Molecular Biomarkers of Gastric Adenocarcinoma
John J. Liang
Dongfeng Tan
INTRODUCTION
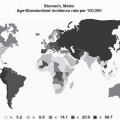
Gastric cancer is a global health problem with a high rate of incidence and mortality. It is the second most common cause of death from cancer, with an estimated 700,000 deaths each year worldwide.1 Its incidence varies widely from country to country (see Chapter 2). The Laurén classification is the most widely used histologic classification of gastric cancer. In 1965, Laurén2 classified gastric carcinoma into the intestinal and diffuse subtypes. The intestinal type is strongly associated with Helicobacter pylori infection and commonly arises within a background of chronic gastritis, glandular atrophy, and intestinal metaplasia (see Chapter 3). In contrast, diffuse-type adenocarcinomas often present with diffuse thickening of the stomach wall rather than a mass.
Although gastric carcinoma has shown a decline in frequency in the United States and Western Europe,1 it is still very common in developing countries. The prognosis for gastric carcinoma traditionally has been poor, mainly due to an advanced stage at diagnosis, with a 5-year survival rate of <5%. In contrast, very early gastric cancer is a curable disease, and the 5-year survival rate of surgically treated early gastric cancer approaches 90% to 95%. Therefore, it is crucial to identify clinically useful molecular markers that can detect gastric cancer at an early stage. Recent studies have demonstrated that many factors are involved in the progression of gastric neoplasia, including genetic and environmental factors (H. pylori infection, diet, and smoking) and predisposing lesions, among others. Adenocarcinoma of the cardia and Barrett-associated adenocarcinomas of the esophagus share similar backgrounds, temporal trends, molecular profiles, and behavioral patterns, which have recently been reviewed.4 In this chapter, we focus on molecular genetics and potential diagnostic molecular biomarkers in noncardia/distal gastric carcinoma.
MOLECULAR MODELS OF GASTRIC ADENOCARCINOMA
The intestinal type of gastric carcinoma that predominates in geographical regions with high incidence is more frequently observed in older patients (>65 years old) and develops from precursor lesions associated with intestinal metaplasia and dysplasia. In contrast, the incidence of the diffuse type is relatively constant, and the tumors have no identifiable precursor lesions. Diffuse cancer occurs more commonly in young patients, is multifocal, and is rarely associated with intestinal metaplasia. Recent studies have demonstrated that many genetic alterations are involved in the development and progression of gastric cancer.3 It appears that there are different genetic pathways leading to intestinal- and diffuse-type gastric adenocarcinoma (GAC). In 1992, Correa5 postulated a multistep and multifactorial model of intestinal-type GAC, involving development of chronic gastritis, atrophy, intestinal metaplasia, and dysplasia and eventually GAC. A number
of molecular abnormalities have been identified, including microsatellite instability (MSI), inactivation of tumor suppressor genes, activation of oncogenes, and reactivation of telomerase.3 On the basis of the findings from prophylactic gastrectomy specimens, Carneiro et al.6 proposed a model for the development of diffuse-type GAC in E-cadherin (CDH1) mutation carriers, involving development of nonatrophic gastritis, in situ signet ring cell carcinoma, pagetoid spread of signet ring cells, and invasive GAC. It is believed that the histology of gastric mucosa is normal in CDH1 mutation carriers until the second CDH1 allele is inactivated by multiple factors.
of molecular abnormalities have been identified, including microsatellite instability (MSI), inactivation of tumor suppressor genes, activation of oncogenes, and reactivation of telomerase.3 On the basis of the findings from prophylactic gastrectomy specimens, Carneiro et al.6 proposed a model for the development of diffuse-type GAC in E-cadherin (CDH1) mutation carriers, involving development of nonatrophic gastritis, in situ signet ring cell carcinoma, pagetoid spread of signet ring cells, and invasive GAC. It is believed that the histology of gastric mucosa is normal in CDH1 mutation carriers until the second CDH1 allele is inactivated by multiple factors.
PROMISING MARKERS IN EVALUATION OF PREMALIGNANT LESIONS
Intestinal-type GAC typically arises in the setting of chronic gastritis. The progression from atrophic gastritis, intestinal metaplasia, and dysplasia to GAC is dependent on continued chronic inflammation.7 Several genetic changes with p16 methylation in intestinal metaplasia have been shown in association with H. pylori infection in gastric precancerous lesions.8 Murata-Kamiya et al. found that E-cadherin expression in H. pylori-infected gastric mucosa was decreased compared with that in the control group. The cytoplasmic and nuclear accumulation of β-catenin induced by interaction of CagA with CDH1 has been implicated in the development of intestinal metaplasia.9 Zavros et al.10 demonstrated that down-regulation of Sonic hedgehog (Shh) by H. pylori leads to the disruption of glandular structure and the gain of a more intestinal phenotype through up-regulation of intestine-related genes, such as CDX2, MUC2, and villin. Gastric intestinal metaplasia can be induced through ectopically expressed Cdx2 in mouse models.11 Studies by Wang and coworkers showed that overexpression of Shh was significantly correlated with premalignant lesions and carcinoma.12 Shh expression was associated with clinical stage, direct tumor invasion, and differentiation of tumor cells.12 These studies suggest that Shh expression is involved in gastric carcinogenesis. The genetic and epigenetic changes in intestinal metaplasia and premalignant lesions could be used as surrogate markers for the evaluation of GAC risk and clinical malignant potential.
