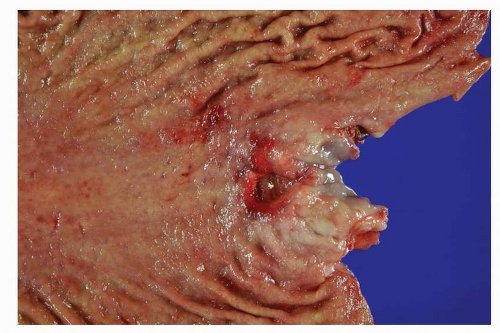
at the GEJ. However, there does not seem to be appropriate justification for separating GEJ tumors from adenocarcinomas of the distal esophagus, especially from a pathophysiologic standpoint. More recently, the International Gastric Cancer Association has endorsed another scheme for classifying these tumors.7 Type I tumors are defined as those in the distal esophagus, type II are tumors of the true gastric cardia, and type III are those in the gastric mucosa of the subcardia (Fig. 5-1). Finally, the recently published 7th edition of the TNM classification by the American Joint Committee on Cancer proposes a novel classification scheme. Cancers whose epicenter is in the lower thoracic esophagus or EGJ, or within the proximal 5 cm of the stomach (i.e., cardia) and extending into the EGJ or esophagus are to be stage grouped similar to adenocarcinoma of the esophagus, while cancers with an epicenter in the stomach >5 cm distal to the EGJ, as well as those within 5 cm of the EGJ but not extending into the EGJ or esophagus, are to be classified and staged as gastric cancer.8
late stages.13 With regard to phenotypic differentiation, differentiated proximal gastric cancers are also more likely to have a gastric immunophenotype. It has been shown that the incidence of human gastric mucin (HGM) expression is significantly higher in proximal tumors at an early stage, whereas in advanced cancers, MUC2 expression is lower than in distal tumors.
metastases.20,21 These lesions are not readily identified by endoscopic examination. In most cases, the neoplastic foci spare the antral mucosa, and there is a marked increase in density and size within the transition zone between the antrum and the body21 There is wide variation in the number of foci both within and between HDGC kindred, from an average over 100 to <20.20,22, 23 and 24 No correlation between patient age and number of foci has been observed. Phenotypic differentiation and proliferation studies support that the neoplasms are developing from the upper isthmus of the neck region of the gastric gland.25
Table 5-1 Hereditary Gastric Cancer Syndromes | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Population-based differences may be seen as well. For example, following the Lauren classification, in high-risk regions of the world, intestinal-type adenocarcinoma is more common than the diffuse type that is relatively more common in low-risk areas. A shift in the proportion of subtypes has also been noted, with the incidence of gland-forming adenocarcinoma decreasing mainly in young patients, and an increasing rate of diffuse-type carcinoma localized to the proximal stomach. Finally, in young individuals, a higher proportion of tumors are of the diffuse type that affects females more often than males.35,36
Table 5-2 Gastric Adenocarcinoma Classification Systems | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
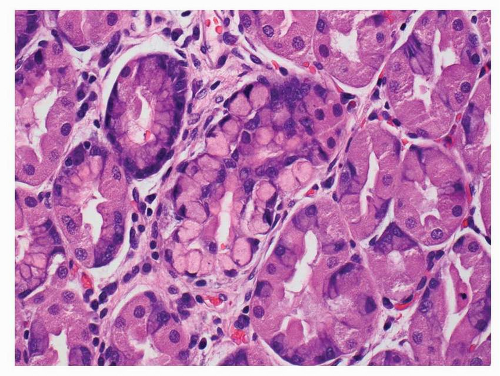
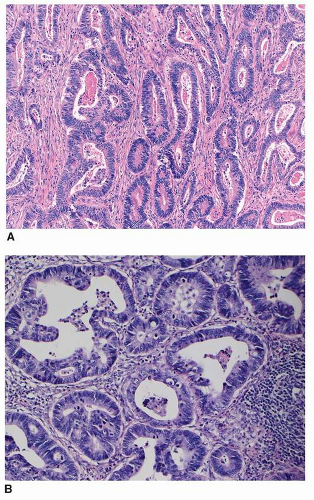
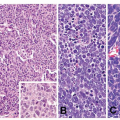
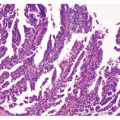
and inflammatory debris can be noted intraluminally. Individual neoplastic cells can be either columnar or cuboidal, or with various degrees of atypia and mitoses. In some well-differentiated cases, the neoplasm can mimic intestinal metaplasia perfectly37 A poorly differentiated variant is sometimes called solid carcinoma (Fig. 5-4). Tubular adenocarcinoma is the most common histologic type of early gastric cancers, representing 52% of the cases.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree