Abstract
Puberty in humans is defined as the period of becoming capable of reproducing. It is marked by maturation of the genital organs, development of secondary sex characteristics, acceleration in linear growth velocity, and, in the female, the occurrence of menarche. Many factors influence the age at onset and the tempo at which puberty progresses. The age at puberty may be associated with health consequences later in adulthood. Normal function of the hypothalamic-pituitary-gonadal (HPG) axis is dependent on the meticulous spatio-temporal orchestration of gonadotropin-releasing hormone (GnRH) neuron development in the hypothalamus. While the neurobiological mechanisms that underlie GnRH pulse generation remain controversial, compelling evidence indicates the fundamental role of KNDy neurons in the arcuate nucleus. Identification and investigation of specific mutations in families with disorders of puberty have established some of the factors involved in GnRH neuron migration and gonadal function. This chapter reviews the disorders of puberty in the context of the development and maturation of the HPG axis. Elucidation of the neurobiology of the GnRH neurons and the developmental processes unfolding during gonadal and adrenal maturation will improve understanding of the pathophysiology of the disorders of puberty and, perhaps, lead to novel therapies.
Key words
Puberty, precocious puberty, hypogonadism, GnRH neuron, KNDy neuron, hypergonadotropic hypogonadism, Turner syndrome, Kallmann syndrome, androgen insensitivity syndrome
Introduction
Puberty in humans is defined as the period of first becoming capable of reproducing, and is marked by maturation of the genital organs, development of secondary sex characteristics, acceleration in linear growth velocity, changes in affect and, in the female, the occurrence of menarche. In humans, the transition into puberty is driven by two physiological processes: gonadarche and adrenarche. Gonadarche comprises growth and maturation of the gonads and is associated with increased secretion of sex steroids and with the initiation of folliculogenesis and ovulation in the female and spermatogenesis in the male. Gonadarche is responsible for thelarche and menarche in girls and testicular enlargement in boys.
Adrenarche , which typically precedes gonadarche, is associated with increased secretion of adrenal androgens and leads to the appearance of sexual hair (pubarche). The major adrenal bioactive C19 androgens are dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate (DHEAS), and androstenedione. Adrenarche occurring late in prepubertal development appears to be peculiar to our own species and to the great apes; and, in humans, the absence of adrenarche does not prevent gonadarche or the attainment of fertility.
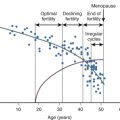
The age at onset of puberty and the tempo at which puberty progresses is dependent on many factors. In girls, increased ovarian and adrenal sex steroid secretion leads to the physical manifestations of puberty, thelarche, and pubarche. In general, these changes occur between 8 and 13 years of age. The mean age at menarche among multiple ethnic groups is between 12 and 13 years old. In boys, the earliest physical manifestation of puberty is an increase in testicular volume, and this usually occurs between 9 and 14 years of age.
The age at puberty may be associated with health consequences later in adulthood. Younger age at menarche generally means longer period of exposure to estrogen. Adverse consequences of this longer exposure to estrogen include increased risks for endometrial and breast cancers. A younger age at menarche has also been associated with increased risk for development of the metabolic syndrome. In the longitudinal 25-year Coronary Artery Risk Development in Young Adults (CARDIA) study, which included both Caucasian and African-American women, the excess adiposity associated with earlier age at menarche was sustained through adulthood. In the Finland Birth Cohort 1966 study involving 2417 males and 2641 females, pubertal timing as estimated by pubertal height growth influenced adult metabolic outcome markers, such as LDL cholesterol, fasting insulin, and fasting glucose. Prepubertal growth explained 19% to 25% of adult body mass index (BMI) variation in this cohort of men and women. The large cross-sectional UK Biobank study reported associations between pubertal timing and subsequent health outcomes. These data showing associations among prepubertal growth, pubertal timing, and adult metabolic outcomes suggest that mechanisms advancing puberty may also contribute to adult metabolic disorders. Additional studies are needed to validate these epidemiological associations.
Not surprisingly, later age at menarche is associated with increased risks for osteopenia and osteoporotic fractures. Possible explanations are that a shorter duration of estrogen exposure predisposes to decreased bone mineral density or that girls with lower body mass tend to experience both later menarche and decreased bone mineral accrual. Areal bone mineral density (BMD) was already decreased during the prepubertal years in girls experiencing later puberty, suggesting that common genetic variants influence both BMD and the timing of puberty. Greater understanding of the mechanisms influencing the timing of puberty might lead to the development of specific prevention strategies for disorders such as osteopenia/osteoporosis.
Traditionally, the diagnosis of precocious puberty is considered when signs of puberty develop prior to 8 years of age in girls and 9.5 years in boys, but these criteria should be used as guidelines to complement the evaluation of individual patients. For girls, the absence of thelarche or menarche by age 13 and 16 years, respectively, is considered to be delayed puberty. For boys, delayed puberty is defined as absence of testicular enlargement by age 14 years. These ages represent 2.5 to 3 standard deviations (SDs) below and above the mean age of puberty as defined by population studies.
While the mechanism underlying the onset of adrenarche remains to be elucidated, it is now established that gonadarche results from the resurgence of activity in the hypothalamic-pituitary axis, which has been relatively quiescent since early childhood. The neuroendocrine regulation of gonadarche in man is similar to that observed in other higher primates. Nonhuman primates (in particular the rhesus monkey) have been extensively employed as paradigms for the study of human puberty. Subsequently, our discussion of the control of the onset of gonadarche will be based on both the human and nonhuman primate literature.
Stages of Pubertal Development, Secular Trends, and Racial and Ethnic Differences
- ◆
A positive secular trend for age at menarche in European and North American girls has been observed.
- ◆
A similar trend for earlier pubertal development, albeit with a smaller magnitude, has been observed also in boys.
- ◆
The National Health and Nutrition Examination Survey (NHANES) III study showed that among American girls, mean ages for breast development and menarche were 9.5 and 12.1 for non-Hispanic black (NHB); 9.8 and 12.2 for Mexican-American (MXAM) girls; and 10.3 and 12.7 years for non-Hispanic white (NHW) girls.
- ◆
For boys in NHANES III, median estimated ages for genital stage 2 were 9.3 for NHB boys; 10.4 for MXAM boys; and 10.1 for NHW youths.
Pubertal Staging
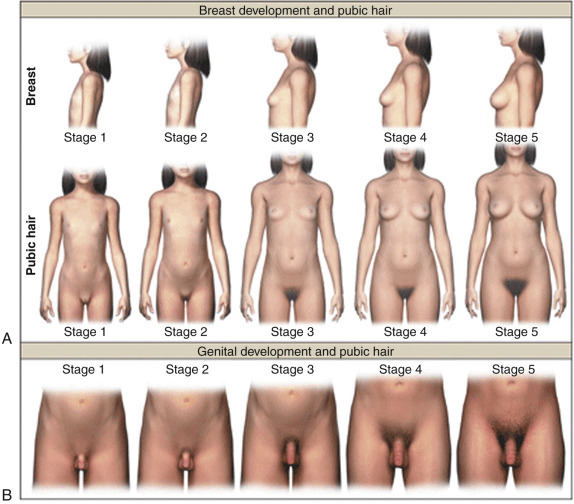
For both sexes, the genital and pubic hair changes that unfold at puberty are classified into five stages: stage 1 is prepubertal and stage 5 is adult ( Fig. 17.1 ; Table 17.1 ). These physical changes may either be the result of gonadarche (as in the case of breast or testicular enlargement) or adrenarche (as in the case of pubic hair development). Although the physical sequelae of gonadarche and adrenarche generally occur concomitantly, discordance of the two processes may also occur in normal development.
| Girls | |||
| Stage | Breast | Pubic Hair | |
| 1 | Prepubertal | No pigmented hair | |
| 2 | Budding with larger areolae | Small amount of coarse, pigmented hair mostly along labia majora | |
| 3 | Enlargement of breast and areolae | Spread of coarse, pigmented hair over mons pubis | |
| 4 | Secondary mound of areolae | Almost adult pattern | |
| 5 | Mature contour | Adult pattern | |
| Boys | |||
| Stage | Genitalia | Pubic Hair | Testicular Volume |
| 1 | Prepubertal | No pigmented hair | <3 mL |
| 2 | Thinning and darkening of scrotum, increased size of penis | Small amount of coarse, pigmented hair at base of penis | 3–8 mL |
| 3 | Increased diameter of penis | Coarse, pigmented hair extends above penis | 10–15 mL |
| 4 | Increased diameter and length of penis | Almost adult pattern | 15–20 mL |
| 5 | Adult size and shape | Adult pattern | >25 mL |
During puberty, increased ovarian estrogen secretion promotes breast development in girls. The development of breast buds with increased areolar diameter is considered to be stage 2; greater enlargement of the breasts occurs in stage 3, accompanied by increased pigmentation of the areolae and nipples. During stage 4, the areolae are mounded above the breast tissue. Recession of the areola to the general breast contour represents breast stage 5. Palpation of the breast is necessary to better differentiate between breast tissue and lipomastia. Additional effects of estrogen at this stage of development include cornification of the vaginal mucosa, uterine growth, and morphogenesis of an adult female body habitus.
Menarche follows an anovulatory cycle and generally occurs 2 to 3 years after the onset of breast development. Menstrual cycles during the first year after menarche are typically irregular and anovulatory, with most ranging in duration from 21 to 45 days. By 3 years postmenarche, over 90% of adolescent females have 10 or more menstrual cycles/year with an average menstrual interval of 36.5 days. Nevertheless, cycles can remain irregular until the fifth year postmenarche.
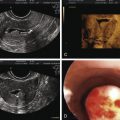
Although primordial and preantral follicles predominate during the prepubertal years, small antral follicles can develop during this phase of maturation. These small follicles are gonadotropin independent. Ovarian volume increases with the onset of puberty, achieves maximum volume soon after (between menarche and age 16 years), and remains stable or decreases slightly thereafter. Polycystic ovary morphology (PCOM) is frequently detected in healthy adolescent girls; this morphology is not associated with decreased ovulatory rate, hyperandrogenism, or metabolic abnormalities. During the early postmenarcheal period, ovarian morphology on transabdominal ultrasound shows multicystic ovaries and increased ovarian volume that differ from ovarian morphology observed in older women.
In girls, increased adrenal androgen secretion is considered to be responsible for the development of darker hairs along the labia, which is classified as pubic hair stage 2. The hair becomes darker and coarser during pubic hair stage 3, spreading over the pubic symphysis with gradual progression to a full female escutcheon. Apocrine odor may precede or accompany the development of pubic hair. Associated findings include axillary hair, acne, and oiliness of skin and hair.
For boys, an increase in testicular volume and enlargement of the scrotum is considered genital stage 2. At stage 2, the testes are approximately 4 to 8 mL in volume with the longest axis being approximately 2.5 cm. The volume of the mature human testis is approximately 20 to 30 mL and represents increased growth of the seminiferous tubule due to Sertoli cell proliferation and differentiation as well as initiation of spermatogenesis. At genital stage 3, further growth of the testes has occurred, and the length and diameter of the penis has increased. At genital stage 4, penile size has increased and the scrotal skin has become darkened. Palpation and use of orchidometer is preferable to inspection. Male pubic hair stage 2 consists of downy hair at the base of the penis. For pubic hair stage 3, the hair is longer, darker, and extends over the junction of the pubic bones. For pubic hair stage 4, the extent of hair has increased, but has not yet achieved the adult male escutcheon. Other secondary sexual characteristics in boys include axillary hair, increased size of the larynx, deepening of the voice, increased bone mass, and increased muscle strength. Approximately 3 years after the appearance of pubic hair, terminal hair appears in androgen-dependent regions on the face and trunk where it may develop for several years thereafter. There is considerable variation in the distribution and density of beard, chest, abdominal, and back hair, presumably reflecting genetic differences. The appearance of spermatozoa in early morning urine specimens (spermaturia) occurs during genital stage 3. Gynecomastia is observed in 50% of boys. Typically this is most prominent in midpuberty when the ratio of circulating concentrations of estradiol to testosterone is relatively high. In most instances, gynecomastia resolves spontaneously by 16 years of age.
The pubertal growth spurt in girls occurs concurrently with the onset of breast development. Usually only 4 to 6 cm of growth occur after menarche. The pubertal growth spurt in boys, with an average height velocity of 9.5 cm per year, occurs around genital stages 3 and 4. In general, the age at peak height velocity shows an inverse relationship with the magnitude of the growth spurt. Linear growth is approximately 99% complete for girls at a bone age of 15 years and for boys at a bone age of 17 years.
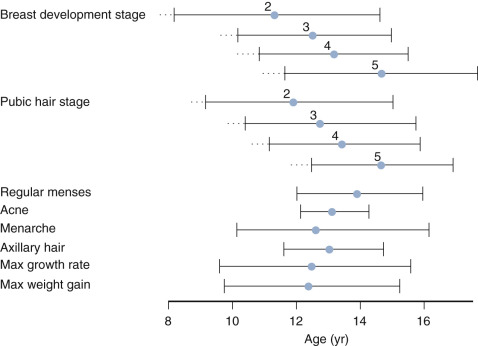
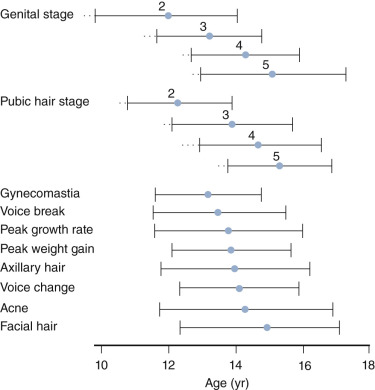
Breast development in girls and testicular enlargement in boys generally precede pubic hair development. Yet, the tempo for pubic hair development is faster such that synchrony between genital and pubic hair development occurs during the later stages of puberty. Schemata for the temporal development of the secondary sexual characteristic and their relationship to growth velocity is shown for girls and boys in Figs. 17.2 and 17.3 , respectively.
Secular Trends and Racial and Ethnic Differences in the Onset and Tempo of Puberty
Reports of secular changes in the onset of puberty have focused on girls and have typically used age at menarche as the biomarker for puberty.
Gluckman and Hanson have suggested that menarche occurred between 7 and 13 years in Paleolithic and Neolithic times. Based on analysis of 994 medieval adolescent skeletons (10 to 25 years) for pubertal stage, it appears that adolescents began puberty around 10 to 12 years with menarche occurring closer to 15 years in rural areas and 17 years in London. Boys experienced protracted pubertal stages during the 10th to 17th centuries. Potential factors that contributed to later puberty during medieval times included poor diet, increased infections, and greater physical exertion.
Available historical data indicate that this was followed by a decline in the age of menarche in Europe and North America from the early 19th century (16 to 17 years of age) to the latter half of the 20th century (13 years of age). This trend has been attributed to the improving socioeconomic conditions during this epoch. Although recent data from North America, several European countries, and other regions of the industrialized world suggest that the trend to earlier menarche has been reduced or halted, breast and pubic hair development are apparently occurring earlier than 50 years ago in both North America and Europe.
The biology underlying this continued positive secular trend in sexual development in girls, which in some populations is loosely associated with a similar trend in growth, is unclear, and may or may not involve an earlier onset of gonadarche or adrenarche. This earlier onset of breast development is not associated with increased gonadotropin or estradiol concentrations, suggesting that this represents a gonadotropin-independent event. Earlier breast development assessed by palpation was reported in NHW girls, which was likely related to the increased BMI of this group. Analogous studies of boys are limited, but no striking sex differences in secular trends in puberty and growth are apparent. Using both genital staging and the orchidometer, the Copenhagen Puberty Study reported pubertal onset occurring 3 months earlier in Danish boys and reported that obesity advanced the onset of testicular enlargement. However, other studies suggest that obesity delays onset of puberty in boys.
The age at onset of puberty varies between ethnic groups. Among American girls, mean ages for breast development, pubic hair development, and menarche were 9.5, 9.5, and 12.1, respectively, for NHB girls; 9.8, 10.3, and 12.2, respectively, for MXAM girls; and 10.3, 10.5, and 12.7 years, respectively, for NHW girls. Data obtained through the cross-sectional Third National Health and Nutrition Examination Survey (NHANES III) between 1988 and 1994 showed that NHB girls enter puberty first, followed by MXAM and NHW girls. Based on the NHANES III study, luteinizing hormone (LH) and inhibin B concentrations associated with onset of breast development were evaluated. As would be anticipated, LH and inhibin B concentrations increased with pubertal progression. Cut-points for Tanner 2 breast development were LH 1.04 mIU/mL (95% confidence interval [CI]: 0.71 to 1.37) and inhibin B 17.89 pg/mL (95% CI: 11.63 to 24.15). The respective median ages at hormonal onset based on LH concentrations were 10.7, 10.6, and 10.1 years for NHW, MXAM, and NHB girls, respectively. Girls with low birthweight and greater postnatal weight gain had relatively earlier onset of puberty based on LH concentrations and a similar pattern regarding pubertal onset was noted based solely on postnatal weight gain.
For boys in NHANES III, median estimated ages for genital and pubic hair stage 2 were 9.3 and 11.1, respectively, for NHB boys; 10.4 and 12.3, respectively, for MXAM boys; and 10.1 and 12.0, respectively, for NHW youths. For genital and pubic hair stage 5, median ages were 14.9 and 15.2, respectively, for NHB boys; 15.8 and 15.7, respectively, for MXAM boys; and 16.0 and 15.6, respectively, for NHW boys. Using the NHANES III data, LH, testosterone, and inhibin B concentrations increased as puberty progressed. Likely reflecting individual and diurnal variation, no single or combination hormone cut-point was found to be predictive of physical pubertal status, either genital or pubic hair stage 2, in this population.
The age at puberty reflects interactions between genetic, prenatal, and environmental factors. Twin studies indicate that heredity is responsible for approximately 50% of the variation in age at menarche. Indeed, pubertal timing of both parents has been demonstrated to influence the timing of pubertal onset of both boys and girls independent of sex. Investigation of genetic variants associated with onset of puberty identified a specific variant, −29G>A, in the promoter region of the follicle-stimulating hormone (FSH) receptor (FSHR) gene; breast development occurred 7.4 months later among homozygous carriers of the −29G>A variant compared to the −29GG+GA carriers.
Physiology of Puberty
- ◆
Synthesis of steroids from cholesterol requires expression of specific enzymes, receptors, co-factors, and other proteins in the adrenal cortex and the gonads under the influence of specific trophic hormones, adrenocorticotropin (ACTH), LH, and FSH.
- ◆
Steroid hormone receptors are ligand dependent transcription factors, comprised of three functional domains: the N-terminal domain serves to modulate function; the DNA-binding domain mediates binding of the receptor to DNA; and the ligand-binding domain binds to the cognate steroid hormone.
- ◆
Puberty is characterized by reactivation of the hypothalamic gonadotropin-releasing hormone (GnRH) pulse generator characterized by the increasing amplitude of intermittent bursts of GnRH secretion into the hypophysial portal circulation. These intermittent bursts promote pulsatile LH and FSH secretion by the pituitary gonadotrophs.
- ◆
Increments in circulating LH and FSH concentrations at the time of puberty drive sex-specific gonadal steroidogenesis, development of secondary sexual features, and gametogenesis.
- ◆
The KNDy neurons in the infundibular (arcuate in nonprimate species) nucleus in the hypothalamus appear to comprise the major elements of the GnRH pulse generator.
- ◆
GnRH pulse generation in the KNDy neurons is achieved by reciprocating stimulatory neurokinin B (NKB) and inhibitory (dynorphin) connections within the arcuate nucleus, while the output of the pulse generator is relayed to GnRH fibers projecting to the median eminence by an intermittent kisspeptin signal.
Steroidogenesis
The biosynthetic pathways for gonadal and adrenal steroids are considered together because of their similarities and the importance in understanding the physiology and pathophysiology of puberty ( Fig. 17.4 ; see Chapter 4 ). Synthesis of steroids from cholesterol requires the expression of specific enzymes, receptors, co-factors, and other proteins in the adrenal cortex and the gonads. Steroidogenesis is regulated by specific trophic hormones, ACTH, LH, and FSH.
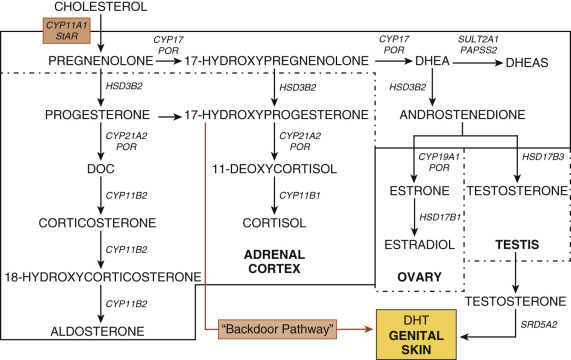
The adrenal cortex consists of three zones, the zona glomerulosa (ZG), zona fasciculata (ZF), and zona reticularis (ZR). The ZG synthesizes aldosterone, a mineralocorticoid, and is primarily regulated by potassium concentrations and renin-angiotensin. The ZF synthesizes cortisol. Steroidogenesis in the ZF is primarily governed by ACTH. The ZR synthesizes C-19 steroids, such as DHEA, DHEAS, androstenedione, androstenediol, and 11β-hydroxyandrostenedione.
ACTH is a peptide derived following proteolytic cleavages of proopiomelanocortin (POMC). Its actions are mediated by the ACTH receptor, a seven-transmembrane G protein-coupled receptor encoded by MC2R. This pathway utilizes cyclic adenosine monophosphate (cAMP)-dependent protein kinase A. The acute effects of ACTH include uptake of plasma low-density lipoproteins, stimulation of cholesterol esterase activity, enhanced synthesis and phosphorylation of steroidogenic acute regulatory protein (StAR), cholesterol transfer across the inner mitochondrial membrane, and increased cortisol secretion. The chronic effects of ACTH involve stimulation of transcription and translation of steroidogenic enzyme genes.
In the gonads, LH and FSH modulate steroid biosynthesis. LH promotes ovarian theca cell and testicular Leydig cell steroidogenesis; its actions are mediated by its cognate receptor, LHCGR. FSH acting through the FSHR stimulates aromatase expression to promote estrogen biosynthesis in the ovary and Sertoli cell growth in the testis. The LH and FSH receptors are both G protein-coupled receptors and contain leucine-rich repeats in their large ectodomains.
Most enzymes involved in steroidogenesis are cytochrome P450s (CYPs) or hydroxysteroid dehydrogenases (HSDs). The rate-limiting step of steroidogenesis is transport of cholesterol into mitochondria mediated by StAR. Within the mitochondria, cholesterol desmolase (also known as side-chain cleavage or P450scc) converts cholesterol into pregnenolone. One enzyme, 17α-hydroxylase/17,20-lyase (P450c17), encoded by the CYP17A1 gene, is the qualitative regulator of adrenal and gonadal steroidogenesis. This enzyme mediates 17α-hydroxylation to convert pregnenolone into 17α-hydroxypregnenolone. In the ZR, ovarian theca, and Leydig cells, this same enzyme catalyzes scission of the C17–20 bond to produce DHEA. Although this one protein is capable of two distinct enzymatic reactions, these enzyme activities are differentially regulated. Factors known to modulate 17,20-lyase activity include: (1) the amount of P450 oxidoreductase (POR); (2) the expression of cytochrome b 5 (CYB5A); (3) the phosphorylation of serine/threonine residues on P450c17; and (4) the phosphorylation of noncanonical P450c17 residues. POR is a protein that transfers electrons from nicotinamide adenine dinucleotide phosphate to microsomal cytochrome P450 enzymes, such as P450c17, P450c21, and aromatase (P450aro). CYB5A modulates adrenal androgen secretion by increasing the 17,20-lyase activity of (P450c17).
The Δ -steroids are converted to the Δ -steroids by 3β-hydroxysteroid dehydrogenase type 2 (HSD3B2), the adrenal and gonadal specific isoform. This enzyme converts pregnenolone to progesterone in the ZG, 17-hydroxypregnenolone to 17-hydroxyprogesterone in the ZF, and DHEA to androstenedione in the ZR. In the ZF, 17-hydroxyprogesterone (17-OHP) is converted to 11-deoxycortisol by 21-hydroxylase (P450c21) and, subsequently, to cortisol by 11β-hydroxylase (P450c11β).
The adrenals, ovaries, and testes synthesize sex steroids. The ZR of the adrenal cortex produces DHEA, DHEAS, androstenedione, androstenediol, and 11β-hydroxyandrostenedione. DHEA sulfotransferase (SULT2A1) converts DHEA to DHEAS; this enzyme is also expressed in the liver. Sulfation of steroids by SULT2A1 requires a sulfate donor, 3′-phosphoadenosine 5′-phosphosulfate (PAPS), and the enzyme PAPS synthase. In the ovary, androstenedione is synthesized in the theca cell and diffuses into the granulosa cell, where it is aromatized by aromatase (P450aro) to estrone and converted to estradiol by 17β-hydroxysteroid dehydrogenase type 1 (HSD17B1). In Leydig cells, androstenedione is converted to testosterone by HSD17B3. In many androgen target cells, such as those in the external genitalia and prostate, testosterone is converted to DHT by 5α-reductase type 2. In other androgen-sensitive tissues, such as bone and adipose, testosterone is converted to estradiol by aromatase. Whereas DHT is the most potent ligand for androgen receptor (AR), some adrenal C-19 steroids can undergo peripheral conversion to hormones capable of binding to the AR, which is encoded by the AR (NR3C4) gene.
The 17β-hydroxysteroid dehydrogenase enzymes comprise a large family of enzymes involved in steroid biosynthesis and metabolism. The differences in tissue distribution, substrate preferences, subcellular localization, and mechanisms of regulation influence the cellular steroid micro-environment. The type 1 isozyme, 17βHSD1, is expressed in ovaries, placenta, endometrium, and liver, where it favors conversion of estrone to estradiol. The type 3 isozyme, 17βHSD3, is expressed in the testis, where it preferentially converts androstenedione to testosterone. The type 5 enzyme, 17βHSD5, is an aldo-keto-reductase (AKR) enzyme (AKR1C3) that is expressed in steroidogenic and peripheral tissues; it can convert androstendione to testosterone.
Through investigations of the tammar wallaby and patients with disordered steroidogenesis, the presence of another pathway leading to dihydrotestosterone (DHT) synthesis was elucidated. In this “alternative backdoor pathway,” 17-OHP undergoes 3α- and 5α-reduction followed by 17,20-lyase, 17β-hydroxysteroid dehydrogenase, and 3α-oxidation steps to generate DHT in the absence of the “classic” intermediates, DHEA, androstenedione, and testosterone. In humans, since 17-OHP is not a favorable substrate for the 17,20-lyase reaction, this pathway acquires functional importance in disorders of steroidogenesis associated with increased 17-OHP concentrations, such as congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency and oxidoreductase deficiency.
Once secreted, sex steroids circulate bound to sex hormone-binding globulin (SHBG) and to albumin. The unbound or free hormone is the bioavailable form that diffuses passively into target cells and interacts with nuclear steroid receptors. Steroid hormone receptors are ligand dependent transcription factors, comprised of three functional domains: the N-terminal domain serves to modulate function; the DNA-binding domain mediates binding of the receptor to DNA; and the ligand-binding domain binds to steroid. Steroid receptor activity is modulated by various tissue-specific cofactors; both coactivators and corepressors can influence receptor function.
Steroids also act through nongenomic mechanisms. For example, testosterone can activate phospholipase C, leading to calcium influx into Sertoli cells, and can activate the mitogen-activated protein kinase as well as other intracellular mediators. Sex steroids can be metabolized to inactive forms by a variety of enzymes. Glucuronidation decreases the biological activity of steroid hormones and increases solubility to facilitate renal excretion. This process, catalyzed by UDP-glucuronyltransferase (UGT) enzymes, involves the transfer of glucuronic acid from uridine diphosphoglucuronic acid to steroid hormones. In humans, the UGT2B isoforms show greater specificity for C19 androgens. A second mechanism is sulfoconjugation, in which DHEA sulfotransferase catalyzes conversion of DHEA to DHEAS, and estrogen sulfotransferase converts estrogens to estrone sulfate. The inactive sulfated steroids can be hydrolyzed to active forms by steroid sulfatase.
Activation of Gonadarche
Gonadarche reflects increased activity of the hypothalamic-pituitary-gonadal (HPG) axis (see Chapter 1 ). The increased pituitary secretion of LH and FSH stimulates gonadal steroidogenesis, development of the physical manifestions of puberty, completion of gametogenesis, and maintenance of fertility. LH and FSH are heterodimeric proteins comprising a common α-subunit and a unique β-subunit; both are glycosylated peptides. Glycosylation appears to modulate hormone stability, protein folding, cellular trafficking, circulating serum half-life, and receptor signaling. The temporal increments in circulating LH and FSH concentrations at the time of puberty, and their relationships to those of the gonadal steroids, testosterone and estradiol, respectively, at this stage of development, have been well documented in both boys and girls. The actions of LH and FSH are mediated by their cognate seven-transmembrane domain G protein-coupled receptors: the LH receptor (LHCGR) and FSHR, respectively.
In the female, LH stimulates androgen production by the thecal cells of the ovarian follicles and progesterone secretion from luteinized granulosa cells of the corpus luteum. FSH is critical for the process of follicular recruitment and selection. In granulosa cells of the developing follicle, FSH induces expression of aromatase, which is responsible for aromatization of the theca-cell-derived androgens into estrogens. FSH also induces LHCGR expression in granulosa cells of the dominant follicle, which selectively amplifies the effect of declining FSH concentrations on the dominant follicle. In the male, LH regulates the secretion of testosterone from Leydig cells.
FSH, together with testosterone, is responsible for initiating and maintaining spermatogenesis. The action of FSH in this regard is indirect and exerted on the somatic Sertoli cell of the seminiferous tubule. While the actions of testosterone are also indirect, several somatic cell types in the testis (Sertoli, Leydig, and peritubular) express AR and are considered to be involved in the control of spermatogenesis.
The pubertal drive to the pituitary-gonadal axis is generated by a diffusely distributed network of hypothalamic neurons expressing GnRH-1, known as the “hypothalamic GnRH pulse generator.” As the name implies, the hypothalamic GnRH pulse generator produces intermittent discharges of GnRH into the hypophysial portal circulation, which is obligatory for gonadotropin synthesis and secretion by the pituitary gonadotrophs. LH and FSH secretion is stimulated by GnRH acting through its receptor, GnRH-R1, located on gonadotropin-secreting cells (gonadotrophs) in the pituitary gland. A unique feature of the human GnRHR, a 7-transmembrane domain G protein-coupled receptor, is its lack of a C-terminal cytoplasmic domain.
A second GnRH gene (GnRH-II) is also expressed by neurons in the primate brain and a GnRH-II receptor has been cloned from the pituitary, but the gene encoding this receptor does not generate a functional protein in humans. The significance, if any, of this second GnRH system in the control of the human pituitary-gonadal axis has not been delineated. Similarly, a hypothalamic RF amide-related peptide (RFRP3) that is inhibitory to gonadotropin secretion in several species and considered to be the homolog of gonadotropin-inhibitory hormone (GnIH) in birds has been identified in mammalian species, GnIH neurons are mainly concentrated in the dorsomedial hypothalamus, form appositions with some GnRH neurons, and signal through its cognate receptor, GPR147. Nevertheless, the potential roles, if any, of GnIH and GPR147 on gonadotropin secretion in man and nonhuman primates remain to be clarified.
Other factors have been studied during puberty. These factors include inhibins, activins, anti-müllerian hormone, insulin-like factor-3 (INSL3), and osteocalcin. Activins and inhibins are members of the transforming growth factor-β (TGF-β) superfamily composed of a common α-subunit and two β-subunits (β A and β B ). The activins are dimers consisting of only the β-subunits; activin A is a dimer of β A subunits and activin B is a dimer of β B subunits. The activins are synthesized in the gonadotropes and influence FSH secretion. Mature inhibins are dimers composed of a common α-subunit covalently linked with one of two β-subunits (β A and β B ). The α/β A and α/β B dimers are known as inhibin A and inhibin B, respectively. The gonadal inhibins, like the gonadal steroids, play both an endocrine role in the regulation of gonadotropin secretion and a paracrine role within the gonads. Inhibin B, which is synthesized in part by the Sertoli cell, is the principal inhibin secreted by the testis. Inhibin B concentrations are low in prepubertal boys and increase with the onset of puberty. The pubertal increase in inhibin B may be attributed to Sertoli cell proliferation and to the initiation of spermatogenesis, both of which reflect the increased gonadotropin drive to the testis at the time of gonadarche.
In girls, circulating levels of inhibin A and B are low or undetectable prior to puberty. Inhibin B begins to rise with the onset of puberty, as does inhibin A in breast stages 3 and 4. Adult levels are attained at approximately 14 to 15 years of age. During the menstrual cycle, inhibin A levels are elevated in the luteal phase, while inhibin B predominates in the circulation of the follicular phase. However, the role of ovarian inhibins in regulating gonadotropin secretion in pubertal and premenopausal women remains to be fully elucidated.
Osteocalcin is secreted by osteoblasts. Most available data regarding the potential actions of osteocalcin are derived from mouse studies. Studies of mice have shown that osteocalcin facilitates testicular testosterone secretion and pancreatic β-cell proliferation. The testicular effects of osteocalcin appear to be independent of the HPG axis as the osteocalcin receptor is not expressed in the hypothalamus or pituitary of mice. Posttranslationally, osteocalcin is carboxylated on three glutamic acid residues. The carboxyated form of the molecule is considered to be biologically inactive whereas the undercarboxylated form is biologically active. Osteocalcin signals through its cognate receptor, GPRC6A, which is expressed by Leydig cells. Interestingly, increased osteocalcin concentrations are associated with the pubertal rise in testosterone concentrations in boys. Osteocalcin shows sexual dimorphism because it modulates Leydig cell testosterone production, but not ovarian estrogen production. The discovery of a missense mutation in the GPRC6A gene in two men with primary testicular failure, oligospermia, and glucose intolerance encourages speculation that osteocalcin influences testicular function in humans.
Anti-müllerian hormone (AMH), also known as müllerian-inhibiting hormone (MIH), is another member of the TGF-β superfamily. AMH signals by binding to a specific type-II receptor (AMHR2); this receptor heterodimerizes with one of several type-I receptors (ALK2, ALK3, and ALK6), leading to recruitment of Smad proteins that are translocated to the nucleus to regulate target gene expression. AMH is secreted by the Sertoli cells of the developing testis and stimulates regression of the müllerian ducts during male fetal development. Postnatally, AMH is secreted by the Sertoli cells of the testis. In boys, AMH concentrations decline at puberty. Although FSH stimulates AMH production, testosterone inhibits Sertoli cell AMH secretion. The decline in AMH concentrations appears to be closely coupled to rising inhibin B concentrations at the onset of puberty in boys—the latter presumably reflecting androgen induced differentiation of the Sertoli cell. Both AMH and inhibin B concentrations are low in boys with bilateral anorchia or complete hypogonadotropic hypogonadism (HH).
Immunohistochemical studies of human ovaries showed no AMH staining in primordial follicles, highest expression in growing preantral and small antral follicles, and disappearance in larger follicles (>8 mm). In ovaries, AMH is primarily secreted by the granulosa cells of the preantral and antral follicles. During infancy, AMH concentrations increase to achieve a plateau during adolescence until age 25 years. Subsequently, AMH concentrations decline and correlate inversely with age. AMH plays a role as a gatekeeper of follicular development.
Data obtained from studies in rodents have demonstrated presence of the AMH receptor, AMHRII, in gonadotropes. One report using rats showed that AMH stimulated FSH secretion in immature female rats. Subsets of GnRH neurons express the AMH receptor. In vitro studies have demonstrated that AMH increases GnRH-dependent LH pulsatility and secretion. Thus AMH may influence gonadotropin secretion.
INSL3, a peptide hormone secreted by Leydig cells, plays a major role in directing the transabdominal phase of testicular descent during gestation. INSL3 signaling is mediated by the G protein-coupled relaxin family peptide receptor 2 (RXFP2). After birth, fetal Leydig cells involute followed by quiescence until puberty. Adult-type Leydig cells derive from a different population of precursors and are dependent on LH stimulation. INSL3 concentrations are low during infancy, rise during puberty, and reflect Leydig cell function during adulthood. Longitudinal data demonstrated that INSL3 concentrations rise with onset of puberty and increasing testicular volume.
INSL3 is also secreted by ovarian theca cells, predominantly by theca cells surrounding medium and large growing follicles. INSL3 and its receptor may play a role in autoregulatory feedback to maintain theca cell androgen production. Despite much variation, INSL3 concentrations tend to rise during late puberty in girls; the variation likely reflects that INSL3 is secreted largely by growing follicles and is a marker of theca cell activity.
In the pubertal and postpubertal individual, the ovaries and testes are governed by feedback control systems. GnRH and the gonadotropins comprise the feed-forward components from hypothalamus to pituitary and from pituitary to gonad, respectively. Steroid and protein hormones from the gonads, in turn, provide the feedback signals that regulate the secretion of LH and FSH. The feedback actions of these gonadal hormones, which involve both negative and stimulatory (positive) actions, may be exerted directly at the level of the pituitary gonadotrophs to modulate expression of the genes encoding LHβ and FSHβ ( LHB and FSHB , respectively). Feedback may also be exerted indirectly at the level of the hypothalamus to regulate the release of GnRH.
In the male, a negative feedback action of testosterone and inhibin B are the major regulators of LH and FSH secretion, respectively. The action of testosterone is predominantly exerted at the hypothalamic level, while that of inhibin appears to occur directly at the pituitary. The role of aromatization of testosterone to estradiol in mediating the negative feedback action of this androgen on LH secretion continues to be an area of active investigation. The feedback control of LH and FSH throughout the menstrual cycle is complicated and involves both negative and positive feedback actions of ovarian steroids at both the hypothalamic and pituitary levels (see also Chapter 8 ). The maintenance of normal ratios of circulating LH and FSH concentrations is important for gonadal function, particularly for folliculogenesis and ovulation.
In conditions under which pulsatile GnRH release is compromised, such as occurs in anorexia nervosa and during periods of strenuous physical training especially in young women, gonadotropin secretion is attenuated and pubertal development is arrested. Thus the pituitary-gonadal axis in both males and females may be viewed as being a slave to the hypothalamic GnRH pulse generator, and this analogy should be held in mind when considering the mechanisms triggering the onset of gonadarche.
Hypothalamic Gonadotropin-Releasing Hormone Pulse Generator
The human hypothalamus contains diffusely distributed GnRH neurons, many of which send their projections to the median eminence, where they synchronously and intermittently discharge their peptide into the primary plexus of the hypophysial portal circulation, thereby providing the pituitary gonadotrophs with the pulsatile stimulation that is essential for maintaining gonadotropin secretion. While the neurobiological mechanisms that underlie GnRH pulse generation remain controversial, compelling evidence indicates the fundamental role of KNDy neurons in the arcuate nucleus (a.k.a. infundibular nucleus). These hypothalamic neurons, named because they coexpress kisspeptin, NKB, and dynorphin, project their axons to the median eminence where they mingle intimately with GnRH fibers en route to the portal vessels. The GnRH fibers that target the median eminence display unique features in that these fibers exhibit characteristics of both axons and dendrites and have been termed “dendrons.” At the median eminences, these fibers are intertwined, encased by tanycytes (specialized ependymal cells of the third ventricle), project to multiple blood vessels, and receive numerous synaptic inputs.
Kisspeptin is an extremely potent GnRH secretagogue and signals through its cognate receptor, KISS1R, which is expressed in GnRH neurons. Kisspeptin fibers project onto GnRH cell bodies and GnRH fibers. Using immunohistochemistry, kisspeptin was detected in the anterior and intermediate lobes of the pituitary in monkeys, but was not apparently colocalized with gonadotrophs, somatotrophs, or lactotrophs. Conflicting data exist regarding the functional relevance of direct kisspeptin action at the level of the pituitary gland. Nevertheless, available evidence suggests that bidirectional central and peripheral signaling modulate reproductive function. Use of Gpr54 KO mice with selective re-introduction of Gpr54 into GnRH cells confirmed that direct effects of kisspeptin on GnRH cells are sufficient to attain fertility, but insufficient to preserve normal functionality of the reproductive axis; kisspeptin does not signal directly at the level of the pituitary.
It has been proposed that GnRH pulse generation is achieved by reciprocal stimulatory (NKB) and inhibitory (dynorphin) connections within the arcuate nucleus, while the output of the pulse generator is relayed to GnRH fibers projecting to the median eminence by an intermittent kisspeptin signal. Thus these neurons may represent the anatomic site of the GnRH pulse generator. Loss-of-function mutations in the genes encoding for kisspeptin (KISS1), the kisspeptin receptor (KISS1R), NKB (TAC3), or the NKB receptor (TACR3) in humans are associated with hypogonadotropic states that are typically manifest at puberty. The importance of these neurons was confirmed by the demonstration that selective ablation of KNDy neurons in the postpubertal rat results in a loss in hypothalamic drive to LH secretion.
The KNDy hypothesis of the GnRH pulse generator was investigated in adult male volunteers by administering kisspeptin-54, NKB, and an opioid receptor antagonist, naltrexone. LH pulsatility was used as a surrogate marker for GnRH pulsatility. Kisspeptin alone potently increased LH and LH pulsatility, which was consistent with previous observations in humans. NKB alone, on the other hand, did not affect gonadotropins. Coadministration of NKB and kisspeptin had significantly lower increases in gonadotropins compared with kisspeptin alone. The combination of naltrexone and kisspeptin significantly increased LH pulse amplitude. These results suggest that interactions between kisspeptin, NKB, and dynorphin influence LH pulsatility and gonadotropin release in humans. In women, NKB appears to influence GnRH/LH secretion in normal women through mechanisms predominantly proximal to kisspeptin in mediating estrogen positive and negative feedback on LH secretion. Overall, these studies have begun to elucidate functional aspects regarding the neurobiology of KNDy neurons. Clarifying the details of the potential interaction of kisspeptin, dynorphin, and NKB warrants further studies.
Curiously, a role for kisspeptin (beyond the GnRH pulse generator) has been implicated as integrating behavior and emotions with reproduction in humans in that kisspeptin administration enhanced limbic brain activity captured on functional MRI in response to sexual images and nonsexual bonding images in healthy volunteer men.
