INTRODUCTION
Chest medicine is inextricably intertwined with cancer medicine as a result of the propensity for cancer therapy or the disease itself to affect the lungs. Pulmonary complications in the cancer patient may manifest as injury to the pulmonary interstitium, alveolar-capillary membrane, pleura, pulmonary circulation, or airways, or, alternatively, may involve multiple intrathoracic structures. This chapter will review cancer-related pulmonary complications, including lung toxicities associated with aggressive chemotherapy and radiotherapy regimens, noninfectious lung disorders arising in the post–stem cell transplant setting, and cancer-related pleural disease, pulmonary vascular disease, and sleep disorders. The focus of this review is to identify, discuss, and provide practical algorithms for the diagnosis and treatment of these complications with emphasis on those issues in which early diagnosis may have a significant impact on patient management and outcome.
CHEMOTHERAPY-INDUCED LUNG INJURY
Injury to the lung due to cancer therapy results in stereotyped histopathologic disease patterns and syndromes (Tables 55-1 and 55-2). Lung toxicity has been described following exposure to conventional chemotherapy as well as molecularly targeted agents and immune modulators. Interstitial and alveolar lung injury patterns are the most frequent. Pleural effusions, pulmonary vascular disease, and, less frequently, drug-induced granulomatous disease and lymphadenopathy have also been described. In addition to direct lung injury, chemotherapy-induced immune suppression may predispose patients to life-threatening pneumonias.
| Agent Class | |||||||
|---|---|---|---|---|---|---|---|
| Pulmonary Syndrome | Alkylating Agents | Antimetabolites | Cytotoxic Antibiotics | Topoisomerase Inhibitors | Podophyllotoxins | Taxanes Microtubule Inhibitors | Other |
| Parenchymal Disease | |||||||
| Interstitial pneumonitis/pulmonary fibrosis | Busulfan BCNU CCNU Cyclophosphamide Ifosphamide Temazolamide Oxaliplatin Melphalan | Methotrexate Azathioprine Cytarabine Fludarabine Azacitabine Gemcitabine | Bleomycin Mitomycin C | Topotecan Irinotecan Amrubicin Daunorubicin Liposomal Doxorubicin | Paclitaxel Docetaxel | ATRA Arsenic trioxide Procarbazine | |
| Eosinophilic pneumonia | Busulfan, Cyclophosphamide, oxaliplatin, | Methotrexate Cytarabine Fludarabine Gemcitabine Pentostatin | Bleomycin | Etoposide | Paclitaxel Docetaxel | ||
| DAD/ARDS/NCPE/DAH | Busulfan, Cyclophosphamide, Ifosphamide, Temazolamide, Oxaliplatin Melphalan | Methotrexate Azathioprine Cytarabine Fludarabine Azacitabine Gemcitabine Pentostatin Pemetrexed Zinostatin | Bleomycin Mitomycin C | Topotecan | Etoposide | Paclitaxel Docetaxel Vincristine Vinblastine Vindesine Vinorelbine Ixabepilone | ATRA Arsenic trioxide |
| Radiation recall pneumonitis | Gemcitabine | Bleomycin | Amrubicin Daunorubicin Liposomal Doxorubicin | Paclitaxel Docetaxel | |||
| Granuloma formation | Oxaliplatin | Methotrexate | |||||
| Airway Disease | |||||||
| Infusion reaction/bronchospasm | Cyclophosphamide Ifosphamide Carboplatin Cisplatin Oxaliplatin | Methotrexate Gemcitabine Pemetrexed | Bleomycin Mitomycin C | Amrubicin Daunorubicin Liposomal Doxorubicin | Etoposide Teniposide | Paclitaxel Docetaxel Vincristine Vinblastine Vindesine Vinorelbine | L-asparaginase |
| BOOP | Busulfan, Cyclophosphamide Ifosphamide Oxaliplatin | Methotrexate | Bleomycin | Topotecan | L-asparaginase | ||
| Vascular Disease | |||||||
| Pulmonary hypertension | Zinostatin | Bleomycin Mitomycin C | |||||
| VTE/DVT | ATRA | ||||||
| Pleural Disease | |||||||
| Pleural effusion | Cyclophosphamide | Methotrexate Gemcitabine | Docetaxel Paclitaxel | ATRA Arsenic trioxide Procarbazine | |||
| Pleural thickening | |||||||
| Other | |||||||
| Opportunistic infections | Temozolamide | Methotrexate Fludarabine | |||||
| MetHemoglobinemia | Cyclophosphamide Ifosphamide | ||||||
| Agent Class | |||||
|---|---|---|---|---|---|
| Pulmonary Syndrome | Monoclonal Antibodies | Tyrosine Kinase Inhibitors | Rapamycin Inhibitors | Proteosome Inhibitors | Immunomodulators |
| Parenchymal Disease | |||||
| Interstitial pneumonitis/pulmonary fibrosis | Cetuximab Panitumumab Alemtuzumab Rituximab | Gefitinib Erlotinib Imatinib Dasatinib Sorafenib Sunitunib Vandetanib Idelalisib Trametinib Crizotinib | Everolimus Temsirolimus | Bortezomib Carlfizomib | Thalidomide IL-2 |
| Eosinophilic Pneumonia | |||||
| DAD/ARDS/NCPE/DAH | Cetuximab Panitumumab Alemtuzumab Rituximab Ofatumumab Ibritumomab Trastuzumab Pertuzumab Gemtuzumab | Gefitinib Erlotinib Imatinib Sorafenib Vandetanib Crizotinib Ruxolitinib | Everolimus Temsirolimus | Bortezomib | Thalidomide Lenolidomide |
| Radiation recall pneumonitis | Panitumumab | Erlotinib Vemurafenib | Bortezomib | ||
| Granuloma formation | Ipilumumab | Everolimus | IFN-γ | ||
| Hemoptysis | Bevacizumab Alemtuzumab Rituximab | Sorafenib Sunitunib Pazopanib | IL-2 TNF IFN-γ | ||
| Airway Disease | |||||
| Infusion reaction/bronchospasm | Cetuximab Panitumumab Bevacizumab Alemtuzumab Rituximab Obinutuzumab Ofatumumab Ibritumomab Trastuzumab Pertuzumab Gemtuzumab Ipilumumab | ||||
| BOOP | Cetuximab Panitumumab | Bortezomib | Thalidomide IFN-γ | ||
| Vascular Disease | |||||
| Pulmonary hypertension | Bortezomib Carlfizomib | IL-2 IFN-γ | |||
| VTE/DVT | Bevacizumab | Dasatinib Ponatinib Pazopanib Crizotinib | Thalidomide Lenolidomide | ||
| Pleural Disease | |||||
| Pleural effusion | Panitumumab | Imatinib Dasatinib Bosutinib | IL-2 IFN-γ | ||
| Pleural Thickening | |||||
| Other | |||||
| Opportunistic infections | Ofatumumab Ibritumomab | Idelalisib Trametinib Crizotinib Vemurafenib Ruxolitinib | Everolimus | ||
| MetHemoglobinemia | |||||
The diagnosis of drug-induced lung injury is hampered by the frequent use of multiagent and multimodality therapies. In addition, overlapping clinical, radiographic, and pathologic manifestations of lung injury caused by infections, aspiration, cancer relapse, radiation, and cancer-induced cardiac disease confound clinical distinctions between these entities and render precise estimates of drug-induced lung injury (DILI) difficult. Other conditions that may mimic DILI include pneumonia, aspiration pneumonitis, diffuse alveolar hemorrhage, and cardiogenic pulmonary edema (1,2). Predisposing factors, such as older age, cumulative dose, concomitant or sequential radiotherapy, oxygen administration, prior lung injury, and the use of multidrug regimens, not only increase the risk of DILI, but also may shorten the latency period between drug exposure and the development of clinical symptoms.
The diagnosis of DILI is suggested by a temporal association between drug exposure and the development of compatible clinical, radiographic, and laboratory evidence of lung injury, coupled with the exclusion of competing diagnoses. Interstitial and mixed alveolar-interstitial opacities, manifested as ground-glass opacities, reticular lines, septal thickening, and mosaic attenuation, typically localize to the peripheral and lower lung zones on chest imaging studies. Upper lobe predominant disease may be seen with hypersensitivity reactions and be accompanied by skin rash and wheezing. Bronchoscopy with performance of bronchoalveolar lavage (BAL) and/or transbronchial biopsies may be helpful in excluding infection or background disease. For example, findings of BAL eosinophilia of greater than 25% are supportive of drug-induced eosinophilic pneumonia. Increased numbers of hemosiderin-laden macrophages on BAL fluid and/or progressively bloody saline aliquots on sequential BAL samples is supportive of diffuse alveolar hemorrhage. A BAL lymphocytosis of greater than 50% with decreased CD4/CD8 ratios on BAL fluid is suggestive of interstitial lung disease; however, these findings are not sufficient to distinguish interstitial lung disease caused by drug toxicity from other causes. Although none of these histopathologic findings are pathognomonic of DILI, a few drugs produce characteristic patterns of involvement. For example, methotrexate, ipilimumab, everolimus, and interferon-γ may cause an acute granulomatous inflammation that mimics opportunistic infection. Histopathologic changes consistent with bronchiolitis obliterans with organizing pneumonia may be seen after exposure to several drugs, including bleomycin, cyclophosphamide, cetuximab, panitumumab, thalidomide, bortezomib, interferon-γ, and methotrexate (Table 55-3).
| Suggested Diagnosis | Histopathologic Findings |
|---|---|
| Eosinophilic pneumonia | BAL eosinophilia (>25%) |
| Diffuse alveolar hemorrhage | Increased hemosiderin-laden macrophages (>20%) |
| Diffuse alveolar hemorrhage | Progressively bloody saline aliquots on sequential BAL samples |
| Hypersensitivity pneumonitis | Increased lymphocytes and plasma cell on BAL fluid; variable numbers of giant cells; small, noncaseating granulomas on biopsy specimens |
| Interstitial lung disease | BAL lymphocytosis (>50%) with decreased CD4/CD8 ratio; interstitial fibrosis, destruction of type I pneumocytes with proliferation of type II pneumocytes following some drug exposures |
| Bronchiolitis obliterans with organized pneumonia | Organized polypoid inflammatory granulation tissue in the small airways |
| Sarcoid-like reactions/Granulomatous pneumonitis | Granulomatous inflammation without necrosis |
Clinical manifestations drug-induced interstitial lung disease include low-grade fever, dry cough, and dyspnea, which typically develop insidiously, usually within weeks to a few months after initiation of the first or subsequent cycles of therapy (3,4). Pulmonary fibrosis may immediately ensue or occur as a late manifestation of DILI months to years after exposure to some agents, such as bleomycin, busulfan, cyclophosphamide, gemcitabine, and carmustine (BCNU). Bronchospasm and allergic reactions are common manifestations of infusion reactions, which typically occur within minutes to hours of therapy.
Evidence-based guidelines in the management of DILI are limited. In most cases, drug withdrawal is recommended once sufficient evidence to implicate the culprit agent with pneumotoxicity is established. Systemic corticosteroids have proven efficacy in the treatment of DILI patterns such as hypersensitivity pneumonitis, eosinophilic pneumonia, and bronchiolitis obliterans organizing pneumonia. In other entities (pulmonary fibrosis, bronchiolitis obliterans), no beneficial role has been established. Steroid therapy should be considered in patients with progressive, steroid-responsive, and/or advanced-stage lung injury patterns. No guidelines for corticosteroid management in DILI are currently available. General recommendations include starting doses of prednisone at 40 to 60 mg or weight-based dosing at 0.75 to 1 mg/kg daily with tapers over a 1- to 3-month time period, pending response to therapy. With few exceptions (see below), drug rechallenge is not recommended. Several of the specific drugs causing DILI deserve separate mention and are discussed below.
