxic T cells and subsequent elimination of cancer cells. However, by interfering with the physiological mechanism for immune tolerance to self-antigens, these agents can lead to irAEs that resemble autoimmune diseases. The various pulmonary toxicities observed with immune checkpoint inhibitors are thought to be a direct consequence of this dysregulation of the immune system; however, the exact mechanism of toxicity and the role of other cells and cytokines is still unknown. As both pathways have synergistic mechanisms of action, combination immunotherapy leads to more potent activation of the adaptive immune system, therefore resulting in a higher rate of toxicity. The lower incidence of pneumonitis in patients with NSCLC treated with PD-L1 inhibitors in comparison to those treated with PD-1 inhibitors may be explained by the vital role of PD-L2 in promoting respiratory tolerance. In contrast to PD-1 inhibitors which block the interaction between PD-1 and both of its ligands, selective PD-L1 inhibition could potentially still allow for the interaction of PD-1 with PD-L2 and preserve immune tolerance in the lungs.
- ■
Radiographic findings: A variety of radiographic findings may be observed with pulmonary toxicities of immune checkpoint inhibitors. In one retrospective study, X-ray of the chest did not detect any abnormalities in close to 25% of patients with pneumonitis ( Figs. 21.1 and 21.2 ); therefore computed tomography (CT) is preferred for evaluation in patients with new or worsening respiratory symptoms. The most common finding on imaging is the presence of areas of ground glass attenuation, which may be diffuse or focal, along with intact bronchovascular markings. Other common findings include organizing pneumonia in which peripheral or subpleural patchy or confluent consolidations may be seen, or increased interstitial markings accompanied by septal thickening and honeycombing, which may be seen in severe cases. Other reported patterns include tree-in-bud nodularity or centrilobular nodules as may be seen with hypersensitivity pneumonitis and other nonspecific patterns.
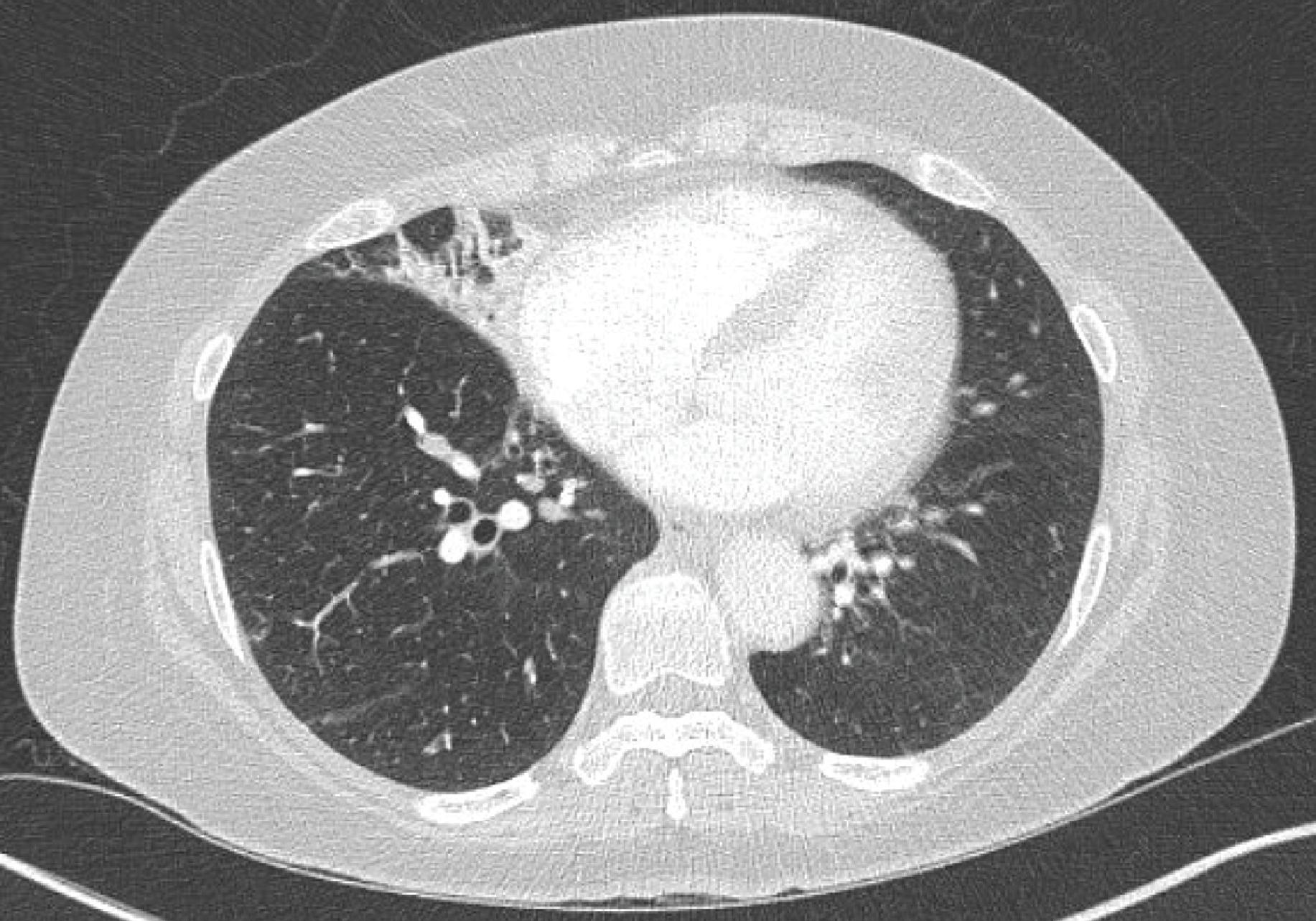
Fig. 21.1
Focal pneumonitis in a patient receiving nivolumab for advanced non–small cell-lung cancer. (Adapted with permission from Sehgal S, Velcheti V, Mukhopadhyay S, Stoller JK. Focal lung infiltrate complicating PD-1 inhibitor use: a new pattern of drug-associated lung toxicity? Respir Med Case Rep. 2016;19:118–120.)
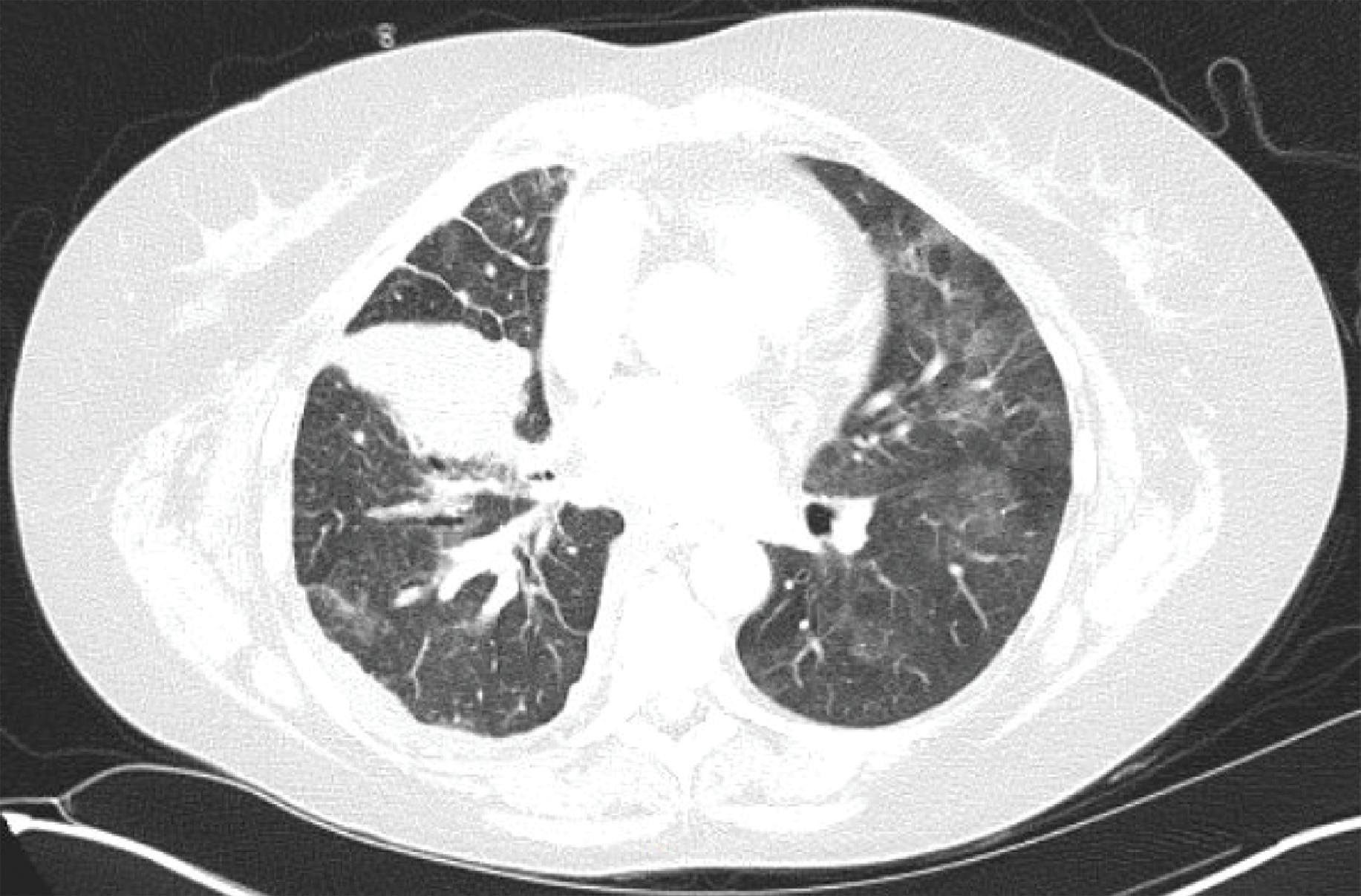
Fig. 21.2
Diffuse ground glass opacities in a patient with Stage IV non–small-cell lung cancer treated with pembrolizumab.
- ■
In a multi-institution retrospective study that evaluated various clinical features of pneumonitis associated with immune checkpoint blockade, ground glass opacities were noted in 37% of the patients, hypersensitivity pattern in 22%, cryptogenic organizing pneumonia in 19%, interstitial pattern in 7%, and pneumonitis not otherwise specified was noted in 15% of patients. Focal radiographic appearances have also been reported with immune-related pneumonitis and thus clinicians should be aware of the possibility particularly because these findings masquerade as infectious pneumonia, and the correct diagnosis may be missed unless there is a high degree of suspicion.
- ■
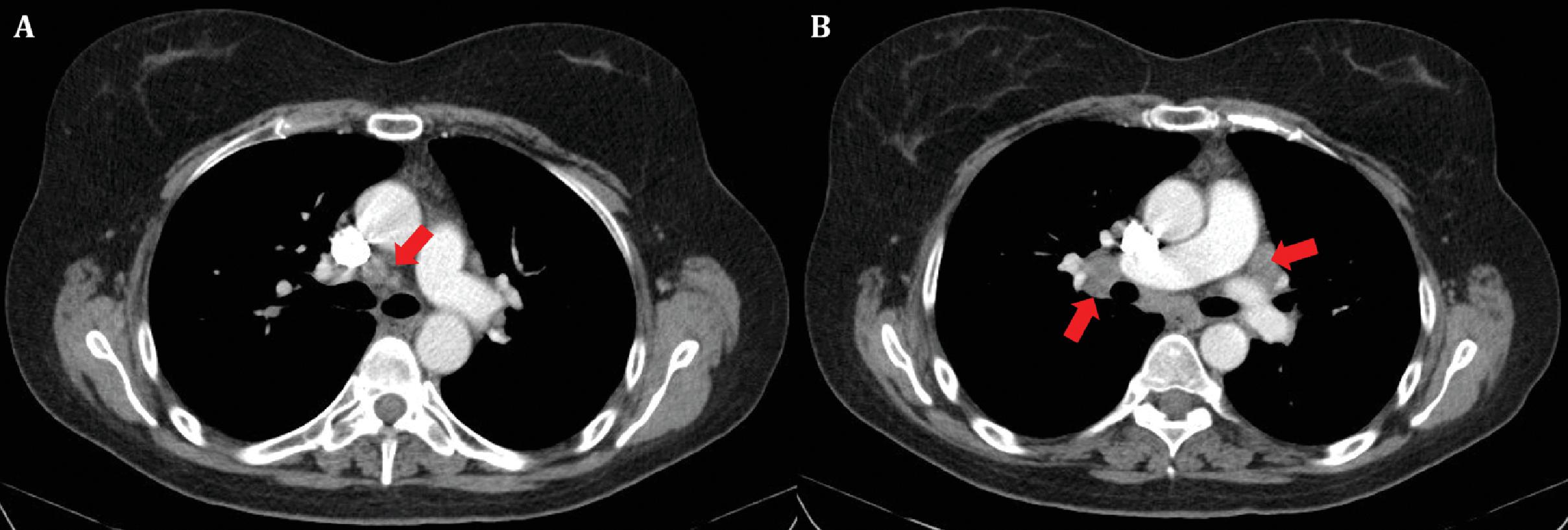
In patients with a sarcoid-like reaction to immunotherapy, enlargement of preexisting lymphadenopathy or new lymphadenopathy may be noted on imaging. As mentioned above, unless clinicians are aware of this potential irAE, lymphadenopathy may be mistaken for disease progression and may lead to discontinuation of a potentially beneficial therapy ( Fig. 21.3 A and B). In a retrospective study of eight patients who developed sarcoid-like adenopathy with ipilimumab, lymphadenopathy resolved on follow-up with a median time to resolution of 3.1 months (range 1.1–5.4 months).