Coordination and interactions among various hypothalamic factors influence GnRH secretion by the GnRH neuron. Both excitatory and inhibitory transsynaptic neuronal inputs modulate the GnRH neuronal system. In addition to neuronal afferents, GnRH neurons maintain close physical contact with glial cells. Thus the secretory activity of GnRH neurons reflects the integrated response to hormones, neurotransmitters, neuromodulators, paracrine interactions, and environmental cues. Leptin, insulin, IGF-1, ghrelin, FGF21, orexigenic peptides, and anorexigenic peptides provide input signals that are deciphered and organized to govern pubertal maturation and ongoing reproductive function.
Neurobiology of Gonadarche
- ◆
GnRH neurons are derived from a heterogenous stem cell population in the embryonic olfactory placode and migrate to their final destinations in the hypothalamus during embryonic development.
- ◆
Several adhesion and guidance molecules and their cognate receptors have been implicated in this process, including anosmin-1 (ANOS1), ephrins, CHD7, FGF8, FGFR1, and prokineticin.
- ◆
A transient reactivation of the HPG axis from 1 through 3 to 6 months of age results in an adult-like endocrine milieu.
- ◆
The control system that dictates the up-down-up pattern of GnRH pulse generator activity from early infancy until puberty may be viewed as a conceptual “neurobiological brake.”
- ◆
The neurobiological brake is less strong in girls than in boys.
- ◆
In the absence of intact kisspeptin or NKB signaling pathways, the output of the GnRH pulse generator will be compromised, resulting in absence of puberty, validating the central importance of the KNDy neuron as the GnRH pulse generator.
- ◆
Available data indicated that the GnRH pulse generator is under the control of an upstream transcriptional gene network.
- ◆
The precise mechanism(s) that governs the timing of the onset of puberty remains a mystery.
- ◆
The “somatometer hypothesis” proposes that attainment of a particular state of somatic maturation initiates puberty. Body fat content, leptin, and insulin have been argued to be relevant for this hypothesis.
- ◆
Another hypothesis invokes a pubertal clock presumably resident in the CNS.
Fetal Development of the Gonadotropin-Releasing Hormone Pulse Generator
Using three-dimensional (3D) imaging and transparent human fetal brains, Casoni et al. have suggested that approximately 2000 GnRH neurons reside in the hypothalamus and approximately 8000 are widely distributed in other areas of the brain. Meticulously orchestrated development of the GnRH neurons and olfactory neurons in conjunction with precise spatio-temporal expression of multiple factors is essential for normal HPG axis function ( Fig. 17.5 ). Specific mutations in families with disorders of puberty and studies of transgenic mice have established some of the factors involved in GnRH neuron migration. Elements essential to GnRH neuron development, migration, and function include cytoskeletal proteins, adhesion molecules, neurotransmitters, growth factors, receptors, and transcription factors. Some factors function in multiple regions and may have diverging effects depending on the context of the molecular environment. Elucidation of the neurobiology and ontogeny of the GnRH neurons will improve understanding of the pathophysiology of HH and, perhaps, lead to novel therapies.
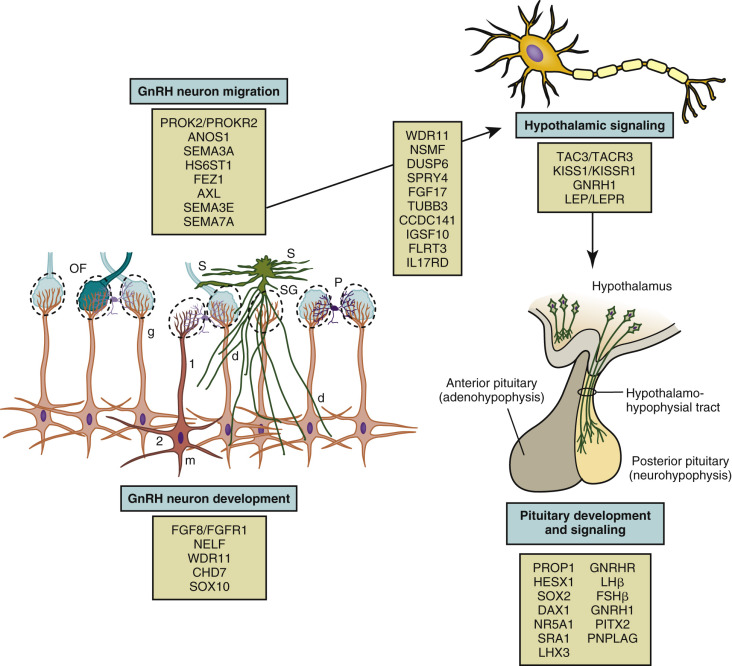
The GnRH neurons are born in the olfactory placode and migrate during early fetal development from the nose through the forebrain to the hypothalamus. The fetal ontogeny of the GnRH neurons can be classified into several stages, each with distinct regulatory mechanisms: (1) differentiation of GnRH neurons; (2) axonophilic migration with axons of the vomeronasal nerve across the cribriform plate and into the forebrain; (3) localization in the hypothalamus and development of processes to the median eminence; and (4) attainment of final location and functionality.
Most vertebrates have two distinct olfactory systems: the main olfactory system responsible for recognition of volatile odorants and the vomeronasal system responsible for detection of pheromones. During the fifth week of gestation in humans, the olfactory placodes develop as thickenings of the ectoderm on the ventrolateral sides of the head. The GnRH progenitor stem cells include cells derived from this embryonic olfactory placode and cells derived from the neural crest.
Two proteins, chromodomain helicase DNA-binding protein 7 (CHD7) and SOX10, influence neural crest cell development and eventual migration. CHD7 is a large protein that participates in chromatin remodeling and transcription; it interacts with other proteins and may regulate genes involved in neural crest cell guidance. In mice heterozygous for Chd7 mutations, Fgfr1 expression in the olfactory placode, GnRH1 and Otx2 expression in the hypothalamus, and GnRHR expression in the pituitary were decreased, supporting a role for CHD7 upstream of Fgf8 and Fgfr1 in the development and maintenance of GnRH neurons.
Other factors involved in the differentiation of GnRH neurons include fibroblast growth factor-8 (FGF8), fibroblast growth factor receptor-1 (FGFR1), and heparan sulfate 6- O -sulfotransferase 1 (HS6ST1). FGF8 influences craniofacial development, neuroendocrine cell proliferation, cell fate specification, and cell survival. In the developing GnRH neuron, FGFR1 is the preferred receptor for FGF8. The FGFR1 is a tyrosine kinase receptor composed of three extracellular immunoglobulin domains, a transmembrane domain, and a cytoplasmic tyrosine kinase domain. Upon ligand binding, FGFR1 and its coreceptor dimerize, leading to autophosphorylation and protein kinase activity. In humans, FGF8 and FGFR1 loss-of-function mutations have been associated with holoprosencephaly, impaired forebrain cleavage, and midline facial anomalies, highlighting the important role of this signaling pathway.
The extracellular domain of FGFR1 interacts with heparan sulfate proteoaminoglycan, its coreceptor. Heparan sulfates are cell membrane and matrix-associated proteoglycans involved in neural development. These polysaccharides undergo nonrandom modifications of the sugar moieties to facilitate cell-to-cell communication. HS6ST1 introduces a sulfate at the 6- O position within heparan sulfate. This action appears to be necessary for FGFR1 function.
GnRH neuronal differentiation occurs between 39 and 44 days of gestation. Between weeks 5 and 6 of human gestation, the migratory mass of neural crest-derived migratory cells and olfactory neurons contains a small number of GnRH neurons. Subsequently, around the sixth week of gestation, the GnRH neurons begin their migration along vomeronasal nerves through the cribriform plate and eventually find their way to the hypothalamus. The GnRH neurons migrate into the brain along two distinct migratory pathways: a ventral pathway directed towards presumptive hypothalamic regions and a dorsal pathway directed toward pallial and subpallial telencephalic regions. These GnRH neurons, accompanied by olfactory ensheathing cells (OECs), travel along with the terminal, vomeronasal, and olfactory nerves into the brain. OECs are glial cells that guide GnRH and olfactory nerves to the forebrain. OECs express several factors important for GnRH migration, such as semaphorin 4D, signaling and neuronal migration factor (NSMF), and stromal derived growth factor 1 (SDF-1). Available data indicate that SOX10 promotes development of OECs.
Correct targeting and movement of GnRH neurons depends on multiple cues provided by many factors. These signals may act directly or indirectly through the scaffold of olfactory neurons. Chemokine gradients likely influence GnRH neuronal movement. Such factors include SDF-1 and gamma-aminobutyric acid (GABA). Curiously, SDF-1 and GABA appear to exert divergent effects to accelerate or retard, respectively, neuronal migration. SDF-1 acts through its receptor, CXCR4, via a G protein-activated inward rectifier potassium channel. SDF-1 has been observed in the nasal mesenchyme (NM), whereas its receptor, CXCR4, has been localized in migrating GnRH neurons and olfactory/vomeronasal nerve axons. Further, CXCR4-deficient mice exhibit a loss of GnRH neurons and impaired migration, suggesting the importance of SDF-1/CXCR4 signaling in the development of this system.
CCDC141 encodes a coiled-coil domain containing protein that is expressed in GnRH neurons and olfactory fibers. Knockdown of Ccdc141 did not change olfactory axon outgrowth, but was associated with decreased GnRH cell migration out of the nasal pit. In mice, Ccdc141 expression, correlated with migration in nasal regions and decreased when GnRH neurons entered the forebrain, appears to affect cellular motility through its interactions with myosin II.
Additional adhesion and guidance molecules include ANOS1, ephrins, and prokineticin 2 (PROK2). The gene encoding ANOS1 (previously known as KAL1 ) is located at Xp22.3 in the pseudoautosomal region of the X chromosome. Anosmin-1 is an extracellular matrix glycoprotein that contains a whey acidic protein-like protease inhibitor domain and four fibronectin type III domains. It promotes the formation of the lateral olfactory tract and neurite development. Anosmin may also serve as (1) an adhesion molecule to guide migrating GnRH neurons; and (2) as a chemoattractant for olfactory axon pathfinding. Also, it may interact with FGFR1. Ephrins are cell surface molecules that play a major role in axon guidance and signal through their cognate membrane tyrosine kinase receptors. PROK2 signals through the prokinecticin receptor 2 (PROKR2), a member of the rhodopsin G protein-coupled receptor family. PROK2 and its receptor (PROKR2) , a G protein-coupled receptor, appear to play major roles in olfactory bulb neurogenesis and GnRH neuron migration. However, neither protein is expressed in GnRH neurons. Based on the findings in mice with targeted PROK2 mutations, the GnRH neurons appear to be trapped with olfactory neurons with arrested migration just after crossoing the cribriform plate.
Semaphorins comprise a large and diverse family of secreted and membrane-associated proteins that influence the navigation of growing axons, and play a role in neural network formation. Four class 3 semaphorins, Sema3A, Sema3B, Sema3C, and Sema3F, are expressed around the developing olfactory/vomeronasal region. Semaphorin-3A (SEMA3A) is a secreted protein with repulsive effects on primary olfactory axons expressing the coreceptor neuropilin-1 (Nrp1), which may influence the migration of GnRH neurons. Semaphorin 3A is also expressed byOECs. Semaphorin-3E (SEMA3E) on the other hand protects maturing GnRH neurons from cell death. Semaphorin 4D is a membrane-bound semaphorin that can also be proteolytically released into the extracellular space in an active form. It can act as a proangiogenic factor through the coupling of its cognate receptor, PlexinB1, with the hepatocyte growth factor (HGF) receptor Met tyrosine kinase (MET). Both semaphorin 4D and PlexinB1 are highly expressed in the developing olfactory placode and the developing NM.
Semaphorin 7A (Sema 7A) appears to play a role in GnRH neuronal migration. Two mechanisms have been described: it can act as a membrane-bound signaling molecule or, following proteolytic cleavage, as a soluble factor. Sema7A can interact with two different receptors, plexin C1 and β1-integrin. Binding to plexin C1 decreases integrin-mediated cell attachment and spreading and interacting with β1-integrin induces integrin clustering and the activation of MAPK pathways. The phenotype of the mouse model with GnRH neuron-specific β1-integrin conditional KO showed impaired migration of GnRH neurons, delayed pubertal onset, and impaired fertility in female mice. In addition to its role in the development of the GnRH system, Sema7A appears to mediate the plasticity of GnRH neurons and tanycytes in the adult median eminence.
IGSF10, a member of the immunoglobulin superfamily, is also implicated in GnRH neuronal migration. Tissue expression studies using mouse embryos showed IGSF10 mRNA expression was localized to embryonic NM during the time that GnRH neurons are migrating through the NM. In a zebrafish IGSF10 knockdown model, loss of IGSF10 led to perturbed migration and failed neurite extension of GnRH3 neurons toward the hypothalamus.
HGF, Axl, and Tyro3 maintain GnRH neuronal survival when the neurons are crossing the cribriform plate region. HGF signals through its receptor, cMet, to promote GnRH neuron migration. Axl and Tyro3 are members of the TAM family of tyrosine kinase receptors and contain a fibronectin domain that binds to heparan sulfate proteoglycans. Mice with targeted Axl/Tyro3 mutations show impaired sex hormone-induced gonadotropin surge, resulting in estrous cycle abnormalities. The protein, growth arrest specific 6 (Gas6) encoded by Gas6, is a ligand that activates Axl and Tyro3. Gas6 is a heparan sulfate proteoglycan activated ligand with similarities to the FGFs and HGF. The phenotype of Gas6 knockout mice is characterized by early loss of GnRH neurons during embryonic development. Despite persistent decrease in GnRH neurons and impaired early stages of sexual maturation, these mice eventually manifested normal fertility.
FEZF1 is a zinc-finger gene encoding a transcriptional repressor that is highly and selectively present during embryogenesis in the olfactory epithelium. Fezf1 -deficient mice have impaired axonal projection of pioneer olfactory receptor neurons that cross the cribriform plate and subsequently innervate the olfactory bulb. These mice have smaller olfactory bulbs and an absence of GnRH neurons in the brain. Thus it appears that the FEZF1 product is required for the olfactory receptor neurons, and hence accompanying GnRH neurons, to enter the brain.
The roles of microRNAs (miRs) in neuronal development and maturation are becoming apparent. Data obtained using mice with targeted deletion of Distal-less-related 5 (Dlx5) gene demonstrated that specific miRs (-9 and -200 class) influence olfactory and GnRH neuron development. Mice with gonadotrope specific deletion of Dicer exhibited suppressed gonadotropin β-subunits and infertility. Another microRNA, miR-7a2, is expressed in pituitary gonadotropes. The phenotype associated with genetic deletion of miR-7a2 in mice includes low gonadotropin concentrations and infertility. miR-7a2 is highly expressed in the pituitary, but does not appear to influence GnRH neuron migration.
Upon arrival in the hypothalamus, the GnRH neurons extend projections to the median eminence to form a network that can secrete GnRH into the primary plexus of the hypophysial portal circulation. LH and FSH reach detectable levels by the 10th week of gestation in the human pituitary, peak in midgestation, and are higher in female fetuses than male fetuses. Although the hypothalamic control of the fetal pituitary gonadal axis has not been extensively studied in higher primates, the GnRH pulse generator is clearly driving the gonadotrophs of the fetal pituitary around the 15th week of gestation. Functional activity of this hypothalamic-pituitary system is essential for fetal testicular testosterone synthesis by the Leydig cell and normal male sex development. In contrast to the fetal testis, the ovary at this stage of development is relatively quiescent, and the absence of gonadal feedback signals likely accounts for the higher gonadotropin levels in the female fetus. As gestation progresses, the secretion of estradiol and other steroids by the fetoplacental unit increases dramatically, and suppresses gonadotropin secretion from the fetal pituitary by exerting an inhibitory action either directly at the pituitary or indirectly on the hypothalamus to restrain GnRH release.
Postnatal Development of Gonadotropin-Releasing Hormone Pulsatility
Following birth, GnRH pulse generator activity is robustly expressed, presumably due to the loss of placental steroids, and the pituitary gonadotrophs of the infant respond with LH and FSH secretion. At the hypothalamic level, kisspeptin and NKB are found in the arcuate nucleus at this stage of development, and a loss-of-function mutation in KISS1R has been associated with HH during infancy. Moreover, in the infant boy, the Leydig cells of the testis are stimulated so that circulating testosterone levels are similar to those observed in adult men. Peak testosterone concentrations occur at approximately 2 to 3 months of age and typically decline by 6 months of age. Among preterm male infants, LH and testosterone concentrations are higher than among full-term infants; phallic growth was positively correlated with urinary testosterone levels, and testicular growth was positively correlated with urinary FSH levels. Despite the “adult-like” endocrine milieu of increased gonadotropin secretion and elevated testosterone concentrations during the first few months of life, sexual hair does not develop and gametogenesis is not initiated, presumably due to limited AR signaling in skin and the immature Sertoli cell. The hypothalamic-pituitary-ovarian axis is active in term and preterm girls, and is associated with a transient increase in antral follicles.
Full hormonal responsivity of the gonad is acquired during childhood, but by this stage of development the GnRH pulse generator has been brought into check, resulting in the hypogonadotropic state that guarantees continued gonadal quiescence until the prepubertal phase of development is terminated by a resurgence of GnRH pulse generator activity ( Fig. 17.6 ). During childhood and juvenile development neither the GnRH neurons, pituitary gonadotrophs, nor the cells of the gonads are limiting to the onset of gonadarche.
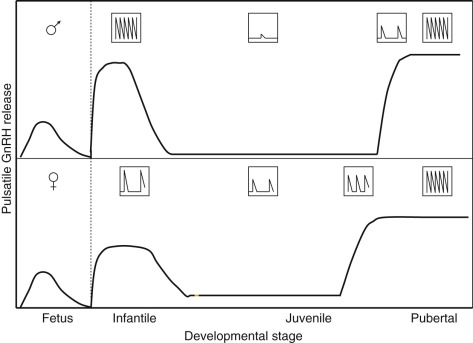
Accordingly, during these stages of development when the primate hypothalamus or pituitary is provided with pulsatile neurochemical, for example, N-methyl-D-aspartate (NMDA), a glutamate receptor agonist, or GnRH stimulation, respectively; or, when the ovary and testis are stimulated directly with LH and FSH, gonadarche may be readily elicited. Blocking endogenous GABA inhibition with the GABA(A) receptor blocker, bicuculline, dramatically increases kisspeptin release, implicating this neuropeptide in the onset of puberty. GABA plays a role in GnRH neuron migration and the onset of puberty. However, the precise details of GABA actions remain to be clarified. These manipulations that trigger premature GnRH secretion are graphically illustrated in children with GnRH-dependent precocious puberty, a disorder discussed later in this chapter.
That the GnRH neuronal network in the juvenile monkey hypothalamus is able upon NMDA stimulation to immediately elicit an adult hypophysiotropic GnRH drive is consistent with the finding that hypothalamic GnRH gene expression and peptide content are maintained throughout this phase of prepubertal development. Thus transcriptional regulation of the gene encoding GnRH from birth to puberty appears to be minimal, and the locus of the developmental control of GnRH release must lie upstream to the GnRH neuronal network.
Because GnRH is secreted in only picogram quantities into the hypophysial portal circulation, changes in the concentration of this neuropeptide in the peripheral circulation do not reflect hypothalamic activity. Therefore studies of the dynamics of the pubertal resurgence of GnRH pulse generator activity in man and other higher primates have generally utilized the high-fidelity relationship that exists between the frequencies of pulsatile GnRH release and episodic LH secretion. The latter may be tracked with relative ease by measuring moment-to-moment changes in LH concentrations in the peripheral circulation. Although the pubertal increase in hypothalamic GnRH drive to the gonadotroph probably involves both frequency and amplitude modulation of the GnRH pulse generator, the relationship between GnRH and LH pulse amplitude is more complex than the relationship between frequency because amplitude modulation of LH release may not always reflect changes in GnRH pulse amplitude. During the initiation of gonadarche in both boys and girls, LH pulse frequency accelerates and LH pulse amplitude increases in association with an amplification of a preexisting sleep-related diurnal pattern in release. This change in neuroendocrine activity may occur before the physical changes of gonadarche are manifest. In boys in particular, LH pulse frequency appears to decline later in pubertal development, probably due to a negative feedback action of rising testosterone concentrations. A longitudinal study of the agonadal monkey suggests that, as in humans, the pubertal acceleration of pulsatile GnRH release is an early neurobiological event in the initiation of gonadarche, and that it is a rapidly completed process. Thus the slow tempo of the overall progression of puberty probably results from mechanisms downstream from the hypothalamus, and particularly at the level of the pituitary.
The control system that dictates the up-down-up pattern of GnRH pulse generator activity from birth until puberty may be viewed as a neurobiological “brake” (or central restraint, as it has been previously described in the pediatric literature) that holds GnRH neuronal activity in check during the greater part of prepubertal development. Here it is important to recognize that the notion of a brake is conceptual; that is, the pubertal resurgence in robust GnRH pulsatility could be occasioned either from the removal of an inhibitory input or by the application of a stimulatory signal to the GnRH pulse generator, or a combination of the two. A similar argument may be applied to the earlier transition between infancy and childhood when GnRH pulsatility is markedly diminished. The neurobiological brake on pulsatile GnRH release throughout childhood and juvenile development is imposed in the absence of the ovary or testis. Consequently, the characteristic pattern of gonadotropin secretion observed during postnatal development in humans with robust gonadotropin secretion during infancy and puberty—separated by a prolonged hiatus in LH and FSH secretion—is maintained in the agonadal situation ( Fig. 17.7 ).
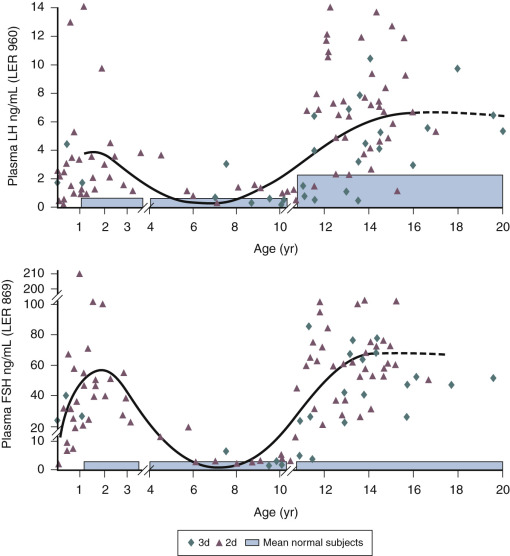
Similarly, in male infants with partial androgen insensitivity, LH concentrations are generally elevated, and this is associated with higher testosterone levels. Yet, infants with complete androgen insensitivity often fail to demonstrate a postnatal rise in LH and testosterone secretion. The later finding is counterintuitive, and an understanding of the molecular basis of this phenomenon may well reveal fundamental insights into the ontogeny of GnRH pulse generation. In agonadal children, the degree of the prepubertal suppression of gonadotropin release is less than that observed in eugonadal individuals. Interestingly, in agonadal children circulating gonadotropin levels are higher in girls than boys, indicating that the intensity of the neurobiological brake imposed on the GnRH pulse generator during prepubertal development is less in the female than in the male. As a result, the gonadotropin drive to the prepubertal ovary stimulates a low level of estradiol secretion, which, through negative feedback action on LH and FSH release, amplifies the relatively weaker neurobiological brake restraining gonadotropin secretion in the prepubertal girl. This sex difference in the strength of the neurobiological brake on prepubertal GnRH release is associated with a shorter duration of the brake in girls, which probably accounts for the relatively earlier age of gonadarche in the female. These and other sex differences in the developmental control of the GnRH pulse generator are presumed to result from greater exposure of the fetal male hypothalamus to testosterone.
Nature of the Neurobiological Brake
The discovery in 2003 that loss-of-function mutations in KISS1R in humans were associated with HH and delayed or absent puberty demonstrated the critical role of kisspeptin in regulating GnRH secretion. Subsequent studies using several different experimental models validated this finding. These data led to the proposal that a major component of the neurobiological brake imposed upon pulsatile GnRH release during the greater part of prepubertal development is due to a hiatus in a stimulatory kisspeptin input to the GnRH neuronal network. This proposal was based on findings in the monkey, that hypothalamic expression of KISS1 and release of kisspeptin in the region of the median eminence increase at the time of the pubertal resurgence in GnRH pulsatility. Additionally, intermittent administration of kisspeptin at hourly intervals during juvenile development elicits a precocious and sustained adult-like pulsatile pattern of GnRH, and the pubertal increase in GnRH release may be suppressed by the administration of a KISS1R receptor antagonist directly to the median eminence.
The finding in humans that loss-of-function mutations in the NKB signaling pathway are associated with a phenotype similar to that reported earlier for inactivating mutations in KISS1R, together with the observation that these two neuropeptides are coexpressed in the same neurons (KNDy neurons) in the arcuate nucleus, have led to the concept that these KNDy neurons are responsible for the generation of GnRH pulsatility. Thus kisspeptin expressing KNDy neurons in the arcuate nucleus comprise a critical component of the GnRH pulse generator. In the absence of an intact kisspeptin signaling pathway, the output of the GnRH pulse generator will be abrogated and pulsatile GnRH release will be compromised, resulting in a delay or absence of puberty. Overall, available data suggest that the KNDy neurons in the arcuate nucleus themselves do not govern the timing of puberty; rather, these neurons appear to be subservient to upstream regulatory mechanisms that govern the developmental pattern of pulsatile GnRH release and the onset of puberty ( Fig. 17.8 ).
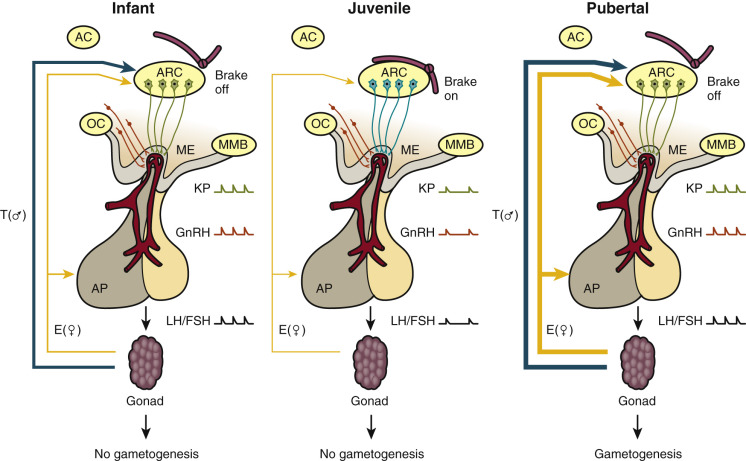
The nature of the upstream pathways that comprise the neurobiological brake on the GnRH pulse generator during childhood and juvenile development remains poorly understood.
Studies of the female rhesus monkey provide evidence that GABA, the major inhibitory neurotransmitter in the brain, is upregulated during juvenile development, and inhibition of GABA tone in the hypothalamus of the prepubertal monkey leads to precocious menarche and ovulation. Interestingly, infusion of the GABA antagonist, bicuculline, into the median eminence of prepubertal female monkeys stimulates release of kisspeptin-54 into this region of the hypothalamus in association with that of GnRH, and the bicuculline-induced GnRH release is blocked by simultaneous infusion of a kisspeptin antagonist. However, it is unclear what reduces GABA inhibition prior to puberty, where the relevant GABAergic neurons are physically located, and how GABA signaling interacts with the GnRH pulse generator.
Other transynaptic signals implicated in the regulation of the pubertal resurgence of GnRH pulse generator activity include glutamate and neuropeptide Y (NPY). Glutamate is the major excitatory neurotransmitter in the brain and, in contrast to GABA, hypothalamic release of this amino acid is increased at the time of puberty in the female monkey, and as discussed earlier in the chapter, repetitive activation of glutamate receptors in the juvenile monkey rapidly leads to the onset of precocious gonadarche. NPY neurons are found in the arcuate nucleus and, in the male rhesus monkey, NPY gene expression in the hypothalamus is inversely related to the up-down-up pattern of GnRH pulse generator activity from birth to puberty. NPY receptors are inhibitory G protein receptors, and their activation leads to hyperpolarization and inhibition of neural activity. However, pharmacological approaches failed to demonstrate that inhibition of NPY signaling in the hypothalamus of the juvenile monkey did not promote GnRH release.
While neuroglia have classically been regarded as subserving only a “supporting role” in the central nervous system (CNS), contemporary views hold that these non-neuronal cells play important functional roles within the brain. Moreover, in the context of the hypothalamus, secretion of TGF-α by astroglia has been postulated to provide the GnRH neuronal network with a stimulatory input at the time of puberty.
Attempts to elucidate the neural mechanism dictating the postnatal pattern of GnRH pulse generation have traditionally led investigators to focus in relative isolation on a “favorite” signaling pathway. Using a systems biology approach, global gene discovery has been combined with computational (in silico) biology to identify functional linked networks of hypothalamic genes that are found to be associated with changes in GnRH pulse generator activity. The initial gene discovery approach was conducted without regard to the phenotype of the cells in which the respective genes are expressed and gene networks are operating. Available data indicate that the developmental changes in the transcriptional factors and gene network relevant to GnRH secretion lie upstream of the KISS1 gene and are, therefore, upstream of GnRH pulse generation. This network of genes serves as a governing hierarchy to orchestrate the resurgence of pubertal GnRH release and, therefore, modulate the timing of puberty. Since such networks of genes are further proposed to function in the absence of signals derived from the periphery, conceptually they may be viewed at a systems level as comprising a pubertal clock (discussed later in the chapter).
Expression of two such transcriptional regulators, enhanced at puberty 1 ( EAP1, also known as interferon regulatory factor 2 binding protein-like) and thyroid transcription factor-1 ( TTF-1, also known as NKX2-1 ), increase in the mediobasal hypothalamus of nonhuman primates at puberty. EAP1 is expressed in kisspeptin neurons in the arcuate nucleus of the monkey. Moreover, its expression in the hypothalamus increases at the time of puberty in the female monkey, and the knock down of EAP1 using a lentivirus approach interrupts menstrual cyclicity in the adult female. Additionally a single nucleotide polymorphism (SNP) upstream of the EAP1 gene has been associated with irregular menses in the monkey. Conditional deletion of Ttf1 from terminally differentiated hypothalamic neurons was associated with delayed puberty, decreased Kiss1 expression, and subfertility. Genetic analysis failed to identify germline mutations in either EAP1 or TTF-1 in patients with HH. Nevertheless, both EAP1 and TTF-1 are functionally connected to genes identified by genome-wide association studies (GWAS) to influence age at menarche.
Additional evidence supporting the concept of a network of genes determining the level of function of the GnRH pulse generator have identified a cohort of genes that encode for a group of transcriptional suppressor proteins known as the polycomb group. In the pubertal rat, expression of these genes is downregulated by DNA methylation, leading to a reduction in the silencer proteins. Two of the polycomb group genes are expressed in the arcuate nucleus, and overexpression of one of these genes resulted in a decrease in Kiss1 expression in association with delayed vaginal opening and a disruption of GnRH pulsatility in mediobasal hypothalamic (MBH) explants. A global inhibition of DNA methylation in prepubertal rats resulted in the delay of vaginal opening, indicating that epigenetic regulation of gene expression may be important for timing puberty. Similarly, data obtained in monkeys indicate that some zinc-finger transcriptional repressors restrain puberty by epigenetically repressing a gene network that operating in the arcuate nucleus controls puberty by governing pulsatile GnRH release.
Additional support for a zinc-finger motif containing suppressor gene holding puberty in check has come from human mutation studies. Using whole exome sequencing (WES), mutations in the gene encoding for makorin RING finger protein 3, MKRN3 , are associated with central precocious puberty (CPP) in seven girls and five boys from 12 families. All affected individuals inherited the disorder-associated allele from their fathers, indicating that only the paternal allele is expressed. These mutations are predicted to result in loss of function of the protein. While the function of MKRN3 is not fully understood, the makorin family of proteins contains a particular zinc-finger motif that has been associated with ubiquitination, a process that is involved in protein trafficking, which in some cases leads to protein degradation. Relevance of this gene to puberty is further supported by demonstrating declining levels of circulating MKRN3 product prior to pubertal onset in Danish girls and boys. Expression of this gene decreases immediately before the onset of puberty in the mouse hypothalamic arcuate nucleus. Thus in mice, makorin-3 may contribute to the neurobiological brake on GnRH secretion. Together, these studies support the concept that transcriptional repression is a core component of the neuroendocrine circuitry that regulates the timing of puberty.
Recently, genome-wide association studies indicate that several specific loci on the human genome are associated with variations in the age at menarche. An SNP that was consistently and strongly associated with an earlier age at menarche was found on chromosome 6 near LIN28B, which encodes for a micro-RNA binding protein. The differences in age at menarche in subjects with and without variants in the region of LIN28B are small (1 to 2 months) compared to the recognized range for the age at menarche in the population at large that may extend from 10 to 16 years of age. A genetic study on girls with early puberty did not find any potentially responsible LIN28B mutations. It is unclear at the moment the exact causal role of LIN28-Let7 if any in timing of human puberty. A follow-up meta-analysis study using genome-wide and custom-genotyping arrays in more than 180,000 European women found strong evidence for 123 SNPs at 106 gene loci to be associated with earlier age at menarche. Surprisingly, only a few known puberty genes overlap with the genes associated with age at menarche. These include MKRN3 , LEPR , IGSF1 , and TACR3 , further implicating biological significance of these genes in pubertal development.
Regardless of the components of the neurobiological brake, which dictate the up-down-up pattern of pulsatile GnRH release from birth until puberty, it is to be anticipated that application and withdrawal of the brake will be associated with a corresponding structural remodeling in those hypothalamic neuronal and glial circuits involved. In this regard, the hypothalamus of the postnatal brain retains its capacity for plasticity, as reflected by the expression of polysialic acid-neural cell adhesion molecule (NCAM).
Putative Physiological Control Systems Governing the Timing of Gonadarche
The physiological control systems that dictate the timing of puberty have intrigued investigators for decades, but the governance of this fundamental developmental event in humans remains largely a mystery. However, two basic schemata have been proposed. In the first, a cue to reawaken the GnRH pulse generator is provided by the attainment of a particular state of somatic maturation. According to this hypothesis, the brain receives this information by way of a signal in the circulation that is tracked by a somatometer resident within the CNS. In the second schema, a pubertal clock (presumably resident in the CNS) generates the signal.
In the case of the somatometer hypothesis, the attainment of a particular proportion of body fat has long been argued to be necessary for the onset of gonadarche. Interest in this hypothesis was rekindled with the discovery of leptin and its receptor. Leptin, encoded by the leptin (LEP) gene, is primarily secreted by adipocytes and regulates feeding behavior and body weight by providing the hypothalamus with information on fat mass and energy status. The leptin receptor (LEPR) is a single transmembrane type I cytokine receptor of the IL-6 receptor family that is encoded by the LEPR gene located at chromosome 1p31.3. Although multiple LEPR isoforms exist, leptin action is primarily mediated by the long form of the LEPR.
Individuals with mutations in leptin signaling, resulting from loss-of-function mutations in either LEP or LEPR, have failed to progress through puberty. The phenotype of LEPR mutations included morbid obesity, abnormal eating behaviors, and lack of spontaneous pubertal development. Over the last 15 years, leptin replacement has been administered to several patients with leptin deficiency due to LEP mutations. When administered at an age appropriate for puberty, leptin promoted pubertal development. Importantly, there has been no evidence of premature puberty in younger children following replacement treatment. Moreover, among children with GnRH-dependent precocious puberty, leptin concentrations correlated with BMI and not pubertal status. These findings provide compelling evidence that the action of leptin, albeit obligatory for the onset of puberty, is nevertheless permissive. This action of leptin may require only low circulating levels of the adipocyte hormone because pubertal development in both male and female subjects with various lipodystrophies has been reported to be normal despite low leptin concentrations.
In girls, it is generally recognized that plasma leptin levels increase progressively through breast stages 1 to 5. In boys, plasma leptin levels also increase during early puberty to reach peak levels between 10 and 12 years of age, but subsequently decline as blood levels of testosterone rise into the adult range. The finding that circulating concentrations of the soluble form of the LEPR progressively decrease during childhood until approximately 11 years of age suggests that the increase in bioavailable leptin during early puberty may be greater than that reflected in total leptin concentrations.
Considerable interest is focused on identification of leptin’s neuronal targets that promote pubertal progression. While it is generally recognized that GnRH neurons do not express LEPR , data obtained in rodents and sheep for the KNDy neurons are inconsistent and the site of action of leptin may be upstream to the GnRH pulse generator. One potential mechanism demonstrated in mice is that leptin induces phosphorylation of neuronal nitric oxide synthase (nNOS) in nNOS-expressing neurons in the preoptic region, which leads to increased LH secretion independent of kisspeptin/KISS1R signaling. Thus NO signaling may play a major role in the crosstalk between leptin and the reproductive axis.
Before leaving the subject of leptin and obesity, it should be noted that although overweight girls tend to “mature” earlier, sleep-related increments in LH secretion, and therefore presumable GnRH pulse generator activity, have recently been reported to be blunted in healthy obese premenarcheal girls.
Other somatic factors have been proposed to serve as signals to the somatometer. The hypothesis that such a factor is skeletal in origin is based on the finding that, in children with an accelerated or retarded maturational tempo, menarche and testicular enlargement correlate better with skeletal age than with chronological age. Skeletal age, also known as “bone age,” is a surrogate marker of biological maturation and may be determined by comparing a radiograph of the left hand to gender-specific standards obtained at various chronological ages. Several caveats are relevant when assessing skeletal maturity. The degree of skeletal maturation at various sites, such as hands, elbows, and knees, may differ. Skeletal maturation of the carpal bones, distal radius, and distal ulna often lag behind the metacarpals and phalanges. Nevertheless, the association between bone age and the onset of gonadarche is maintained in disorders of growth. In children with constitutional delay of growth and with true isolated growth hormone (GH) deficiency, gonadarche occurs at a late chronological age but at a normal skeletal age. On the other hand, when skeletal maturation is advanced, as may occur in association with CAH or familial testotoxicosis, secondary GnRH-dependent precocious puberty may develop. Although proteins synthesized in bone enter the vascular compartment, the ability of osteocalcin and other bone proteins to modulate the activity of the GnRH pulse generator has not been addressed.
GH secretion during childhood is relatively stable, but GH release is amplified up to threefold in boys and girls with the initiation of gonadarche and the rise in circulating levels of sex steroids. The pubertal increase in GH, in combination with the increased circulating concentrations of insulin-like growth factor-1 (IGF-I), estrogens, and androgens, contribute to the adolescent growth spurt. The increased secretion of GH at the time of gonadarche is not sustained; by late puberty, GH levels begin to decline. During puberty, height velocity, IGF-1 concentrations, and sex steroid concentrations rise with synchronization of these changes within individuals. Because the increases in GH and IGF-I appear to be in response to the initiation of gonadarche, and particularly to increased gonadal steroid secretion at this time, neither GH nor IGF-I represent compelling candidates for the signal responsible for the resurgence of GnRH release.
At the time of gonadarche, insulin resistance increases. This insulin resistance is greatest among children in Tanner stages 2 and 3 when compared with prepubertal children and adults. Manifestations of insulin resistance appear to be limited to effects on carbohydrate metabolism and are associated with compensatory hyperinsulinemia and normal disposition index. Disposition index, a function of insulin sensitivity and insulin secretion, reflects beta-cell response for a given insulin sensitivity. Using euglycemic-hyperinsulinemic clamp studies in conjunction with investigation of substrate utilization, one longitudinal study found that puberty was associated with decreased insulin sensitivity, increased insulin secretion, increased total body lipolysis, decreased glucose oxidation, and increased IGF-1 concentrations. In a longitudinal prospective cohort study involving healthy children, insulin sensitivity decreased before the onset of physical features of puberty. In one study, no changes in insulin’s ability to suppress hepatic glucose production were noted during puberty, indicating that decreased insulin sensitivity is limited to peripheral glucose uptake. Although cross-sectional studies suggest that the magnitude of insulin resistance is influenced by BMI (body weight/height [kg/m 2 ]), gender, and ethnic background, these associations have not been consistently noted in longitudinal studies. IGF-1 concentrations during puberty have been reported to mirror those in insulin sensitivity. Mean 24-hour serum GH and IGF-I concentrations positively correlate with the degree of insulin resistance during puberty, and the pubertal changes in insulin sensitivity may be partially mediated by increased GH and IGF-I concentrations. Yet, while it is generally recognized that increased gonadal steroid levels are responsible for activation of the GH/IGF1 axis at puberty (discussed earlier in the chapter), a causal relationship between testosterone or estradiol and insulin resistance has not been demonstrated.
Treatment of nonobese Catalunyan girls with premature adrenarche and advanced skeletal maturation with the insulin sensitzer, metformin, was associated with later onset of breast development and menarche. Moreover, in this relatively homogenous ethnic population, metformin treatment for 3 years slowed pubertal tempo among low birth weight girls who are at risk for early puberty and shorter adult stature. Taken together, the foregoing considerations raise the possibility that decreased insulin sensitivity may represent a component of the cue that times the onset of gonadarche.
Ghrelin is a small peptide, secreted predominantly by the stomach, which circulates in two forms. The active form is acetylated and promotes GH secretion. Ghrelin influences food intake, sleep, body weight, gastrointestinal mobility, and reproduction. Ghrelin suppresses LH pulsatility in the pituitary. Circulating ghrelin concentrations are higher during fasting, and decrease after food intake, indicating that ghrelin signals energy deficient states and modulates appetite and carbohydrate metabolism. Ghrelin concentrations peak during the first 2 years of life and decrease during puberty. Negative correlations were found between ghrelin concentrations and both age and pubertal stage. The ghrelin receptor, GH secretagogue receptor-1a (GHSR-1a), is expressed in human hypothalamus, pituitary, testis, and ovaries.
Ghrelin and leptin appear to act as reciprocal regulators of energy homeostasis exerting opposing influences on the HPG axis. Adipokines, hormones secreted by adipocytes, include resistin, adiponectin, leptin, and visfatin. Adiponectin concentrations are inversely related to insulin resistance and have been reported to decrease in pubertal males. Resistin is secreted by adipocytes and peripheral blood mononuclear cells and signals through the Toll-like receptor 4. Although details regarding its physiologic role are unclear, resistin appears to promote insulin resistance. The respective roles, if any, of resistin and visfatin on gonadarche or adrenarche remain to be determined.
Activation and Timing of Adrenarche
- ◆
The primary signal for adrenarche remains to be elucidated.
- ◆
Adrenarche appears to occur independently of developmental changes in the HPG axis (gonadarche).
- ◆
Adrenarche is characterized by increased DHEAS secretion by the ZR associated with increased expression of CYB5A.
Adrenarche is characterized by development of the ZR in the adrenal cortex and increased DHEA, DHEAS, and androstenedione secretion. DHEA and DHEAS are not bioactive androgens; DHEA is a precursor for more potent sex steroids. Prior to birth, the fetal zone of the adrenal cortex produces large amounts of DHEAS, which serves as the precursor for placental estrogen synthesis. Following involution of the fetal zone after birth, DHEAS concentrations remain low until 6 to 7 years of age at which time they begin to increase. Rising DHEAS secretion is the earliest hormonal manifestation of adrenarche. Despite no obvious changes in ACTH or cortisol secretion, the failure of children with ACTH receptor mutations to experience adrenarche implicates ACTH in this process.
The onset of adrenarche is associated with increased 17,20-lyase activity and decreased 3β-hydroxysteroid dehydrogenase activity. Available data suggest that, at the time of adrenarche, changes in expression of CYB5A, DHEA sulfotransferase (SULT2A1), and 3β-hydroxysteroid dehydrogenase (HSD3B2) play essential roles in DHEA and DHEAS production. Histologically, increased thickness of the ZR occurs concurrently with the increase in DHEAS concentration. Using more sensitive methodology, such as urinary GCMS, studies indicate that concentrations of DHEA and its metabolites show a continuous rise beginning at 3 to 4 years old. These data contradict conventional concepts and imply that adrenarche is a gradual process beginning earlier than previously considered. DHEAS concentrations continue to rise and peak between 20 and 25 years of age, followed by progressive decline. Although the findings have been inconsistent, clinical studies suggest that insulin, IGF-I, and GH concentrations influence the timing, onset, and progression of adrenarche. Comparison of IGF-I concentrations among prepubertal children have shown higher concentrations in African-American children. Whether these ethnic differences contribute to the earlier onset of adrenarche, or the increased incidence of premature pubarche in African-American girls, is unknown. Clinical observations regarding associations between adrenarche and body size and fatness have been inconsistent; one longitudinal study showed that DHEAS concentrations increased commensurate with the largest increase in BMI, whereas another longitudinal study found no association between DHEAS and weight, BMI, or body surface area.
Despite numerous hypotheses, the primary signal for adrenarche remains to be elucidated. Hypothalamic releasing factors, such as corticotropin-releasing hormone (CRH) and vasopressin (antidiuretic hormone [ADH]), play important roles in governing hypothalamic-pituitary-adrenal axis function. However, in contrast to the role of hypothalamic GnRH in gonadarche, CRH and ADH do not appear to trigger the onset of adrenarche. No specific adrenal androgen-stimulating factors have been isolated from the pituitary.
Moreover, adrenarche occurs independently of developmental changes in the HPG axis. For example, children with gonadal dysgenesis experience normal adrenarche and pubarche, whereas children with primary adrenal insufficiency may have normal gonadarche. Nevertheless, despite the long-established notion that gonadarche and adrenarche are independent events, recent data hint that adrenal androgens may influence pubertal timing. Specifically, using methodology with lower limits of detection, these data show that higher prepubertal urinary androgen excretion correlated with an earlier onset of breast development and penile growth, respectively, and with a shorter duration of pubertal growth spurt.
Genetics and Puberty Genes
- ◆
Temporal correlation between pubertal stages in families indicates genetic influences on the timing of puberty.
- ◆
GWAS studies identified several loci associated with age at menarche.
- ◆
Epigenetic factors, methylation status, noncoding regulatory sequences, enhances, insulators, and other factors modify expression of genes involved in the pubertal process.
The observations that (1) the temporal correlation in somatic maturation and attainment of pubertal stages in monozygotic twins is more robust than that in dizygotic twins ; (2) the age of menarche in mothers and daughters is correlated; (3) pubertal onset in children is influenced by pubertal timing of both parents ; (4) precocious gonadarche in girls may be familial and transmitted in an autosomal-dominant mode ; (5) paternally inherited precocious puberty is associated with MKRN3 mutations ; and (6) the age of menarche varies with racial group, underline the major influence of genetic factors for timing the onset of puberty. However, the nature of the genetic factors that directly dictate the timing of the pubertal resurgence of GnRH release remains to be elucidated. These genetic factors could include a network of clock genes in analogy to circadian time keeping and/or could affect the balance between stimulating and repressive factors that govern GnRH release.
Despite the accelerating knowledge regarding the genetic causes of disorders of pubertal development, the identity of the specific puberty genes that dictate the timing of the resumption of pulsatile GnRH release (and therefore determine the age of gonadarche) and those that regulate the developmental increase in adrenal androgen secretion that determine the age of pubarche have yet to be identified. The whole genome can be interrogated by linkage and GWAS without a priori hypotheses regarding specific candidate genes. This approach has enabled examination of millions of loci across the genome. However, these data explain <3% of the variance in age at menarche.
Four GWAS studies identified genomic loci associated with age at menarche. Among more than 100 loci identified in those GWAS, only several loci, LEPR , GNRH1 , TACR3 , and IGSF1, were near genes known to be associated with disorders of puberty ( Fig. 17.9 ). To date, the largest genetic effect on pubertal onset has been associated with FSHR and FSHB . However, none of the approximately 50 genes associated with central disorders of puberty timing genes appear to be responsible for triggering the pubertal resumption of pulsatile GnRH secretion.
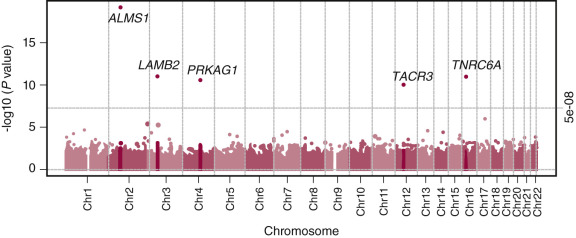
An increasing number of factors have been identified that modulate and regulate gene expression through epigenetic mechanisms. As described above, some growth regulatory genes show parent-of-origin effects due to genomic imprinting. Developmental disorders, such as Prader-Willi syndrome, in which the timing and tempo of puberty is altered (discussed later in the chapter), are associated with abnormal expression of imprinted genes. Maternal nutritional status can influence fetal gene expression through epigenetic mechanisms; animal studies suggest that this effect may be transmitted to subsequent generations. Environmental factors, such as nutrition, hormone and chemical exposures, and physical elements, can alter gene expression through these epigenetic modifications. These disorders may represent the “tip of the iceberg” for epigenetic influences on the genes that dictate or modulate the timing of puberty.
Genetic information and transcriptional regulation are influenced by DNA (CpG) methylation, chromatin packaging, small RNA or micro-RNA effects, noncoding regulatory sequences, long range transcriptional regulation (enhancers and insulators), and long noncoding RNA. Interactions between genes and proteins provide another mechanism to modify gene expression. In a longitudinal puberty study involving healthy children, changes in peripheral blood DNA methylation were detected. Several differentially methylated CpGs were found to be associated with changes in testosterone, FSH, AMH, LH, and inhibin B concentrations in boys. One region, situated between Solute Carrier Family 12 Member 9 (SLC12A9) and Thyroid Hormone Receptor Interactor 6, 7q22 (TRIP6), at chromosome 7q22, was coordinately regulated as a function of pubertal development. The function of the protein encoded by SLC12A9 is unclear. Curiously, circulating TRIP6 concentrations were noted to increase during puberty.
Although many genes have been associated with puberty, none have been confirmed to regulate reactivation of the GnRH pulse generator. Hence, the term “puberty” gene should be restricted to those genes that specifically regulate the timing of either adrenarche or gonadarche. For gonadarche, such genes would determine the age of the pubertal resurgence of pulsatile GnRH release by regulating the timing of the application or withdrawal of the neurobiological brake to the GnRH pulse generator of the juvenile hypothalamus. Puberty genes could time the resurgence of pulsatile GnRH release not only by triggering a hypothalamic signal at puberty but also potentially by determining the duration of the prepubertal brake on pulsatile GnRH release or by timing the “turn off” of the GnRH pulse generator during infancy. In the case of adrenarche, the developmental increase in adrenal androgen secretion would presumably be timed by genes that are responsible for the changes in adrenal steroidogenesis manifested by increased ZR activity.
Factors Modulating the Timing of Puberty
- ◆
Adequate energy stores are essential for puberty. The association between extreme energy expenditure, undernutrition, and delayed gonadarche, especially in the female, is well established.
- ◆
Endocrine disrupting chemicals (EDCs), for example, polychlorinated biphenyl (PCB), polybrominated biphenyl (PBB), bisphenol A (BPA), and similar chemicals have been suspected to be modulators of pubertal timing. EDCs have recently gained considerable attention. However, additional data are needed to establish a direct causative link.
In general, the majority of studies that have examined the influence of various parameters on the onset and tempo of puberty have focused on the process of gonadarche. Considerably less attention has been paid to the timing and progression of adrenarche. Additionally, many factors that influence the onset and tempo of puberty (e.g., nutritional status) also modulate hypothalamic GnRH pulse generator activity in the majority of adults. Therefore, a modulator of puberty is not necessarily a component of the mechanism that initiates this developmental event. Rather, it is more likely that such factors play permissive, albeit in some cases obligatory, roles to allow the process of puberty to unfold once the signal responsible for the resurgence in pulsatile GnRH secretion has been activated.
Nutrition and Diet
Adequate energy stores are essential for the accelerated linear growth and achievement of reproductive competence at puberty. Neuropeptides and hormones provide information regarding energy status. Nutritional state modulates the onset of gonadarche in girls, as reflected by the findings that menarche is delayed in malnourished girls, and menarche tends to occur at a particular or “critical” body weight rather than at a set age. Obesity is associated with early breast development and menarche. Rapid weight gain during the first year of life is associated with obesity and early menarche in a prospective UK cohort study. In addition, the timing of adrenarche may also be influenced by nutritional status. In the female rhesus monkey, high calorie diets were associated with a precocious increase in nipple volume, sex-skin swelling, and menarche in association with increased concentrations of leptin and IGF-1 concentrations, and an increased BMI. While the impact of undernutrition on gonadarche in boys has received less attention, there is no reason to suspect that the male axis is unaffected in this regard.
Leptin and osteocalcin appear to communicate energy status to several tissues including adipose tissue, bone, CNS, and gonads. In its undercarboxylated (active) form, osteocalcin promotes insulin secretion, improves insulin sensitivity, induces β-cell proliferation, increases adiponectin secretion, and decreases lipolysis. In osteoblasts, insulin signaling increases bone formation, bone resorption, and secretion of active osteocalcin production.
Differences in the timing and tempo of gonadarche unrelated to racial or ethnic background have been reported even among well-nourished girls. Researchers have suggested that diet may account for such variations. Attention has been drawn to subtle relationships between diets high in animal protein and early menarche, and between diets high in vegetable grains and delayed onset or tempo of gonadarche. Moderate to vigorous exercise in the absence of weight restriction appears to have a negligible impact on the timing and tempo of puberty in either sex. However, breast development, menarche, and skeletal maturation can be delayed in girls involved in strenuous physical training. The association between extreme energy expenditure and delayed gonadarche in the female is particularly marked in ballerinas, long-distance runners, and figure skaters, who must maintain their body weight within strict limits. Such girls may experience amenorrhea secondary to HH. The factors responsible for compromising pulsatile GnRH release in pubertal children who exercise vigorously are presumed to be similar to those resulting from undernutrition. The life of the young female dancer or athlete is stressful; therefore stress may also represent a contributing factor underlying exercise-induced delayed gonadarche. Because certain physical and psychological characteristics are necessary for outstanding athletic performances, there may be a significant contribution of self-selection involved in the decision of girls to participate at such an exceptional level. This can be complicated by eating disorders, which are more common in elite artistic athletes such as gymnasts. In a study of young female gymnasts and their parents, menarche in the mothers was found to be delayed relative to that of mothers of sedentary girls. Therefore, the possibility of a genetic contribution to this phenomenon cannot be totally excluded. The effect of physical training on the timing of pubarche in girls has been less studied, and conclusive data are lacking.
Although the relationship between strenuous physical training and male puberty has received less attention, this developmental process is apparently less susceptible in boys than it is in girls. This may be related, in part, to sex differences in the age at which training is initiated, and to the intensity of the exercise. Nevertheless, in sports like wrestling that require weight control achieved with a combination of strenuous exercise and dietary restriction, impaired testosterone secretion may occur.
Environmental Disruptors
Increased attention has been focused on EDCs as modulators of pubertal timing. Most EDCs have estrogenic or anti-androgenic activities. Many occur as mixtures rather than single agents and may have multiple actions. Sexually dimorphic actions can occur. Dioxins, PCB, PBB, BPA, plasticizers (phthalates), pesticides, fungicides (vinclozolin), alcohol, tobacco, and pharmaceutical agents (diethylstilbestrol) are considered to be EDCs. Phytoestrogens, which can function as selective estrogen receptor modulators (SERMS), are found in soybeans, flaxseed, peanuts, and some vegetables. Most are diphenolic compounds with structural features common to estrogenic steroid agonists and antagonists. EDCs can affect peripheral reproductive systems, such as inducing breast development. These chemicals may also exert neuroendocrine effects to influence hypothalamic and pituitary function. EDCs can act through hormone receptors, can affect enzymatic processes, and may disrupt the complex interactions of endogenous hormones at multiple levels. Available data suggest that EDCs are associated with both early puberty and delayed completion of puberty. The specific consequences of EDCs may depend on age and duration of exposure. Further investigations into EDCs, defining mechanisms of action and consequences, are necessary.
Prenatal Influences
The roles of prenatal influences and environment on postnatal development including puberty are increasingly recognized. Children with intrauterine growth retardation (IUGR) or born small for gestational age (SGA) have increased risks to develop insulin resistance, hypertension, diabetes mellitus, metabolic syndrome, and coronary artery disease in adulthood. Increased weight gain during the first few years of life exaggerates these risks. Increased adrenal androgen levels and in some cases precocious or exaggerated adrenarche has been reported in both boys and girls born SGA. Some, but not all, girls with premature adrenarche have an increased risk to develop polycystic ovary syndrome (PCOS) during adolescence. Reports are inconsistent regarding the timing of menarche in girls born SGA compared to girls born at an appropriate size for gestational age. The timing of puberty in boys born SGA appears to be normal, although subfertility has been reported after they reached adulthood. Perinatal HIV infection is associated with later onset of puberty. Potential mechanisms responsible for the consequences of abnormal fetal growth are multifactoral and not mutually exclusive. Suggested causes include genetic variations, differential methylation, alterations in microRNA, altered histone acetylation, and other long-range regulatory effectors.
The neuroendocrine consequences of prenatal androgen exposure have been best characterized in sheep. Findings include decreased sensitivity to estradiol and progesterone feedback, decreased content of dynorphin and NKB in KNDy neurons, and altered morphology of KNDy neurons. Whether these data are applicable to humans and whether prenatal androgen exposure alters GnRH pulse generator function is unclear.
Adoption or Migration from Developing to Developed Countries
Several European countries have reported precocious gonadarche in a relatively dramatic proportion of children—particularly girls—adopted from developing world regions like Asia and South America. This increased endocrine activity in the hypothalamic-pituitary-ovarian axis prior to the onset of physical manifestations of puberty indicates a central origin to the condition. The cause of the precocity appears to be complex, but improved nutritional and social conditions may contribute to this phenomenon. Precocious gonadarche is also seen in immigrant girls arriving with their parents and lacking evidence of earlier compromised growth and nutrition. Moreover, ethnic background does not appear to influence this trend. A study of immigrant girls in Belgium with premature gonadarche reported an association with previous exposure to organochlorine pesticides. A study in Spain found an increased relative risk for precocious gonadarche in adopted girls (both national and international), but not in immigrant girls. However, this outcome is inconsistent because adopted Chinese girls experienced menarche at ages comparable to nonadopted Chinese girls.
Disorders of Puberty
- ◆
Approximately 90% of cases of CPP in girls are idiopathic in girls versus 50% to 70% of cases in boys.
- ◆
Hypothalamic hamartomas are a common etiology of CPP.
- ◆
Paternally inherited heterozygous makorin 3 (MKRN3) mutations are associated with CPP in boys and girls. Circulating makorin-3 concentrations decline immediately prior to puberty among healthy girls and boys.
- ◆
The nonclassic or milder forms of the virilizing CAHs primarily present with signs of excessive androgen secretion rather than the symptoms of glucocorticoid and mineralocorticoid deficiencies typical of the classical forms.
- ◆
The protein encoded by the POR gene functions as an electron donor to cytochrome P450 enzymes. Therefore, POR loss-of-function mutations present with a clinical picture showing various combinations of deficiencies of 21-hydroxylase, 17α-hydroxylase, and aromatase enzymes.
- ◆
Constitutional delay in growth and puberty (CDGP) may be a normal variation of pubertal timing.
- ◆
Delayed puberty can be due to gonadal failure (hypergonadotropic), neuroendocrine dysfunction (hypogonadotropic), or other endocrine disorders.
- ◆
In some instances, the underlying pathophysiology may be similar for both constitutional delay and idiopathic HH.
- ◆
Oligogenic etiology for HH (i.e., more than one HH-associated gene mutation is present in a patient) accounts for 10% to 20% of all cases.
- ◆
Mutations in genes influencing GnRH neuron development and migration may be associated with HH.
- ◆
Approximately 10% to 15% of individuals with HH who carry loss-of-function mutations experience a clinical recovery following the initiation of sex steroid replacement therapy.
- ◆
Primary gonadal failure, disorders of steroidogenesis, or defects in steroid hormone action result in loss of negative feedback inhibition by gonadal steroids at both the hypothalamic and pituitary level leading to hypergonadotropic hypogonadism.
- ◆
Turner syndrome (TS) is due to deletions or structural rearrangements of the X chromosome and the most common form of gonadal dysgenesis seen in 1 : 2000 to 1 : 5000 liveborn females.
- ◆
In 46,XY gonadal dysgenesis, the degree of testosterone deficiency varies; therefore, the external genital development can range from normal female external genitalia to undervirilization of male external genitalia.
- ◆
KS (47,XXY karyotype) is associated with failure in both testosterone production and spermatogenesis and may manifest before or after puberty.
- ◆
Hereditary disorders in adrenal and/or gonadal sex steroid production may lead to ambiguous genitalia or delayed puberty.
- ◆
Complete androgen insensitivity syndrome (CAIS) is usually associated with mutations in the AR (NR3C4) gene.
- ◆
To identify the cause of delayed puberty may require reevaluation over time.
- ◆
The goal of gender-specific sex hormone replacement therapy is to mimic the normal pattern of gonadal secretion to induce secondary sexual characteristics.
- ◆
Fertility issues should also be frankly discussed.
- ◆
Fertility preservation should be considered if appropriate.
The terminology used to describe disorders of puberty has evolved as the pathophysiology and molecular etiologies of these disorders have been clarified ( Box 17.1 ; Table 17.2 ). The term “CPP” or GnRH-dependent precocious puberty refers to premature resurgence of GnRH pulse generator activity, which we have labeled as GnRH-dependent precocious gonadarche (CPP). The terms “partial,” “incomplete,” “peripheral,” “pseudo,” and “GnRH-independent precocious puberty” have been used to describe other etiologies of premature sexual development. We refer to these disorders as GnRH-independent precocious puberty. Isosexual refers to the development of sexual characteristics typical for the patient’s gender. Heterosexual refers to the development of sexual characteristics typical of the other gender (e.g., feminizing tumors in males). Delayed puberty has been categorized as being either hypogonadotropic (low gonadotropin concentrations) or hypergonadotropic (elevated gonadotropin concentrations). With expanding knowledge of the functional genomics of the pubertal process and the molecular genetics underlying its pathophysiology, the classification of the etiologies of disorders of puberty will continue to evolve.
GnRH-Dependent Gonadarche (CPP)
Idiopathic: Progressive, nonprogressive
Congenital CNS lesions:
- •
Hypothalamic hamartoma
- •
Septooptic dysplasia
- •
Arachnoid cysts
- •
Suprasellar cysts
- •
Genetic:
- •
KISS1R activating mutation
- •
KISS1 activating mutation
- •
MKRN3 mutation
- •
Chromosome 14q32 variants
- •
Acquired CNS disorders:
- •
Postinflammatory
- •
Postradiation therapy
- •
Postinfectious
- •
Hydrocephalus
- •
Posttrauma
- •
Tumors (astrocytoma, pineal tumor, optic glioma, craniopharyngioma)
- •
Neurofibromatosis type 1 (optic glioma)
- •
Tuberous sclerosis
- •
Sturge-Weber syndrome
- •
Histiocytosis X
- •
Chronic exposure to androgens:
- •
Congenital adrenal hyperplasia
- •
Familial male-limited precocious puberty
- •
GnRH-Independent Gonadarche
McCune-Albright syndrome
Feminizing disorders:
- •
Estrogen-secreting tumors
- •
Ovarian: granulosa cell, Peutz-Jeghers syndrome, gonadoblastoma/dysgerminoma, carcinoma, cystadenoma, theca cell, lipoid
- •
Adrenal
- •
- •
Feminizing disorders:
- •
Estrogen secretion unrelated to tumors: aromatase mutation
- •
Exposure to exogenous sex steroids or endocrine disruptors
- •
Isolated premature menarche:
- •
Estrogen-secreting cyst
- •
Tumor
- •
McCune-Albright syndrome
- •
Primary hypothyroidism
Premature thelarche:
- •
Variant of normal development
- •
Rubinstein-Taybi syndrome
- •
Kabuki make-up syndrome
Primary hypothyroidism
Virilizing disorders
- •
Premature adrenarche
- •
Congenital adrenal hyperplasias
- •
21-hydroxylase deficiency
- •
3β-hydroxysteroid dehydrogenase deficiency
- •
11β-hydroxylase deficiency
- •
- •
Other disorders affecting steroidogenesis
- •
Oxidoreductase deficiency
- •
Apparent cortisone reductase deficiency
- •
Apparent DHEA sulfotransferase deficiency
- •
Inherited glucocorticoid resistance
- •
- •
Familial male-limited precocious puberty (testotoxicosis)
- •
Androgen-secreting tumors
- •
Adrenal sex steroid-secreting tumors such as adenoma and carcinoma
- •
Ovarian tumors such as arrhenoblastoma
- •
Testicular Leydig cell tumor
- •
hCG-secreting tumors (e.g., hepatoblastoma/hepatoma)
- •
Dysgerminoma
- •
Teratoma
- •
Choriocarcinoma
- •
- •
Cushing syndrome
- •
Cushing disease associated with increased ACTH secretion
- •
Adrenal tumors/disease
- •
Ectopic secretion of CRH or ACTH
- •
Prolonged excessive exogenous steroid use
- •
ACTH , Adrenocorticotropin; CNS, central nervous system; CRH , corticotropin-releasing hormone; DHEA , dehydroepiandrosterone; GnRH, gonadotropin-releasing hormone; hCG , human chorionic gonadotropin.
| Gene | Locus | Phenotype |
|---|---|---|
| CYP21A2 | 6p21 | CAH due to 21-hydroxylase deficiency |
| CYP11B1 | 8q21 | CAH due to 11β-hydroxylase deficiency |
| CYP19A1 | 15q21.1 | Precocious puberty/gynecomastia |
| DLK1 | 14q32.2 | Paternally imprinted precocious puberty |
| GNAS1 | 20q13.2 | McCune-Albright syndrome |
| GRL | 5q31 | Inherited glucocorticoid resistance |
| HSD3B2 | 1p13.1 | CAH due to 3β-hydroxysteroid dehydrogenase deficiency |
| KISS1 | 1q32.1 | Precocious puberty |
| KISS1R | 19p13.3 | Precocious puberty associated with activating mutations |
| LHR | 2p21 | Familial male-limited precocious puberty |
| MKRN3 | 15q11.2 | Paternally imprinted precocious puberty |
| STK11/LKB1 | 19p13.3 | Peutz-Jeghers syndrome |
Disorders of Early Puberty
Gonadotropin-Releasing Hormone-Dependent Precocious Pubertal Development
Progressive Precocious Gonadarche or Central Precocious Puberty
Although this disorder is labeled GnRH-dependent precocious puberty or puberty (central precocious puberty [CPP]), it represents precocious gonadarche due to either premature resurgence or incomplete suppression of the hypothalamic GnRH pulse generator. It occurs more often in girls than in boys. This sex difference is probably related to the lesser prepubertal suppression of the GnRH pulse generator in girls than boys. The sequence of pubertal development is typical of a normal puberty including adrenarche in some cases, but it begins at an earlier-than-normal age. Observational data collected from 2008 to 2010 through a stringent Spanish hospital-based registry reported an annual incidence ranging from 0.02 to 1.07 cases per million.
Approximately 90% of cases of CPP in girls are considered to be idiopathic whereas 50% to 70% of cases in boys are associated with identifiable etiology. The discovery of genes involved in GnRH and gonadotropin secretion has allowed for identification of specific gene mutations in patients with CPP. A heterozygous missense mutation, p.Arg386Pro, in the KISS1R gene was associated with precocious puberty in a girl. This mutation located in the C-terminal tail of the receptor was associated with delayed degradation of the receptor yielding a prolonged duration of action. In addition to this activating mutation in the receptor, a mutation in the KISS1 gene was identified in a young boy who presented at 17 months of age with CPP. This variant, p. Pro74Ser, appears to prolong the half-life due to resistance to degradation. To date, KISS1 and KISS1R mutations are extremely rare in children with CPP.
As described above, paternally inherited MKRN3 mutations are associated with CPP in boys and girls. This gene is mapped to chromosome 15q11.2 in the Prader-Willi Syndrome critical region. The maternal allele is methylated and silenced resulting in expression of the paternal allele. The gene encodes a protein involved in ubiquitination and cell signaling. Several unique mutations have been reported among different ethnic groups. Mutations in this gene appear to be the most common familial etiology of CPP. The median age of pubertal onset is 6.0 years (3.0 to 7.5) in girls and 8.25 years (5.9 to 9.0) in boys. No abnormalities of the CNS have been reported. Curiously, despite the physical proximity of MKRN3 to the Prader-Willi syndrome locus, no major signs of Prader-Willi syndrome have been described. A GWAS study looking at the parent of origin effects on puberty identified a signal in the region of MKRN3 gene; the paternal allele of a specific SNP (rs12148769, G>A) affects age at menarche in healthy girls suggesting that variants in this region affect pubertal timing within the normal range. Circulating makorin-3 concentrations were found to decline prior to puberty among healthy girls and boys ( Figs. 17.10 and 17.11 ).
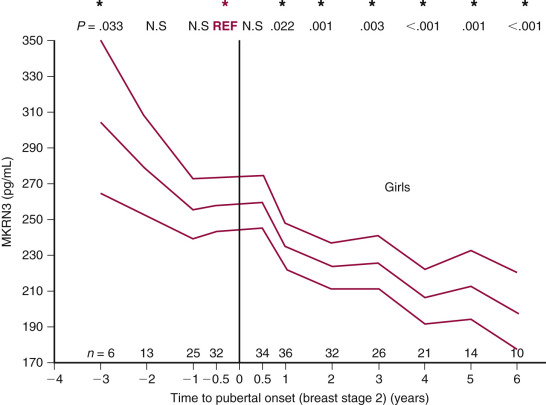
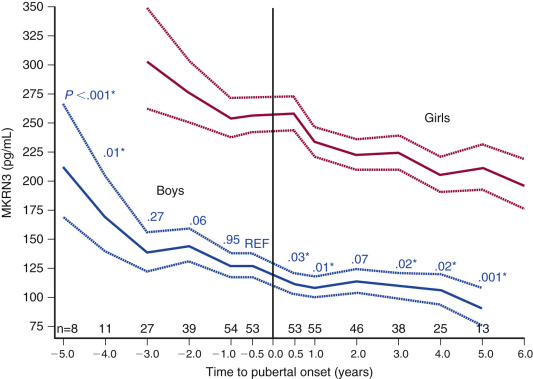
CPP has been described in the Williams-Beuren syndrome, histiocytosis X, and maternal uniparental disomy for chromosome 14. Evaluation of a family with CPP led to identification of a deletion/duplication mutation in the delta-like 1 homologue (DLK1) gene located at chromosome 14q32. The four affected individuals presented with thelarche between the ages of 6.4 to 8.0 years of age. An additional investigation revealed undetectable serum DLK1 concentrations (<0.4 ng/mL) compared to control group (1.9 to 20 ng/mL). DLK1 is a paternally expressed gene located within the genetic locus associated with Temple and Kagami-Ogata syndromes. Temple syndrome is characterized by IUGR, hypotonia in infancy, CPP, and short stature. Genetic findings in Temple syndrome include maternal uniparental disomy, paternal deletion, or loss of differential methylation at the DLK1/MEG3 region on chromosome 14. Patients with Kagami-Ogata syndrome is associated with prenatal overgrowth, developmental delay, abdominal wall defects, but disorders of pubertal timing do not appear to be a prominent feature. Based on available animal and human data, the opposing directions of regulation of MKRN3 and DLK1 expression prior to puberty may underlie interactions between these factors that modulate the timing of puberty.
Hypothalamic hamartomas, congenital malformations composed of a heterotropic gray matter, neurons, and glial cells usually located on the floor of the third ventricle or attached to the tuber cinereum, are a common etiology of CPP. Two potential mechanisms have been hypothesized for the association between CPP and hypothalamic hamartomas; one is increased GnRH secretion from tissue emancipated from suppression by the prepubertal brake and the other is that factors such as TGF-α provide an ectopic drive to GnRH neurons with a normal distribution in the hypothalamus. The hamartomas can be classified as parahypothalamic , attached or suspended from the floor of the third ventricle, or as intrahypothalamic , in which the mass is enveloped by the hypothalamus and distorts the third ventricle. The lesions do not grow over time, do not metastasize, and do not produce β-human chorionic gonadotropin(β-hCG) and α-fetoprotein. Extreme precocity suggests a hamartoma. Although gelastic or laughing seizures can be associated with precocious puberty due to hypothalamic hamartomas, the majority of patients with hypothalamic hamartomas do not exhibit neurological symptoms. Among patients in whom hamartomas were removed to treat intractable seizures, hamartomas associated with CPP were more likely to contact the infundibulum or tuber cinereum and were larger than hamartomas not associated with CPP. All hamartomas expressed GnRH, TGF-α, and GnRHR. No differences were detected in the expression of KISS1 and GPR54 between hamartomas associated with CPP and those not associated with CPP. Gene expression profiling of hypothalamic hamartomas associated with precocity may provide clues regarding genes, proteins, and regulatory pathways associated with the timing of puberty.
Hypothalamic hamartomas are generally sporadic, but may occur as a feature of several dysmorphic syndrome including Pallister-Hall syndrome and oral-facial-digital syndrome (OFD) types I and VI. Pallister-Hall syndrome is an autosomal-dominant disorder associated with mutations in the GLI3 gene located at chromosome 7p13. Additional features include pituitary anomalies, hypopituitarism, imperforate anus, and polydactyly. In the presence of sonic hedgehog (SHH), the full-length GLI3 translocates to the nucleus where it functions as a transcriptional activator. When SHH is absent, GLI3 is phosphorylated and cleaved; the cleaved protein translocates to the nucleus to repress specific target genes. The truncated GLI3 protein showed stronger repressor activity than the wild type protein, suggesting that these mutations are associated with constitutive repression of SHH signaling. Mutations in additional genes in the SHH pathway, especially PRKACA, have been detected in HH associated with epilepy.
In addition to hypothalamic hamartomas, optic gliomas, suprasellar cysts, arachnoid cysts, previous head trauma, static cerebral encephalopathy, CNS infections, CNS radiation, hydrocephalus, meningomyelocele, and neurodevelopmental disabilities may also be associated with CPP. For hypothalamic-pituitary disorders, the endocrine symptoms of precocious puberty may precede neurological symptoms. Three predictors associated with CNS lesions in girls are: (1) age younger than 6 years; (2) absence of pubic hair; and (3) estradiol concentrations greater than 110 pmol/L. The type of CNS lesion influences the presentation of GnRH-dependent precocious puberty presumably due to differences in the mechanisms inducing puberty and to the hypothalamic-pituitary deficiencies associated with the initial lesion or its treatment.
Optic gliomas are associated with neurofibromatosis type 1 (NF1), an autosomal-dominant disorder diagnosed based on clinical features that include size and number of café-au-lait spots, macrocephaly, and family history of the disorder. The mechanism of action has been presumed to be a mass effect. Pineal tumors can be associated with precocious puberty due to tumor-induced hydrocephalus or germ cell tumors. CNS radiation, dose range 18 to 50 Gray, used to treat intracranial tumors or used prophylactically for malignancies, can induce precocious gonadarche, perhaps as a result of an astroglial response with increased TGFα production. In the situation of CPP, simultaneous GH deficiency (GHD) may be masked by the accelerated growth velocity associated with increased gonadal sex steroid secretion.
Other situations associated with progressive CPP include children with virilizing disorders (such as CAH) and familial male-limited precocious puberty (testotoxicosis) in whom skeletal maturation is usually markedly advanced. In these situations, the precocious gonadarche is considered to be secondary to the virilizing disorder, but the mechanism through which the GnRH pulse generator is prematurely activated is unclear.
Treatment of Central Precocious Puberty
The treatment of choice for children with progressive precocious gonadarche is a GnRH receptor agonist (GnRH-Ra). In situations of coexisting GHD, combined treatment with recombinant human GH may be helpful to preserve height potential. GnRH-Ras are modifications of the native GnRH decapeptide, have greater resistance to degradation, and possess increased affinity for the GnRH-R. Therefore, they are perceived by the pituitary as a continuous GnRH stimulation, which induces down regulation of GnRH-R function and leads to decreased gonadotropin secretion. GnRH-Ras are available as daily injections or depot forms that are currently administered every 28 days. The depot formulation of leuprolide acetate is commonly used in the United States; the recommended dose is 0.3 mg/kg administered every 28 days. Another GnRH-Ra, histrelin, has been formulated as a hydrogel subdermal implant that is surgically inserted into the inner aspect of the upper arm. Over the course of 1 year, histrelin diffuses from the 50 mg implant. Studies indicate excellent gonadotropin suppression. Additional depot preparations available in the United States, Canada, the UK, and Europe include triptorelin, goserelin, and buserelin.
When the progressive nature of the disorder is equivocal, serial evaluations are necessary to detect a sustained acceleration in tempo of pubertal development, including skeletal maturation (and therefore loss of height potential), before initiating therapy. This strategy is necessary because children with nonprogressive precocious puberty do not benefit from treatment. The major goals of treatment are to prevent further pubertal progression until appropriate for chronological age and to achieve improved adult height.
Clinically, the cessation of pubertal progression is apparent within 3 months of initiating treatment. The signs related to adrenarche neither regress nor are they prevented; they may even progress. In girls, breast size typically decreases but may not completely regress. Vaginal bleeding secondary to estrogen withdrawal, and acne may occur during the first month of treatment. Subsequently, there should be no further vaginal bleeding even if menarche occurred prior to initiation of therapy. The ovaries and uterus decrease in size. In boys, testicular volume decreases. Linear growth velocity and the rate of bone mineral accretion decrease. Longitudinal evaluation of a cohort of children with precocious gonadarche showed increased lumbar bone mineral density and BMI SD scores at the initiation of therapy. After cessation of therapy in children treated with GnRH-Ra, bone mass, bone turnover, and percent body fat were normal, suggesting that peak bone mass would be appropriate.
Adequacy of treatment is judged by prepubertal estradiol/testosterone concentrations and prepubertal gonadotropin response to GnRH stimulation. Monitoring to confirm efficacy of therapy includes interim history, physical examination to ascertain height, weight, and stage of pubertal development, and bone age x-rays. In addition, GnRH stimulation tests should be repeated at regular intervals (3 to 12 months) to document gonadotropin suppression. This monitoring is necessary because bone maturation may progress despite regression of the clinical features associated with gonadarche. Urinary gonadotropin determinations do not provide adequate sensitivity to judge the efficacy of therapy.
The duration of GnRH-Ra therapy should be individualized with the decision to discontinue therapy based on chronological age, skeletal maturation, projected adult height, and psychosocial readiness for the resumption of puberty. For girls, menstruation usually occurs 9 to 15 months after discontinuation of GnRH-Ra therapy, with earlier onset in those who had experienced menarche prior to treatment. Studies indicate that final height is improved over initial predicted height at diagnosis but is still less than that based on mid-parental height. Rapidly progressive pubertal development, advanced skeletal maturation, predicted compromise of adult height, and psychosocial considerations justify treatment.
Among the adverse effects of treatment, parents may note increased emotional lability and moodiness just prior to the GnRH-Ra injection. Local or systemic allergic reactions or sterile abscesses may occur, but are uncommon. Some children show increased weight gain disproportionate to their linear growth. Intermittent therapy, often due to poor compliance, may have the deleterious effect of increasing gonadotropin and gonadal steroid secretion, leading to progressive skeletal maturation and further compromise of adult height. Since affected children appear older than their chronological age, parents and teachers may have inappropriate expectations regarding psychosocial development and abilities. In a small study, girls treated with GnRHa (median age 10.4 years) showed comparable cognitive performance, behavior, and psychosocial characteristics compared to age-matched control girls with the exception of higher emotional reactivity on one of the two emotional reactivity tasks. However, cognitive and emotional development are normal for chronological age. Thus the guilelessness and naiveté of such children exposes them to an increased risk of sexual abuse, with affected girls at risk of becoming pregnant.
Long-term overall positive experience has now accrued for GnRH-Ras. Pregnancies with normal offspring have been observed. Although no major adverse effects on reproductive function have been noted, some girls appear to have an increased risk to develop PCOS. However, similar findings in a cohort containing both treated and untreated girls suggest the possibility of an underlying predisposition to PCOS preceded CPP.
Nonprogressive Precocious Gonadarche
Some children experience a nonprogressive (or slowly progressing) form of precocious gonadarche attributed to a premature but intermittent or transient activation of the hypothalamic GnRH pulse generator. Among this latter group of children, basal gonadotropin concentrations and gonadotropin responses to GnRH stimulation may be normal for chronological age but can overlap values observed among children with progressive precocious gonadarche. Because the physical signs of pubertal development do not always correlate with GnRH-stimulated gonadotropin responses, physical findings alone cannot differentiate between progressive and nonprogressive precocious gonadarche. In general, children with this nonprogressive form of precocious gonadarche show no evidence of pubertal responsiveness to GnRH stimulation, no loss of height potential, and do not usually benefit from GnRH-Ra therapy.
Gonadotropin-Releasing Hormone-Independent Precocious Pubertal Development
Precocious pubertal development may occur independently of pulsatile GnRH secretion. In these situations of peripheral precocious puberty, inappropriate gonadal or adrenal steroid secretion or exposure to exogenous steroids induces the physical signs of puberty. In most instances, the pubertal development is incomplete and fertility is not attained.
McCune-Albright Syndrome
The classical clinical triad of McCune-Albright syndrome (MAS) is precocious pubertal development, café-au-lait spots, and bony fibrous dysplasia. The café-au-lait lesions are generally large, do not cross the midline, and have irregular “coast of Maine” margins, but exceptions to the classic features can occur. Precocious pubertal development is not observed in all cases and appears to be more common among girls than boys. This disorder is due to constitutive activation of the G s α protein that is coupled to membrane-bound glycoprotein hormone receptors and is associated with autonomous function of endocrine glands. The syndrome is due to postzygotic somatic cell mutations in the GNAS1 gene; missense mutations Arg201His and Arg201Cys are among the most common. Partial or atypical forms are increasingly recognized; among 113 children with 1 to 3 features typical of MAS, a PCR-based mutation analysis protocol identified a missense mutation at codon 201 in 90% when an affected tissue (e.g., ovarian, bone, or adrenal tissue) was analyzed. These gain-of-function mutations in the G s α gene lead to constitutive activation of gonadotropin receptors, and subsequent increased autonomous ovarian estrogen and testicular testosterone secretion in affected girls and boys, respectively.
Vaginal bleeding, with or without breast development, is the typical presentation in prepubertal girls. The vaginal bleeding represents estrogen withdrawal following the resolution of an estrogen-secreting cyst. Estrogen concentrations may be elevated and pelvic ultrasound may confirm a unilateral ovarian cyst. The frequency of recurrent episodes of ovarian cysts and vaginal bleeding is variable. Because the precocious pubertal development is GnRH-independent, GnRH-Ra treatments are ineffective. Treatment with aromatase inhibitors, estrogen receptor modulators, and medroxyprogesterone acetate have been tried. Aromatase inhibitors have been tried; anastrozole had limited efficacy whereas letrozole showed sustained beneficial effects on skeletal maturation, growth velocity, and predicted adult height. A partial estrogen antagonist, tamoxifen, has been helpful. Preliminary results using fulvestrant, a pure estrogen blocker, have been promising to decrease vaginal bleeding and slow bone maturation. One outcome study showed variable gonadal function in affected adult women, with some having regular menses and fertility while others had persistent autonomous gonadal function associated with irregular menses and infertility.
Among boys with MAS, precocious puberty is less common. In a cohort of 54 males, 11 (21%) presented with precocious puberty. Gonadal involvement occurred in approximately 80%. Testicular embryonal carcinoma is rare. Macroorchidism may be noted on examination. Ultrasound features can include macroorchidism, hyperechoic lesions, hypoechoic lesions, and testicular microlithiasis. Leydig cell hyperplasia can occur in postpubertal men, but is rare in boys. AR blockers, aromatase inhibitors, and blockers of sex steroid synthesis have been used to treat the precocious puberty. A conservative approach to MAS-associated testicular lesions has been endorsed with emphasis on serial imaging and testicular preservation. Combined treatment with bicalutamide and anastrozole in one boy was well tolerated and associated with normalization of growth velocity and prevention of pubertal progression.
The typical bone lesion is polyostotic fibrous dysplasia. Lesions tend to be asymmetric and can affect any bone, including the skull. Skeletal disease is generally apparent by 10 years of age. The cystic bone lesions can lead to pathological fractures and deformities. Pseudoarthrosis can occur. With involvement of the skull, hyperproliferation of the preosteoblastic cells results in impingement into cranial foramina, leading to compression of cranial nerves. Blindness, deafness, facial asymmetry, or ptosis can result. Rather than being associated with a single abnormality of bone, the specific histopathology and clinical consequences vary depending on anatomical location: axial or appendicular skeleton, cranial bones, or gnathic bones. Total body bone scintigraphy can be performed to identify and determine the extent of bone lesions before they are visible on x-rays. Hypermetabolic bone disease is indicated by increased serum osteocalcin and alkaline phosphatase concentrations, as well as increased urinary hydroxyproline concentrations. Studies suggest that bisphosphonate therapy may benefit the bone disease with reduced fracture rate, decreased bone pain, and radiological evidence of long bone healing.
Hypophosphatemic rickets can occur secondary to increased production of the phosphaturic hormone, FGF23, by the bone lesions. There is a positive relationship between disease activity and FGF23 concentrations. Renal tubulopathy with some phosphate wasting may be evident. However, significant hypophosphatemia is uncommon. Treatment of the hypophosphatemia includes phosphate and vitamin D. Other endocrine manifestations include nodular thyroid hyperplasia with hyperthyroidism, multiple pituitary adenomas associated with gigantism, acromegaly or hyperprolactinemia, and parathyroid adenoma or hyperplasia with hyperparathyroidism. Cushing syndrome is extremely rare and almost always occurs during infancy.
Feminizing Disorders
Estrogen-Secreting Tumors
Estrogen-secreting tumors are a rare cause of premature or abnormal pubertal development. Types of tumors include granulosa cell, gonadal stromal cell, ovarian sex cord stromal, and theca cell tumors. The majority of juvenile granulosa cell tumors are large, can be palpated on bimanual examination, and are limited to the ovary at the time of diagnosis. Rapid pubertal progression is common. Estrogen concentrations may be very elevated, gonadotropin concentrations are suppressed, and circulating tumor markers such as α-fetoprotein, inhibin, CA-125, or hCG may be detected. Somatic mutations involving AKT1 have been identified as a major event in the pathogenesis of juvenile granulosa cell tumors; 60% (i.e., 10/16) of the ovarian samples carried in-frame duplications that activated AKT1. The primary treatment is surgical excision with staging that includes peritoneal cytology.
Rarely, gonadoblastomas in streak gonads, lipoid tumors, cystadenomas, and ovarian carcinomas can secrete estrogens, androgens, or both. Elevated serum inhibin and AMH concentrations, and the finding of AMH immunoreactivity in the tumor, indicate that the tumor cells are of granulosa or Sertoli cell origin. Expression of FOXL2 , SOX9 , and other specific genes can be helpful to discriminate between gonadoblastomas and intratubular germ cell neoplasias; FOXL2 expression suggests that a gonadoblastoma has a granulosa cell component. If originally positive, these markers may be useful to recognize recurrence.
Sex cord tumors with annular tubules are common in patients with Peutz-Jeghers syndrome (PJS), an autosomal-dominant disorder characterized by mucocutaneous pigmentation and gastrointestinal polyposis. PJS is associated with mutations in the STK11/LKB1 gene on chromosome 19p13.3, which encodes a serine-threonine kinase. The endocrine tumors, which may be multifocal and bilateral, can differentiate into granulosa cell or large cell calcifying Sertoli tumors with the potential to secrete estrogen. Thus affected females may present with precocious puberty, and affected males may present with gynecomastia. Although usually benign, granulosa or Sertoli cell tumors can undergo malignant changes. Affected individuals have an increased risk for colon, stomach, small intestinal, breast, and pancreatic cancers. Aromatase inhibitors were associated with decreased gynecomastia in a small series of boys with large cell calcifying Sertoli cell tumors.
Sertoli cell tumors have been found in association with both PJS and the Carney Complex (CNC). CNC is often associated with mutations in the protein kinase, c-AMP dependent, regulatory, type 1, alpha (PRKAR1A) gene, which encodes the regulatory subunit type 1 of protein kinase A. Among boys, adrenal, testicular, or hepatocellular tumors can express aromatase, leading to secretion of estradiol and estrone. In instances where gynecomastia is excessive, prolonged, and apparent at a time other than midpuberty (around stage 3), further evaluation may be warranted. The evaluation should include testosterone, estradiol, hCG, LH, FSH, thyroid-stimulating hormone (TSH), and DHEAS measurements. In addition to tumors, the differential diagnosis of gynecomastia includes Klinefelter syndrome, impaired testosterone biosynthesis, androgen insensitivity, and hyperprolactinemia.
Estrogen Secretion Unrelated to Tumors
In one family, autosomal-dominant familial gynecomastia was due to aberrant transcription of the aromatase gene; the affected girl developed GnRH-independent precocious pubertal development. Autosomal-dominant aromatase excess syndrome is characterized by high systemic estrogen levels, short stature, prepubertal gynecomastia, and testicular failure in males, and premature breast development, macromastia, and uterine pathology in females. Small chromosomal arrangements in the promoter region of the aromatase gene appear to be associated with increased promoter activity, resulting in increased aromatase activity. Novel cryptic deletions and duplications were identified; phenotypic severity in this gain of function disorder is primarily determined by the tissue expression pattern of CYP19A1 .
Isolated Premature Menarche
Isolated premature menarche refers to vaginal bleeding at an inappropriately early age in the absence of other signs of puberty. The duration of bleeding is usually limited to a few days. The most common endocrine etiology is spontaneous resolution of an estrogen-secreting ovarian cyst. Often, the ultrasound shows no abnormality because the cyst has resolved by the time the study is obtained. Most instances are self-limited, remit spontaneously, and are associated with normal pubertal development. Typically, isolated menarche due to an estrogen-secreting cyst is a sporadic event that usually occurs once. However, such cysts and episodes of vaginal bleeding may recur. The bloody vaginal discharge noted in female infants during the first week of life is a physiological event secondary to estrogen withdrawal.
Tumors or trauma usually do not cause cyclic bleeding. The other major differential diagnoses of isolated vaginal bleeding include sexual abuse, vaginal foreign bodies, vaginal infection, MAS, and primary hypothyroidism. Neoplasms such as rhabdomyosarcoma and sclerosing stromal tumors can present with isolated vaginal bleeding.
Premature Thelarche
Premature thelarche is defined as isolated breast development without other signs of pubertal maturation. Typically, the parents or pediatrician note breast development, either unilateral or bilateral, between 9 and 18 months of age. No significant nipple development or pigmentation occurs, and the vaginal mucosa remains pink and shiny.
Breast ultrasound can help distinguish breast tissue from cysts, fibroadenomas, neurofibromas, or other less common lesions, but is usually not needed. Pelvic ultrasound may show a bilateral increase in the number of ovarian follicular cysts. Using a recombinant cell bioassay with increased sensitivity, estradiol concentrations are higher among girls with premature thelarche than healthy controls. However, estradiol concentrations in such patients are still low and remain below the assay detection limit for most radioimmunoassays. FSH concentrations may be increased for chronological age, but LH concentrations and LH responses to GnRH stimulation are prepubertal. No acceleration in linear growth velocity or skeletal maturation occurs, and the breast development usually regresses spontaneously over time. Although premature thelarche prior to 2 years of age is often transient, closer observation is indicated if growth velocity is accelerated or if the basal LH concentration is greater than 0.3 IU/L. In most instances, onset of puberty, adult height, and adult reproductive function are normal. Premature thelarche can usually be considered as a normal variant. Nevertheless, longitudinal evaluation is helpful to ensure the nonprogressive nature of this disorder.
Premature thelarche has been described in Rubinstein-Taybi syndrome (RTS), an autosomal-dominant disorder characterized by short stature, psychosocial retardation, a characteristic fades, broad thumbs and halluces, and increased risk for neoplasia. RTS has been associated with mutations in CREB binding protein (CREBBP) and E1A binding protein p300 (EP300) genes. Premature thelarche and precious puberty have been reported in girls with the Kabuki make-up syndrome, which is characterized by a peculiar facies with eyes reminiscent of Kabuki actors, mental retardation, and decreased growth velocity. Kabuki syndrome has been associated with mutations in the KMT2D and KDM6A genes.
Hypothyroidism
Girls with primary hypothyroidism can, on rare occasions, present with breast development or isolated vaginal bleeding. This constellation of clinical features was first described by Van Wyk and Grumbach in 1960. On ultrasound, enlarged multi-cystic ovaries may be noted. Additional features may include delayed bone age, ascites, and pleural and pericardial effusions. This is the only etiology for precocious puberty associated with delayed bone age. Thyroid hormone replacement therapy is associated with regression and resolution of the cysts; surgical treatment is not indicated. The mechanism underlying the ovarian stimulation is unclear. One possibility is that the excessively elevated TSH concentrations cross-react with the FSHR to promote estrogen secretion. Another explanation is that increased FSH secretion observed in the hypothyroid state is responsible.
Hypothyroid boys may show increased testicular volume (macroorchidism) for age. Interestingly, after thyroxine replacement to a cohort of such boys and the subsequent attainment of stage 5, testicular volume was found to be considerably greater than that in controls. The macroorchidism associated with prepubertal hypothyroidism is probably the result of an expanded population of undifferentiated Sertoli cells resulting from increased FSH signaling in response to elevated concentrations of either FSH or TSH.
It should be noted that at the hypothalamic-pituitary level, hypothyroidism leads to a delay in the pubertal resurgence of LH secretion, which presumably accounts for the delayed puberty that is generally associated with chronic hypothyroidism (discussed later in the chapter).
Exogenous Hormone Exposures
Exposure to exogenous estrogenic steroids or estrogen receptor agonists can induce pubertal development. Information regarding possible environmental exposures from tea tree oil, lavender oil, or personal care products should be elicited because use of these substances has been associated with thelarche and gynecomastia. Potential sources of estrogenic steroids include oral contraceptives, creams, shampoos, and various lotions. In addition, phytoestrogens found in a variety of foods, and phthalate esters present in plastics are environmental endocrine disruptors with estrogen agonist activity. Epidemics of premature breast development reported in Puerto Rico and Italy have been attributed to increased exposure to estrogenic steroids, phthalates, phytoestrogens, or estrogenic mycotoxins. Several case reports have described precocious puberty and virilization in children accidentally exposed to the testosterone gels.
Mycotoxins are naturally occurring substances that can be found as environmental contaminants in cereals, corn, and nuts. Certain drugs, such as marijuana, isoniazid, spironolactone, ketoconazole, and cimetidine, can induce gynecomastia by a variety of mechanisms. It has been suggested that prenatal exposure to endocrine disruptors may influence fetal programming of the endocrine system and, therefore, may influence the timing and tempo of puberty. Potential mechanisms of action include binding to nuclear hormone receptors, influencing co-factor recruitment, altering steroidogenesis, and epigenetic modifications.
Virilizing Disorders
Premature pubarche is defined as the development of pubic hair, axillary hair, and apocrine body odor prior to age 8 years in girls and age 9.5 years in boys. The differential diagnosis includes premature adrenarche, CAH, and androgen-secreting tumors.
Premature Adrenarche
While the age of adrenarche varies considerably among ethnic groups, it is generally considered premature when it occurs prior to age 8 years in girls and age 9.5 years in boys. As in normal adrenarche, pubic hair, axillary hair, adult type apocrine odor, and acne may develop, whereas skeletal maturation may be appropriate for chronological age or slightly advanced. Clitoromegaly and marked phallic enlargement are unusual findings in patients with premature adrenarche. Usually, gonadarche occurs at an appropriate chronological age and subsequent pubertal development proceeds normally. More girls tend to be referred for evaluation than boys. Despite BMI comparable with controls, one recent study reported that girls with premature pubarche appear to have increased total and central fat mass. In one study of Spanish and Italian girls, mean age at menarche for girls with documented premature pubarche was 6 months earlier than healthy controls.
Typically androgen concentrations are elevated for chronological age but within normal limits for the stage of pubic hair development. However, a subset of girls with premature adrenarche show persistent hyperandrogenism upon gonadarche. Insulin resistance, hyperinsulinemia, and dyslipidemia have been described in these girls. These girls may develop chronic anovulation, hirsutism, insulin resistance, hyperinsulinemia, severe acne, and an increased LH:FSH ratio. These features are suggestive of incipient PCOS. Subsequently, adolescent girls with PCOS have an increased risk to develop impaired glucose tolerance and type 2 diabetes mellitus. In some populations, the frequency of heterozygosity for mutations in the 21-hydroxylase (CYP21A2) gene is higher among children with premature pubarche and adolescent girls with incipient PCOS.
Premature pubarche due to premature adrenarche is a diagnosis of exclusion. The majority of children with premature adrenarche require no pharmacological intervention. However, at this time, the ability to predict outcome and risk for PCOS is imperfect. Because lifestyle interventions involving food choices and exercise programs decrease the progression from impaired glucose tolerance to diabetes mellitus among adults, it seems prudent to counsel children with premature pubarche to adopt healthy lifestyles.
Rett Syndrome
Rett syndrome is a rare neurodevelopmental disorder, which is characterized by loss of hand skills, loss of acquired spoken language, gait abnormalities, and stereotypic hand movements. This disorder is associated with heterozygous mutations in the Methyl-CpG-binding protein 2 (MECP2) gene, which is mapped to the X chromosome. Affected girls enter puberty early but they experience delayed menarche. Based on data generated through the multicenter RTT Natural History study, median duration of puberty, from thelarche to menarche, was reported to be 3.9 years.
Disorders of Steroidogenesis
Virilizing Congenital Adrenal Hyperplasias
The virilizing CAHs are a group of autosomal-recessive disorders in which cortisol synthesis is impaired due to decreased 21-hydroxylase, HSD3B2, or 11β-hydroxylase activity (see also Chapter 4 ). The specific pattern of circulating steroid hormone concentrations reflects which steroid enzyme is involved. Approximately 90% to 95% of cases are due to 21-hydroxylase deficiency, which is due to mutations in the CYP21A2 gene. Mutations in HSD3B2 and 11β-hydroxylase (CYP11B1) genes account for the remaining 5% to 10% of patients with virilizing CAH.
In all cases, decreased cortisol concentrations lead to loss of negative feedback inhibition, increased ACTH secretion, and increased adrenal androgen biosynthesis. The clinical spectrum for these disorders ranges from complete loss-of-function mutations, which typically present during infancy with genital ambiguity, to partial loss-of-function mutation, which may present in childhood, adolescence, or adulthood. Here, discussion is limited to nonclassic CAH (NCAH) where the major symptoms are secondary to hyperandrogenism, rather than to adrenal cortical insufficiency, as in the classical forms of this disease.
During childhood, boys or girls with NCAH can present with premature pubic hair, adult type apocrine odor, increased growth velocity, and tall stature. In contrast to premature adrenarche, clitoromegaly or phallic enlargement and advanced skeletal maturation are more common. In female patients, the symptoms of NCAH are similar to those of PCOS. Males with NCAH are often not identified unless sisters with NCAH have been diagnosed.
21-Hydroxylase Deficiency
The reported incidence of NCAH is approximately 1 in 1000 patients, whereas the reported incidence of the more severe forms is approximately 1 in 14,000. In 21-hydroxylase deficiency, decreased 21-hydroxylase activity leads to increased concentrations of 17-OHP, 17-OH-hydroxypregnenolone, DHEA, androstenedione, and testosterone. The gene coding for 21-hydroxylase, CYP21A2 , maps to chromosome 6p21. A highly homologous nonfunctional pseudogene, CYP21A1P , is located in close proximity to the functional gene. The majority of mutations associated with 21-hydroxylase deficiency represent gene conversion events in which the functional gene has acquired deleterious nucleotide sequences from the pseudogene. To date, over 200 mutations have been reported, but approximately 10 mutations account for the majority of affected alleles. Most affected individuals are compound heterozygotes and carry different CYP21A2 mutations on each allele. For individuals with nonclassical CAH, the missense mutation, V281L, accounts for at least one of the CYP21A2 alleles for most patients with nonclassical CAH. Phenotype–genotype correlations are fairly consistent, with the phenotype usually representing the least severe mutation.
For children with symptoms suggestive of NCAH, early morning basal 17-OHP values have been suggested as an effective screening test. Armengaud et al. reported 100% sensitivity and 99% specificity with a threshold value of 200 ng/dL (6 nmol/L) to diagnose NCAH in children with premature pubarche. Defective 21-hydroxylase activity also promotes accumulation of other steroid hormone intermediates, such as 21-deoxycortisol and 16α-hydroxyprogesterone, 11-ketoandrostenedione, and 11-ketotestosterone. Although random 17-OHP concentrations can be diagnostic (especially in the more severe forms), ACTH-stimulation tests may be necessary to confirm the diagnosis of milder forms. Despite the increased availability of CYP21A2 genotype analyses, the complexity of this locus precludes routine use of molecular diagnostics. Techniques that genotype each allele or inclusion of parental DNA samples to segregate the alleles are helpful because a single CYP21A2 allele can carry multiple mutations. Two mutations on the same allele can act synergistically to impair enzyme activity to a greater extent than would be anticipated for each mutation individually.
3 β -Hydroxysteroid Dehydrogenase Deficiency
In this form of virilizing CAH, decreased activity of the adrenal and gonadal specific form of HSD3B2 leads to increased concentrations of 17α-hydroxypregnenolone and DHEA. This disorder is due to mutations in the gene coding for HSD3B2 . Patients with classical HSD3B2 deficiency have been found to have mutations in the HSD3B2 gene. However, nonclassical CAH due to HSD3B2 mutations is extremely rare. Consequently, the correlation of molecular genotype data with hormonal responses has led to the adoption of more stringent criteria for the diagnosis of 3 β -HSD deficiency. In general, ACTH-stimulated 17-OH-hydroxypregnenolone and DHEA responses are elevated.
11 β -Hydroxylase Deficiency
The clinical features associated with 11β-hydroxylase deficiency are similar to 21-hydroxylase deficiency. Patients with this type of virilizing adrenal hyperplasia may develop hypertension attributed to increased deoxycorticosterone secretion. This disorder is due to mutations in the CYP11B1 gene located on chromosome 8. Nonclassical CAH due to CYP11B1 mutations is extremely rare. The incidence of 11β-hydroxylase deficiency has been estimated to be 1 : 100,000 among Caucasians, but the incidence among Israeli Jews of Moroccan origin is reported to be higher. Elevated basal and ACTH-stimulated 11-deoxycortisol concentrations are typically found in this form of CAH. Serum 17-OHP, androstenedione, and testosterone concentrations may be mildly elevated. Plasma renin activity concentrations are low or suppressed.
Other Disorders Affecting Steroidogenesis
Oxidoreductase Deficiency
This autosomal-recessive (AR) disorder of steroidogenesis is characterized by a steroid profile suggesting combined 17α-hydroxylase and 21-hydroxylase deficiencies. The more severe phenotype is characterized by ambiguous genitalia, adrenal insufficiency, and skeletal anomalies and is known as the Antley-Bixler syndrome. The skeletal abnormalities can include craniosynostosis, midface hypoplasia, choanal atresia, low-set ears, pear-shaped nose, arachnodactyly, clinodactyly, and radiohumeral synostosis. This disorder is due to mutations in the POR gene located at chromosome 7q11.2. The protein encoded by the POR gene functions as an electron donor to cytochrome P450 enzymes. POR loss-of-function mutations influence the activities of 21-hydroxylase, 17α-hydroxylase, and aromatase enzymes.
Prenatal virilization of female fetuses occurs, but there is minimal postnatal virilization. Maternal virilization during pregnancy may occur and has been attributed to the aromatase deficiency. Curiously, some patients, both male and female, present with delayed puberty; and ovarian cysts can occur in adolescent females. One adolescent girl presented with breast development, primary amenorrhea, bony anomalies, and cystic ovaries. Basal and ACTH-stimulated steroid profiles are variable because oxidoreductase deficiency affects multiple steroidogenic enzymes; serum 17-OHP concentrations tend to be elevated. Urinary analysis of steroid excretion using gas chromatography and mass spectroscopy (GC/MS) can provide hormonal confirmation of the diagnosis. Cortisol deficiency may occur; affected individuals may benefit from glucocorticoid replacement therapy. Mutations in the FGFR2 gene are associated with similar skeletal anomalies, but have normal steroidogenesis and normal external genitalia.
Apparent Cortisone Reductase Deficiency
Cortisol is the active glucocorticoid secreted by the adrenal ZF. Concentrations of cortisol and its inactive metabolite, cortisone, are modulated by the enzymes, 11β-hydroxysteroid dehydrogenase type 1 (HSD11B1) and type 2 (HSD11B2). Apparent cortisone reductase deficiency (ACRD) is due to hexose-6-phosphate dehydrogenase (H6PD) deficiency and has been associated with premature adrenarche. This disorder is due to loss-of-function mutations in the H6PD gene, which interferes with HSD11B1 oxo-reductase activity and prevents local conversion of cortisone to cortisol. The net result is accelerated peripheral clearance of cortisol resulting in decreased negative feedback inhibition of the hypothalamic, pituitary, adrenal axis, increased ACTH secretion, and increased ACTH-mediated adrenal androgen secretion. DHEAS, androstenedione, and testosterone concentrations are increased. Urinary steroid metabolite profiling by combined gas chromatography and mass spectrometry may be helpful in the differential diagnosis of ACRD.
Apparent Dehydroepiandrosterone Sulfotransferase Deficiency (PAPSS2)
In the adrenal cortex, DHEA can be converted to DHEAS by the enzyme DHEA sulfotransferase (SULT2A1). The catalytic activity of SULT2A1 depends on the availability of sulfate donor PAPS. The isoform predominantly expressed in the adrenals and liver is PAPSS2 . Sequencing of the PAPSS2 gene in a girl with premature adrenarche who subsequently developed a PCOS-like phenotype revealed compound heterozygous mutations. Several patients have been reported to have a bone phenotype characterized by spondyloepimetaphyseal dysplasia. The bone dysplasia has been attributed to impaired proteoglycan sulfation, which disrupts extracellular matrix formation.
Diagnosis and Treatment of Congenital Adrenal Hyperplasia and Other Disorders of Steroidogenesis
Steroid hormone responses to ACTH stimulation help to differentiate between premature adrenarche and CAH. To perform an ACTH-stimulation test, a pharmacological dose of synthetic ACTH (0.25 mg cosyntropin) is administered after a basal blood sample has been obtained. A second sample is collected 30 to 60 minutes later. Basal and stimulated steroid hormone concentrations and hormone ratios provide important information. Stimulated 17-OHP responses of less than 500 ng/dL at 30 minutes are within normal limits. Responses greater than 1500 ng/dL are consistent with 21-hydroxylase deficiency. Intermediate responses, 500 to 1500 ng/dL, are consistent with heterozygosity for 21-hydroxylase deficiency. Children with premature pubarche due to HSD3B2 mutations have had ACTH-stimulated 17-OH-hydroxypregnenolone values greater than 9000 ng/dL. Genetic testing is necessary to confirm the molecular diagnosis.
Treatment of the virilizing CAHs involves hormone replacement therapy with glucocorticoids. The goal of treatment is suppression of excessive ACTH and adrenal androgen secretion without hypercortisolism. Hydrocortisone or a synthetic glucocorticoid, such as prednisone or dexamethasone, can be used. During childhood, hydrocortisone is generally the preferred glucocorticoid because linear growth in the growing child is extremely sensitive to glucocorticoid levels. Longer-acting glucocorticoids, such as prednisone and dexamethasone, may interfere with linear growth velocity. Typically, hydrocortisone dosages range from 7 to 15 mg/m 2 per day and must be administered 3 times daily because of the hormone’s short duration of action. This relatively short duration of action is the major disadvantage of hydrocortisone because patients may have significant variation in serum hormone concentrations between doses.
The use of a reverse diurnal dosing has been suggested so that the highest dose is administered at night, whereas others suggest that the highest dose should be administered in the morning. Older adolescents and young adults may like the convenience of fewer daily doses and use prednisone (5.0 to 7.5 mg divided into two daily doses) or dexamethasone (0.25 to 0.5 mg daily). The growth suppressive potencies of prednisone and dexamethasone are greater than their antiinflammatory potencies. It is also important to remember that the mineralocorticoid activity of these glucocorticoids varies, with prednisolone having less mineralocorticoid activity than hydrocortisone and dexamethasone having no mineralocorticoid activity.
Long-term management involves interim medical history, physical examination with assessment of growth velocity, and hormone measurements to assess for adequate adrenal suppression. Glucocorticoid therapy alone is generally sufficient for children with milder forms of CAH. For patients with oxidoreductase deficiency, ACRD, and PAPSS2, the decision to initiate glucocorticoid therapy needs to be individualized and based on clinical features, ACTH-stimulated cortisol response, and skeletal maturation.
Stress doses are indicated for fever, persistent vomiting, serious injuries, and surgery. Families should have injectable hydrocortisone readily available for situations in which oral medications are not tolerated. General guidelines for oral stress doses are 3 to 4 times the maintenance doses or approximately 50 mg/m 2 per day. Parenteral stress dosing varies by age. All affected individuals should wear a Medic-Alert identification badge to alert emergency health care providers to their disorder.
Children with CAH who develop a secondary GnRH-dependent precocious gonadarche may benefit from GnRH-Ra treatment. Children with overt salt loss, as well as those with simple virilizing forms, benefit from receiving mineralocorticoid replacement therapy.
Now that increased numbers of children with CAH or other disorders of sexual differentiation survive into adulthood, the medical and psychosocial aspects of their health care are undergoing reevaluation. Thus interest in the determinants of gender identity, approach to genital reconstructive surgery, and outcome has increased. Hence issues related to gender identity, surgery, sexuality, and outcome should be discussed when evaluating patients with these disorders.
Because CAH is one of the most common disorders associated with aberrant external genital differentiation, most of the literature in this area relates to outcome in women with classical CAH. Adult women with CAH have tended to be unmarried, have fewer children than healthy controls, and have negative self-images. Girls with CAH tend to have female gender identity with preferences for male-type play and male career choices. Preliminary data from a limited number of outcome studies indicate that honesty, extensive education, and counseling benefit these patients with disorders of sexual differentiation and their families.
Testicular adrenal rest tumors (TARTS) may develop in the testes of boys with CAH. These testicular tumors are more common in those boys who are undertreated or poorly compliant. The tumors tend to be benign, bilateral, and they are believed to arise from aberrant ACTH-responsive adrenal cells. Due to their location in the mediastinum testis, obstruction of the seminiferous tubules leading to gonadal dysfunction and infertility can occur.
Inherited Glucocorticoid Resistance
Familial glucocorticoid resistance, also known as Chrousos syndrome, is an autosomal-dominant disorder due to loss-of-function mutations in the glucocorticoid receptor ( GRL or NR3C1 ) gene. Consequently, in the face of normal or elevated cortisol concentrations, there is loss of negative feedback inhibition leading to increased ACTH secretion and adrenal androgen biosynthesis, similar to that seen in CAH patients. The predominant symptoms of this disorder are due to excessive androgen and mineralocorticoid secretion rather than glucocorticoid deficiency. Affected children may present with premature pubarche, hypertension, fatigue, or hypokalemia. Laboratory investigation shows elevated plasma concentrations of cortisol, adrenal androgens, aldosterone, and ACTH; these levels recede after administration of a high dose of dexamethasone. Treatment involves high-dose therapy with a potent glucocorticoid (i.e., dexamethasone), leading to suppression of ACTH release and decreased adrenal androgen secretion.
Familial Male-Limited Precocious Puberty
This autosomal-dominant disorder is due to inherited or de novo constitutive activating mutations of the LHCGR gene that alter the tertiary confirmation of the receptor protein, leading to increased cAMP signaling in the absence of ligand. Clinical manifestations are limited to male patients and include phallic enlargement, increased testicular volume, pubic hair, body odor, accelerated growth velocity, and advanced skeletal maturation. These characteristics may present within the first few years of life. Testosterone concentrations are high, while gonadotropin concentrations are low. Short-term treatment with steroid synthesis inhibitors like testolactone or ketoconazole can be used in conjunction with spironolactone, which blocks androgen action. Another intervention involves the combination of bicalutamide, a nonsteroidal anti-androgen that blocks androgen action and a third-generation aromatase inhibitor. If a secondary GnRH-dependent gonadarche develops, GnRH-Ra treatment can improve height prediction. Long-term treatment with cyproterone acetate or ketoconazole revealed similar outcomes without major side effects in 10 boys with testotoxicosis; effects on final height outcome were variable. The lack of a female phenotype is puzzling; perhaps the most attractive possibility is that expression of LHCGR by the prepubertal ovary is low.
Androgen-Secreting Tumors
Leydig cell tumors secrete testosterone, which leads to precocious pubertal development in boys. Testicular volume may be asymmetric because the tumors are often unilateral. Because some tumors are too small to be palpated, ultrasonography may be helpful to localize the tumor. The majority of Leydig cell tumors are benign. Malignant tumors tend to be larger, show greater cell atypia, and tend to infiltrate beyond the testis. In rare instances, the tumor can secrete large quantities of other steroids like 17-OHP, which may confound the diagnosis. A novel somatic cell-activating mutation of the LHCGR gene has been found in some adenomas. The D578H mutation associated with familial male-limited precocious puberty has been found in association with Leydig cell adenomas and nodular hyperplasia of Leydig cells in prepubertal boys. Alterations in other factors, such as AMH, inhibin, and other growth factors, may also contribute to the development of such tumors. Hence, the etiologies of tumors at the molecular level are heterogeneous. If possible, the tumor should be excised without removing the testis. Ovarian androgen-secreting tumors are a rare cause of virilization in girls. Sertoli-theca cell tumors are sex cord stromal tumors that have been reported in girls with PJS.
Human Chorionic Gonadotropin-Secreting Tumors
In boys, hCG-secreting tumors induce testicular testosterone secretion, leading to precocious pubertal development. These tumors are frequently hepatic in origin. In girls, hCG-secreting tumors in the absence of pubertal levels of FSH are not usually associated with precocious pubertal development. However, hCG secreting tumors associated with precocious pubertal development in girls have been rarely reported. The rarity of precocious puberty due to hCG secreting tumors in girls is consistent with the concept that LHCGR expression in the prepubertal ovary is low.
Cushing Syndrome
Cushing syndrome is characterized by excessive glucocorticoid concentrations, whether endogenous or exogenous in origin. Although the predominant features of glucocorticoid excess in children and adolescents are arrested pubertal development and growth failure, precocious virilization may occur when the hypercortisolism is accompanied by hyperandrogenism. This occurs in Cushing disease, in cases of ectopic secretion of ACTH or CRH, and in rare cases of adrenal tumors. Individuals with chronic conditions like rheumatoid arthritis, steroid dependent nephropathies, and asthma requiring high-dose steroid treatment may develop signs and symptoms due to glucocorticoid excess. Intranasal steroids can cause iatrogenic Cushing syndrome and adrenal suppression.
Among children younger than 8 years of age, adrenal tumors are the most common cause of Cushing syndrome. Such tumors are often malignant. The combination of rapid pubertal development and hypercortisolism suggests an adrenal tumor. Such tumors, which are rare, can secrete a mixture of steroid hormones. Nevertheless, virilization is a common presentation for childhood adrenocortical tumors. There is a female predominance. Complete surgical excision is the treatment of choice; complete resection is required for cure. Residual or metastatic disease is associated with a poorer prognosis. Histological examination may not be able to accurately differentiate benign adenomas from carcinomas. Cushing disease due to excessive pituitary ACTH secretion is extremely rare in young children but can occur in adolescents.
Primary pigmented adrenocortical nodular disease (PPNAD) is often associated with the periodic or episodic hypercortisolism. The adrenal glands are characterized by multiple pigmented autonomously functioning nodules. PPNAD is associated with inactivating mutations of the PRKAR1A gene. The majority of benign lesions of the adrenal cortex appear to be associated with abnormalities of the cAMP signaling pathway, whereas adrenocortical carcinomas are linked to aberrant expression of IGF II, tumor protein p53, and related molecules. Syndromes associated with adrenocortical tumors include CNC, Li-Fraumeni syndrome, and Beckwith-Wiedemann syndrome.
Confirmation of excessive glucocorticoid secretion is essential prior to imaging studies. Loss of diurnal variation in cortisol secretion, excessive 24-hour urinary free cortisol excretion, and inadequate dexamethasone-induced suppression are valid diagnostic studies. In some instances, due to periodic hypercortisolism, these studies need to be repeated. Treatment is directed by the underlying disorder.
Approach to the Child With Precocious Pubertal Development
Identification of the etiology of sexual precocity begins with a thorough medical history, with questions focusing on the physical manifestations of puberty and the age and sequence of their appearance ( Figs. 17.12 and 17.13 ). Does the patient have neurological symptoms or gelastic seizures? Has the patient received radiation therapy? Was the patient exposed to exogenous hormones? Obtaining a complete family history is important because some disorders, such as familial male-limited precocious puberty, glucocorticoid resistance, and NF1, show autosomal-dominant inheritance.
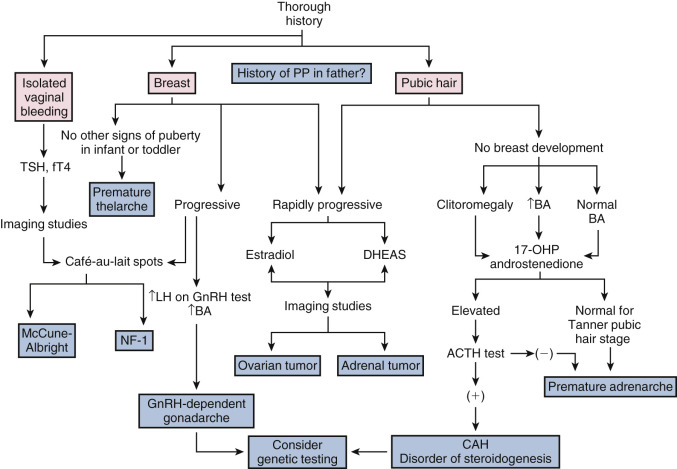
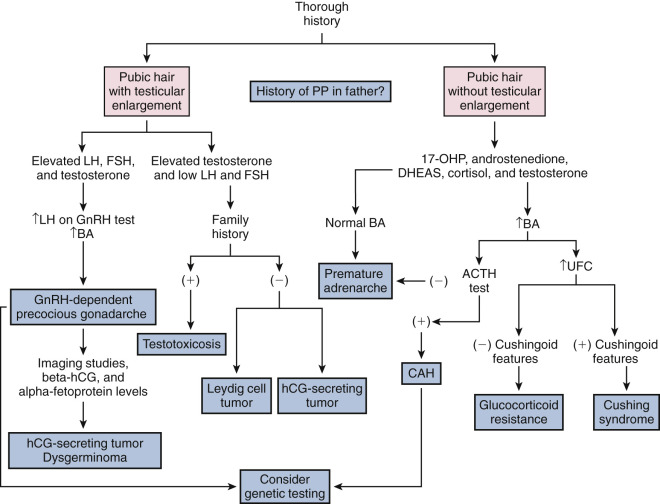
Next, a complete physical examination, including auxological measures, should be performed; particular attention should be paid to development of secondary sexual characteristics. For girls, are breast and/or pubic hair development present? For boys, is testicular volume increased? Because increased growth velocity occurs concomitantly with breast development among girls, observation of accelerated growth velocity helps to differentiate GnRH-dependent precocious gonadarche from nonprogressive gonadarche or premature thelarche. The physical examination should be directed to detect physical signs indicating onset of gonadarche or adrenarche, as well as features suggestive of specific disorders such as café-au-lait spots in MAS or NF1. Does the patient have manifestations of hypothyroidism?
Laboratory Assessment
Information obtained from the medical history, family medical history, and physical examination should direct the evaluation for individual patients. Typically, boys with precocious puberty should undergo more detailed evaluations because of the increased likelihood of organic etiologies. For example, testicular enlargement in boys directs the evaluation towards a gonadotropin-dependent etiology. In contrast, a boy with phallic enlargement, pubic hair, and prepubertal testes is more likely to have a GnRH-independent disorder, such as virilizing CAH.
Laboratory studies are used to confirm or exclude specific disorders based on the possible differential diagnoses. The degree of skeletal maturation as ascertained on a bone age x-ray is usually, but not always, advanced among patients with GnRH-dependent precocious gonadarche and the virilizing CAHs. Typically, bone maturation is not significantly advanced among subjects with premature thelarche or premature adrenarche. In healthy individuals, the bone age interpretation should lie within two SDs of the normative data.
In addition to x-ray assessment of skeletal maturation (bone age), gonadotropin, IGF-1, and sex steroid concentrations may be helpful. Because secretion is pulsatile, random gonadotropin concentrations may provide only limited information. In some instances, GnRH-stimulation tests may be necessary to differentiate GnRH-dependent precocious gonadarche from premature thelarche; an LH predominant response is typical of GnRH-dependent precocious gonadarche, whereas an FSH predominant response is typical of premature thelarche. Traditionally, synthetic GnRH (Factrel), at doses of 2.5 µg/kg or 100 µg/m 2 (maximum 100 µg), was administered to demonstrate a pubertal pattern of gonadotropin responses. Following Factrel, LH and FSH concentrations typically rise by 20 to 30 minutes.
The limited availability of GnRH (Factrel) led to development of stimulation tests utilizing an alternate GnRH-R agonist (GnRH-Ra), such as leuprolide as the provocative agent. Leuprolide (500 µg, subcutaneous [s.c.] or 20 µg/kg, s.c.) or triptorelin (0.1 g, s.c.) may be used when Factrel is unavailable; the time to the peak gonadotropin response is more variable and is typically later than with Factrel. A stimulated LH predominant response confirms the increased GnRH pulse generator activity typical of CPP.
Most current assays for gonadotropins are sandwich assays specific to the β-subunit. In girls, when gonadotropins are measured using third-generation monoclonal fluorometric assays, basal and GnRH-stimulated LH concentrations greater than 0.6 and 6.9 U/L, respectively, are 70% and 92% sensitive for the diagnosis of GnRH-dependent precocious gonadarche. Indeed, using the newer third-generation gonadotropin assays, random LH concentrations greater than 1.0 U/L in girls with features consistent with precocious puberty may be sufficient to diagnosis CPP in most instances. Nevertheless, stimulation testing may be warranted for some girls with inconclusive basal gonadotropin concentrations. Abbreviated stimulation tests using only GnRH-agonist-stimulated LH and FSH values are being utilized. It is important to be aware of the normal values for the specific methodology used.
Girls with CPP have higher IGF-1, fasting insulin, and oral glucose-stimulated insulin concentrations compared to healthy nonpubertal girls; these differences persist after adjustment for chronological age, skeletal age, and pubertal stage. The IGF-1 concentrations do not decrease to prepubertal values despite adequate treatment.
For patients manifesting androgenic effects or virilization, measurement of C19 bioactive androgens is essential. Elevated androgen concentrations suggest adrenal or testicular etiologies. Elevated random 17-OHP concentrations may be diagnostic for some patients with 21-hydroxylase deficiency. If the diagnosis of CAH or another disorder of adrenal steroidogenesis is suspected, an ACTH stimulation test should be performed. Following collection of a basal sample, 0.25 mg Cortrosyn can be administered. ACTH-stimulated hormone concentrations can be obtained at 30 or 60 minutes.
Imaging Studies
Magnetic resonance imaging (MRI) and computerized tomography (CT) imaging are helpful to identify anatomical lesions such as hypothalamic hamartomas. On CT or MRI, hamartomas appear as an isodense, abnormal fullness. Imaging with MRI is superior to CT, but the lesions do not enhance with contrast material. For boys, earlier onset of puberty, greater bone age advancement, and/or neurologic symptoms are associated with a higher risk for pathological CNS lesions. Thus CNS imaging remains a valuable diagnostic tool for boys with CPP. For girls greater than 6 years of age, the likelihood of detecting a tumor or brain abnormality is extremely low. Therefore routine MRI screening may not be indicated in girls with CPP between 6 and 8 years of age.
Pelvic ultrasound is helpful for the evaluation of girls with precocious puberty because the size and morphology of the uterus and ovaries reflect function and hormone exposure. If estradiol concentrations are extremely elevated, imaging is important to assess for ovarian and adrenal tumors. The prepubertal uterus is tubular in configuration; the cervix is similar or slightly larger than the fundus in size. Estrogen exposure causes a disproportionate enlargement of the fundus so the uterus acquires a pear-like shape. The mean ovarian volume in prepubertal girls between 2 and 7 years of age is 1 cm 3 . Small follicles (less than 9 mm) may normally be present. In GnRH-dependent precocious puberty, uterine and ovarian changes can provide evidence of ovarian gonadotropin stimulation. However, the overlap between prepubertal and puberty features limits the usefulness of ultrasonography. Among girls, pelvic ultrasound can assess for pubertal development of the ovaries and uterus; it can also detect tumors and ovarian cysts.
Similarly, if testosterone, DHEAS, or other C19 bioactive androgen concentrations are elevated, testicular and adrenal imaging might be beneficial to assess for an androgen-secreting tumor. Boys with elevated hCG concentrations may require imaging of the head, chest, and abdomen to detect an extratesticular hCG-secreting tumor. Testicular ultrasound to assess for Leydig cell tumors or microlithiasis may be indicated, especially when the testicular volumes are discordant. Adrenal ultrasound to assess for adrenal tumors or adrenal hyperplasia is appropriate for children with premature pubarche and extremely elevated DHEAS concentrations. As noted above, excessive glucocorticoid secretion needs to be established before performing imaging studies in patients suspected to have Cushing syndrome.
For patients with suspected disorders of steroidogenesis, liquid-chromatography-tandem mass spectrometry (LC-MS/MS) is rapidly becoming the method of choice for clinical steroid analysis because of the higher sensitivity, better reproducibility, greater specificity, and low sample volume compared to immunoassay techniques. This technique offers the ability to analyze multiple steroids simultaneously. Prior to clinical use, reliable reference ranges need to be established for specific analytes according to gender and pubertal stage.
Delayed Puberty
In most instances, delayed puberty is the result of delayed gonadarche and is defined as development of secondary sexual characteristics at a chronologic age greater than two SDs above the normal population. Thus for girls, lack of breast development by age 13 years or menarche by 16 years may be viewed as delayed. For boys, lack of increased testicular volume at age 14 years is considered to be delayed. Pubertal delay can be broadly subclassified as constitutional, hypogonadotropic, or hypergonadotropic.
Atypical, delayed, or absence of pubertal development during the adolescent years may also reveal disorders of sexual differentiation that escape diagnosis during infancy. Failure to complete puberty within 5 years also warrants evaluation. In general, treatment of delayed puberty involves steroid hormone replacement, which is discussed in detail at the end of the section ( Box 17.2 ; Table 17.3 ).