Nonspecific interstitial pneumonitis (NSIP) is the most common morphologic pattern of interstitial lung disease. Dry cough and progressive dyspnea develop insidiously, over weeks to months following drug exposure. Radiographic findings may include pleural-based, lower lobe ground-glass attenuations, reticular lines, mosaic patterns, and nodules. Injury to epithelial and endothelial cells leads to alveolar edema and diffuse alveolar damage early on, which may progress to end-stage fibrotic lung disease, despite drug withdrawal and corticosteroid therapy.
Bleomycin-induced interstitial pneumonitis (BIP) has been well studied. This cytotoxic antibiotic is widely used in the treatment of germ cell tumors, lymphomas, and a variety of squamous cell carcinomas, particularly those of head and neck and esophageal origin. The lungs and skin are targets of bleomycin-induced lung injury due to the lack of the inactivating enzyme, bleomycin hydrolase, in these two organ systems. Bleomycin-induced interstitial pneumonitis is the most common pattern of bleomycin lung injury, occurring in up to 20% of treated patients, typically 4 to 10 weeks after bleomycin administration (Fig. 55-1) (5). Risk factors for BIP include age greater than 70 years, cumulative dose greater than 400 U, concomitant or sequential radiation therapy, uremia, multiagent therapy, and high inspired oxygen administration (6,7). Evidence suggesting an association between hyperoxia and increased BIP risk and/or severity is largely anecdotal. Questions regarding the threshold dose of oxygen and duration of oxygen therapy that confer an increased risk of bleomycin lung toxicity are unknown. In addition, the latency period between bleomycin and high oxygen exposure that mitigates the risk of increased toxicity has not been established. Nonetheless, general recommendations regarding supplemental oxygen therapy in bleomycin-exposed patients includes titration to achieve oxygen saturations at or above 89% to 92%. Declining values of diffusing capacity of the lung for carbon monoxide (DLCO) are thought to be early markers of bleomycin lung injury, although threshold cut-offs for drug withdrawal based on declining DLCO have not been established. Pulmonary function tests (PFTs) with DLCO should be considered in patients with known lung disease and/or abnormal lung function at baseline. Serial monitoring of DLCO is recommended as cumulative doses of bleomycin approach 400 U. Drug withdrawal is the mainstay of therapy with or without the institution of corticosteroids. The grade of pneumotoxicity should be used to guide the need for corticosteroid therapy. In patients with moderate (grade 2 or greater) interstitial pneumonitis, prednisone dosed at 0.75 to 1 mg/kg/d or its equivalent is recommended.
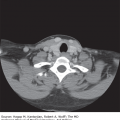
FIGURE 55-1
Bleomycin lung injury. A 26-year-old woman with progressive shortness of breath, hypoxia, and decline in diffusing capacity of the lung for carbon monoxide on lung function testing following the fifth cycle of bleomycin-based chemotherapy for Hodgkin lymphoma. Sagittal (A) and standard (B) views on chest computed tomography imaging showed bilateral, lower lobe predominant ground-glass opacities and parenchymal consolidation. Bronchoalveolar lavage cultures showed no growth. The patient was treated with high-dose steroids for presumed bleomycin-induced lung injury but succumbed to respiratory failure.
Rates of DILI following BCNU approach 50% among patients receiving cumulative doses of this drug in excess of 1,500 mg/m2. Carmustine toxicity is unique in its predilection for middle and upper lobe disease, which may occur years after BCNU exposure. Late-onset pneumonitis and fibrosis have also been described following cyclophosphamide and busulfan administration. Gemcitabine and paclitaxel are also known causes of interstitial pneumonitis, which may be fatal in some cases. Among the molecular targeted therapies, the mammalian target of rapamycin (mTOR) inhibitors (everolimus, temsirolimus) and epidermal growth factor receptor (EGFR) inhibitors (gefitinib, erlotinib, cetuximab, panitumumab) are most frequently implicated in the development of interstitial pneumonitis (8,9,10,11).
Hypersensitivity pneumonitis (HP)-like reactions typically occur after repeated drug exposure to an offending agent Associated symptoms of fever dyspnea, dry cough, and rash typically occur over the first 3 to 4 weeks following drug exposure and may wax and wane without adjustments in therapy. Poorly formed granulomas and BAL lymphocytosis are common histopathologic findings. Upper lobe predominant disease is characteristic, particularly in chronic forms of the disease. Methotrexate is the prototype agent associated with HP, which may develop following oral, intravenous, intrathecal, and intramuscular routes of methotrexate administration. Drug withdrawal and steroid therapy typically produce favorable outcomes, with complete resolution of clinical signs and symptoms in most cases.
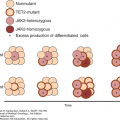
Drug-induced injury to the alveolar-capillary membranes may result in capillary leak and a permeability (noncardiogenic) pulmonary edema. Acute respiratory distress syndrome (ARDS) and its histologic hallmark, diffuse alveolar damage (DAD), may ensue as the disease progresses. These reactions may be unrelated to drug dosage or duration of therapy. Busulfan, bleomycin, cyclophosphamide, molecularly targeted agents (gefitinib, erlotinib, cetuximab), antilymphocyte monoclonal antibodies (rituximab, alemtuzumab, ofatumumab), and rapamycin inhibitors (everolimus, temsirolimus) are most often implicated in the development of drug-induced noncardiogenic pulmonary edema (NCPE). Noncardiogenic pulmonary edema leading to ARDS has been described following ruxolitinib, a novel JAC1/2 inhibitor, as a result of a cytokine rebound reaction. This reaction is mitigated with the preemptive use of corticosteroids and supportive therapy (12,13). Cytokine storm has also been described following all-trans-retinoic acid (ATRA) and arsenic trioxide therapies in the treatment of acute promyelocytic leukemia (APL). The so-called differentiation syndrome occurs in up to 25% of APL patients undergoing induction therapy, which is characterized by potentially fatal NCPE and ARDS. Unlike many of the lung injury processes, in patients with ATRA- and arsenic-related differentiation syndrome, de-escalation of drug dose, rather than drug withdrawal, along with systemic steroid therapy has been associated with successful resolution of toxicity in patients with mild to moderate forms of this syndrome (14,15). Diffuse alveolar hemorrhage (DAH) is typically seen as sequela of alveolar-capillary membrane injury and, thus, in the setting of ARDS/DAD. Occasionally, bland alveolar hemorrhage has been described in the absence of DAD following rituximab and alemtuzumab therapy (12,16). Massive, and sometimes fatal, bleeding has been reported during bevacizumab therapy for treatment of central airway tumors (17).
Drug-induced pleural effusions may occur as an isolated toxicity to the pleura (following methotrexate, dasatinib, bosutinib, docetaxel, ATRA, or granulocyte colony-stimulating factor [GCSF] administration) or as a manifestation of a generalized pleuroparenchymal abnormality (18,19). These small to moderate-sized effusions are typically exudative and lymphocyte predominant and may be unilateral or bilateral. Withdrawal of the offending agent may result in spontaneous resolution in some cases.