MSI, HYPERMETHYLATION, AND TARGET GENES
MSI is a marker of the presence of replication errors in simple repetitive microsatellite sequences due to DNA mismatch repair (MMR) deficiency. MMR proteins include the MutS proteins hMSH2, hMSH3, and hMSH6 and the MutL proteins hMLH1, hPMS1, hPMS2, and hMLH3.13 Tumors are classified as MSI-high (MSI-H) when at least 30% of the microsatellite markers examined show MSI, whereas tumors with <30% of the markers showing instability are described as MSI-low (MSI-L). Those with no instability at any loci examined are considered stable (MSS). Several studies have reported that MSI is present in both familial and sporadic GAC and that about 20% to 30% of GACs have MSI.14 MSI occurs at the stage of chronic gastritis, several years before a diagnosis of GAC.15 Therefore, MSI analysis is promising as a valuable marker of the risk of progression to cancer.
MSI analysis of colonic carcinomas showed that MSI-H tumors were associated significantly with inactivation of hMLH1.16 Genome-wide hypomethylation and selective hypermethylation of DNA sequences have been recognized as hallmarks of human cancers.17 Recent studies have demonstrated that hypermethylation of gene promoters occurs along the pathway from chronic gastritis, intestinal metaplasia, and adenoma to GAC.18 Loss of expression of hMLH1 associated with promoter hypermethylation is the underlying mechanism causing MSI in gastric adenomas and early gastric cancer, similar to findings for advanced gastric cancers. Baek and co-workers found, by using immunohistochemistry, that about 87% to 88% of MSI-positive gastric adenoma and GAC show absent or decreased hMLH1 expression and that all of these tumors have methylation of the hMLH1 gene promoter.19a Carvalho et al.19b reported that hypermethylation was
detected in about 30% of COX2 and hMLH1 and in about 50% to 60% of CDH1 and MGMT gene promoters in sporadic GAC. These results indicate that inactivation of different gene promoters by hypermethylation plays an important role in carcinogenesis of GAC. MSI-H GAC is associated with antral location, intestinal-type differentiation, relatively old patients, and a better prognosis.20 In a large series of GAC studies, MSI was detected in 16% of cases and was significantly associated with long survival. Therefore, MSI analysis has clinical prognostic utility (see Chapter 17).
detected in about 30% of COX2 and hMLH1 and in about 50% to 60% of CDH1 and MGMT gene promoters in sporadic GAC. These results indicate that inactivation of different gene promoters by hypermethylation plays an important role in carcinogenesis of GAC. MSI-H GAC is associated with antral location, intestinal-type differentiation, relatively old patients, and a better prognosis.20 In a large series of GAC studies, MSI was detected in 16% of cases and was significantly associated with long survival. Therefore, MSI analysis has clinical prognostic utility (see Chapter 17).
There are hundreds of thousands of mutations in MSI carcinomas due to genetic instability. Approximately 70% of TGFβRII genes, 25% of IGFIIR genes, and 30% of BAX genes were found to be mutated in GAC.21 Transforming growth factor-β (TGF-β) family members are involved in the regulation of cell proliferation, differentiation, motility, and apoptosis. TGF-β action starts after its secretion in the extracellular matrix as a latent protein complex. TGF-β signaling is initiated by the binding of TGF-β ligands to type II TGF-β receptors (TGFβRII). Once bound to TGF-β, TGFβRII recruits and phosphorylates the type I TGF-β receptor TGFβRI, which stimulates TGFβRI protein kinase activity.
The high frequency of TGFβRII gene mutations suggests that the alteration of TGFβRII gene mutations occurs earlier than for IGFIIR, BAX, and TCF-4 genes in gastric carcinogenesis and that it is associated with intestinal-type GAC.22 There are two types of TGF-β serine/threonine kinase receptors: type I and type II, both necessary for downstream signal transduction. Mutations of the type II receptors can interrupt the signal transduction passway, resulting in growth stimulation rather than growth restriction. In one study, frame-shift mutations of TGFβRII were detected in 38% of MSI-H adenomas, but no frame-shift mutations of hMSH6 and IGFIIR were observed.23 Kim et al.24 reported that MSI-positive adenomas coexisting with cancer showed a higher mutation rate of the TGFβRII gene than those not coexisting with cancer (88% compared with 40%), suggesting that gastric adenoma with TGFβRII gene mutation may be more likely to transform into carcinoma.
IGFIIR is a multifunctional protein with important functions in lysosomal enzyme trafficking, endocytosis, and activation of TGF-β.25 Mutations of IGFIIR poly(G) 8 were detected in about 30% of MSI gastric tumors and were significantly associated with low frequency of lymph node metastasis and serosal invasion.26 These findings raise the possibility that the IGFIIR mutation is a prognostic marker for GAC.
INACTIVATION OF TUMOR SUPPRESSOR GENES
APC and p53
Up to 60% of intestinal-type gastric tumors and approximately 25% of gastric adenomas harbor mutation and/or loss of heterozygosity (LOH) of APC, but such are rare in diffuse-type GAC.27 Consistently, β-catenin mutations have also been detected in intestinal-type gastric tumors but are absent from diffuse-type tumors.28 Furthermore, Abraham et al.29




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree