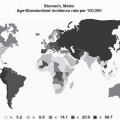
Proteins categorized according to Cell Cycle and Cell Proliferation Regulation, Cell Migration and Metastasis, Cell Structure and Motility, DNA Repair Immunity Defenses, Oncoprotein, Signal Transduction, and Transcription and Translation (d: downregulated; u: upregulated) |
Studies: 1. Zhang et al.7 2. Chen et al.13 3. He et al.19 4. Yoshihara et al.20 5. Nishigaski et al.6 6. Kon et al.8 7. Chan et al.12 8. Chen et al.14 9. Yang et al.1710. Wang et al.16 |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
Sample size (N) |
|
84 |
44 |
10 |
5 |
14 |
24 |
10 |
|
|
|
Specimen type (T: tissue, J: gastric juice, C: cell line) |
T |
T |
T |
T |
T |
J |
C/T |
C |
C |
C |
Protein |
Function |
|
|
|
|
|
|
|
|
|
|
Cell Cycle and Cell Proliferation Regulation |
14-3-3 β/α |
Regulator of cell cycle |
|
|
|
|
|
|
u |
|
|
|
Cell division control protein 42 homologue |
Regulates cadherin-mediated cell-cell adhesion |
|
|
|
|
|
|
|
d |
|
|
Cell division kinase 6 |
Related to cell proliferation, tumor heterogeneity, invasion, and metastasis |
|
|
|
|
|
|
|
u |
|
|
Foveolin precursor FOV (gastrokine-1) |
Growth factor |
|
|
|
d |
|
|
|
|
|
|
Microtubule-associated protein, RP/EB family, member 1 |
Regulation of cell cycle, cell proliferation, microtubule-binding, APC protein C-terminus binding |
|
|
|
|
u |
|
|
|
|
|
Mitotic checkpoint protein isoform MAD1a |
Cell proliferation, mitotic spindle checkpoint, centrosome |
|
|
|
|
d |
|
|
|
|
|
Prohibitin |
Inhibits cellular proliferation |
|
|
u |
|
|
|
|
|
|
|
Proteasome activator PA28 b-chain |
Immunoproteasome assembly and antigen processing |
|
u |
|
|
|
|
|
|
|
|
SEPT 2 protein |
Cytokinesis, mitosis |
u |
|
|
|
|
|
|
|
|
|
S-phase kinase-associated protein 1A |
Mediates the ubiquitination of proteins involved in cell cycle progression, signal transduction, and transcription |
|
|
|
|
|
|
|
d |
|
|
T-complex protein beta |
Related to p53 and activates DNA damage checkpoints |
|
|
|
|
|
|
|
d |
|
|
Tumor RMS cell line RD specific product (CYR61) |
Insulin-like growth factor binding, extracellular |
|
|
|
|
d |
|
|
|
|
|
Cell Migration and Metastasis |
Annexin I |
Regulates cell proliferation, promotes membrane fusion, calcium-dependent phospholipid binding, inhibition of phospholipase A2 |
d |
|
|
|
|
|
|
d |
u |
|
Catechol O-methyltransferase |
Cancer progression and lymph node metastasis |
|
|
|
|
|
|
|
u |
|
|
Galectin-1 |
Promotes cancer cell invasion and metastasis |
|
|
|
|
|
|
|
u |
|
|
High mobility group protein 1 |
Interacts with transcription factors and regulates transcription related to tumor growth and invasion |
|
|
|
|
|
|
|
u |
|
|
Laminin γ-1 chain precursor |
Induces collagenase IV, matrix metalloproteinase (MMP-9) |
|
|
|
|
|
|
u |
|
|
|
Platelet-derived endothelial cell growth factor |
Angiogenesis |
|
u |
|
|
|
|
|
|
|
|
S100 calcium-binding protein A11 |
Calcium-binding, actin filament bundle formation, and cell motility |
|
|
|
|
|
|
u |
u |
|
|
|
|
Tropomyosin (1: isoform; 2:3/4) |
Stabilizes and binds actin filaments |
u |
|
u1 |
|
|
|
|
d2 |
|
|
Vimentin |
Intermediate filament in mesenchymal cells related to migration |
|
|
|
|
|
|
|
u |
|
|
Cell Structure and Motility |
ACTG1 protein |
Cytoskeleton |
u |
|
|
|
|
|
|
|
|
|
Actin alpha2 |
Structural protein |
u |
|
|
|
|
|
|
|
|
|
Alpha-actinin |
Anchors actin to intracellular structures |
|
|
|
|
|
|
|
u |
|
|
Cytokeratin 8 |
Intermediate filaments |
|
|
|
u |
|
|
|
|
|
|
Cytokeratin 20 |
Intermediate filaments |
|
|
|
d |
|
|
|
|
|
|
Cytoskeletal 5 |
Epidermis development, cytoskeleton organization and biogenesis, cellular morphogenesis |
|
|
|
|
|
|
|
u |
|
|
Cytoskeletal 17 |
Marker of basal cell differentiation |
|
|
|
|
|
|
|
d |
|
|
Tubulin alpha 6 |
Microtubules |
|
u |
|
|
|
|
|
|
|
|
WDR1 protein |
Induces disassembly of actin filaments |
|
u |
|
|
|
|
|
|
|
|
DNA Repair |
Uracil DNA glycosylase |
Excise uracil residues from the DNA |
|
u |
|
|
|
|
|
|
|
|
Manganese superoxide dismutase (MnSOD) |
Protection of DNA from oxidative damage |
|
d |
|
u |
|
|
|
|
|
|
Immunity Defenses |
α defensin |
Microbicidal peptides |
|
|
|
|
|
u |
|
|
|
|
FK506-binding protein 4 (FKBP4) |
Immunoregulation, protein folding/trafficking, binds FK506/rapamycin |
|
|
|
|
|
|
u |
|
|
|
MHC class I antigen |
Inhibits evasion of the immune system and enhances tumor growth |
|
|
|
|
|
|
|
d |
|
|
Oncoprotein |
18-kDa Antrum mucosa protein (AMP-18) |
Human stomach specific, epithelial cell mitogen |
|
|
|
d |
|
|
|
|
|
|
Signal Transduction |
Actin filament-binding protein (frabin) |
Signal transduction |
|
|
|
|
|
|
|
|
|
u |
Catenin, 120ctn |
Interacts with cadherin to regulate cell adhesion properties |
|
|
|
|
|
|
|
u |
|
|
Catenin, alpha-1 |
Actin crosslinking at adherens junctions |
|
|
|
|
|
|
|
d |
|
|
Catenin, beta |
Modulates of cytoskeletal dynamics and cell proliferation |
|
|
|
|
|
|
|
u |
|
|
Chloride intracellular channel protein1 (CLIC1) |
Signal transduction, Ion homeostasis, cell volume regulation, transepithelial transport. |
|
u |
|
|
|
|
|
|
|
d |
Eukaryotic translation initiation factor 5A |
Signal transduction |
|
|
|
|
|
|
|
|
u |
|
Integrin alpha 6/beta 4 |
Cell-matrix adhesion |
|
|
|
|
|
|
|
u |
|
|
N-myc downstream regulated gene 1 |
Signal transduction |
|
|
|
|
|
|
|
|
d |
|
Peroxiredoxin 5 |
High antioxidant efficiency to effect cell differentiation and apoptosis |
|
|
|
|
|
|
|
u |
|
|
Raf kinase inhibitor protein |
Suppresses metastasis, angiogenesis, and vascular invasion |
|
|
|
|
|
|
|
d |
|
|
Ras GTPase-activating-like protein IQGAP1 |
Actin cytoskeleton assembly and E-cadherin-mediated cell adhesion |
|
|
|
|
|
|
|
d |
|
|
Rho-related GTP-binding protein RhoG |
Small GTPase-mediated signal transduction, positive regulation of cell proliferation, actin cytoskeleton organization |
|
|
|
|
|
|
|
d |
|
|
RMD5 homolog B (RMND5B) |
Signal transduction |
|
|
|
|
|
|
|
|
|
d |
Tyrosine 3/tryptophan 5-monooxygenase activation protein |
Activates protein kinase C and Ca2+/calmodulin-dependent protein kinase II |
|
u |
|
|
|
|
|
|
|
|
Vinculin |
Mediates the interactions between integrins and the actin |
|
|
|
|
|
|
|
d |
|
|
Transcription and Translation |
Elongation factor 2 |
Transcription & translation |
|
|
|
|
|
|
|
|
d |
|
HnRNP A2 |
RNA trafficking, telomere maintenance |
u |
|
|
|
|
|
|
|
|
|
HnRNP-E1 |
RNA-binding protein, RNA trafficking |
d |
|
|
|
|
|
|
|
|
|
MADS box transcription enhancer factor 2, polypeptide C |
Regulation of transcription, transcription from Pol II promoter, nucleus |
|
|
|
u |
|
|
|
|
|
|
Nucleophosmin |
Ribosome assembly and transport |
|
|
|
|
|
|
|
|
u |
|
Translation elongation factor EF-Tu (EF-Tu) |
Translation factor, cell growth, chaperone activity |
|
u |
|
|
|
|
|
|
|
|