The development of thromboembolic disease, pulmonary hypertension, and pulmonary veno-occlusive disease (PVOD) has been described following conventional chemotherapeutic, molecularly targeted, and immune-modulating agents. Increased rates of venous thromboembolism (pulmonary embolism and deep venous thrombosis) have been reported with the ALK inhibitor crizotinib, the Bcr-Abl inhibitor ponatinib, and the vascular endothelial growth factor (VEGF) inhibitors bevacizumab, sunitinib, sorafenib, and pazopanib (17,20,21,22,23). In addition, the angiogenesis inhibitors (thalidomide, lenalidomide, pomalidomide) in combination with steroids, doxorubicin, or BCNU are associated with a 14% to 43% increased risk of thromboembolic events. Other agents, including hormonal therapies, growth factors, and erythropoietic agents, contribute to cancer-associated venous thromboembolism. The development of pulmonary arterial hypertension (PAH) has been associated with several drugs, including bleomycin, busulfan, BCNU, interferon, and dasatinib. Severe PAH following dasatinib, a multikinase Bcr-Abl tyrosine kinase inhibitor (TKI) is well described. Once dasatinib-associated PAH develops, drug withdrawal without rechallenge is recommended. There have been no reports of PAH following exposure to the more selective Bcr-Abl–targeted TKIs (imatinib and nilotinib), which may be safely used in dasatinib-induced PAH (24,25,26,27). Bleomycin and BCNU have also been implicated in the development of PVOD, an irreversible and often fatal form of pulmonary hypertension that is characterized by fibrous obliteration of pulmonary venules.
Virtually all chemotherapeutic and targeted agents may trigger an infusion reaction (IR), a sometimes life-threatening acute reaction that may be associated with dry cough, dyspnea, wheezing, chest pain, and hypoxia. Infusion reactions may manifest as IgE-mediated, type 1 hypersensitivity reactions (carboplatin, oxaliplatin, and l-asparaginase) or as anaphylactoid reactions, mediated by cytokine release. The latter reaction is often seen following the administration of many of the monoclonal antibodies (mAbs). Infusion reactions may be triggered by the drug itself or in response to the vehicle in which the drug is formulated. This is particularly true of the taxane class of drugs. For example, paclitaxel is formulated in Cremophor EL, a highly allergenic polyoxyethylated castor oil solvent. Docetaxel is formulated in polysorbate 80. Both vehicles may induce mast cell/basophil activation and subsequent hypersensitivity reaction. Other drugs that are formulated in Cremophor EL (cyclosporine, teniposide, ixabepilone) or polysorbate 80 (etoposide) may trigger similar reactions and should be avoided in patients with a history of IRs following taxane administration (28,29). Histamine receptor antagonists and steroids are recommended as standard prophylaxis prior to taxane administration, which has reduced the incidence of taxane-induced bronchospasm from 30% to 2% (30,31). Infusion reactions may occur within minutes to several hours following drug exposure. Close monitoring during and immediately following drug infusion is critical, as breakthrough IRs may occur despite prophylaxis. Although vinca alkaloids are rarely associated with lung toxicity, severe bronchospasm has been described when these agents are given with concurrent or sequential administration of mitomycin therapy.
RADIATION-INDUCED LUNG INJURY
Clinically significant radiation-induced lung injury (RILI) is the most common dose-limiting complication of thoracic radiation therapy (RT), occurring in 5% to 20% of patients. Recent advances in radiation techniques and delivery systems, such as proton therapy, three-dimensional conformal RT (CRT), intensity-modulated RT (IMRT), and stereotactic body RT (SBRT), purport lower lung injury rates while delivering higher target doses of radiation to the lung. Factors associated with radiation delivery (total radiation dose, dose per fraction, volume of irradiated lung, and beam characteristics and arrangements) as well as clinical factors (preexisting lung disease, underlying poor pulmonary reserve, prior radiotherapy, multimodality regiments, rapid steroid withdrawal) all potentiate the appearance and severity of radiation pneumotoxicity. Radiographically apparent lung injury is common with total doses of radiation that exceed 40 Gy and is rare at doses below 20 Gy (32,33). Hyperfractionated radiation doses delivered to the smallest lung volume is recommended.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree