Patients at increased risk of breast cancer have a range of treatment options available to decrease their risk of breast cancer development. The absolute risk reduction by any of these strategies is dependent on the individual woman’s actual risk of breast cancer development. Statistical models for risk stratification, as well as the availability of genetic testing, enable women and their physicians to evaluate the risk of breast cancer. A number of risk-reducing treatment options exist for these women, and they vary in efficacy. Options include frequent surveillance with clinical examination and imaging, chemoprevention, prophylactic salpingo- oophorectomy (PSO), and prophylactic mastectomy (PM). The individuals most likely to benefit from bilateral PM are those with the highest risk—BRCA carriers and those with a strong family history of breast cancer. Women with a personal history of breast cancer are also at higher risk for a second primary breast cancer in the contralateral breast and may choose to pursue contralateral prophylactic mastectomy (CPM) to decrease this risk as well as for cosmetic and psychological reasons. As a preventive measure PM remains controversial. There are no randomized controlled trials (and likely will not be in the future) to substantiate the potential benefit or harm associated with PM. Since PM is an irreversible procedure, providers and patients must understand its consequences, benefits, limitations, and available alternatives. This chapter discusses which patients may consider prophylactic surgery and summarizes data on the efficacy of PM for the prevention of breast cancer and its effect on survival.
The absolute risk reduction afforded by PM is greatest in the women at highest risk of breast cancer development. The average lifetime risk of breast cancer for women in the United States is 12.7% with a lifespan of 85 years. This risk is greatest after the sixth decade of life.1 Most women, however, tend to overestimate their risk of breast cancer.2 It is important that women have realistic risk estimates in order to help them make informed decisions regarding risk reduction. For women without a personal or family history of breast cancer, the Gail model is the most commonly used tool to predict risk of breast cancer in women, and is viewed 20,000 to 30,000 times per month.3 The Gail model is accurate for predicting breast cancer incidence in populations of women; however, its major limitation in terms of clinical use is its poor accuracy when used for risk prediction in individuals.4 Also, the Gail model does not apply to women with a history of lobular carcinoma in situ (LCIS) or ductal carcinoma in situ (DCIS) and has been shown to underpredict risk for women with atypical hyperplasia.5
Factors associated with a generalized increased risk of breast cancer above the population average include LCIS, atypical hyperplasia, and increased breast density. Atypical ductal or lobular hyperplasia found at breast biopsy (preferably confirmed by a breast pathologist) is associated with a 4-fold increased relative risk compared to the general population,6 with increasing foci of atypia correlating with greater risk.7 Increasing mammographic breast density is associated with increasing risk of breast cancer8 as well as identification of interval cancers.9 Thus the occasional woman with combined features of very dense breast tissue, high-risk histology, and multiple prior biopsies may request PM due to concerns about inadequate detection with the use of standard imaging modalities.
Individuals with deleterious mutations of BRCA1 or BRCA2 genes are a special subset of women with highly elevated risks of breast cancer development. The average cumulative risk of breast cancer in BRCA1 and BRCA2 mutation carriers by age 70 years is 65% and 45%, respectively (Fig. 11-1).10 These individuals are also at a higher risk of ovarian cancer, with lifetime risks of 40% and 20%, respectively, for BRCA1 and BRCA2 carriers. Some women with a strong family history may choose not to undergo BRCA testing. Additionally, some women with a strong family history may test negative for deleterious mutations, or a variant of unknown significance may be found. Claus tables can be used to provide estimates of breast cancer risk for individuals with a strong family history.11 A negative test for a BRCA mutation is only reassuring in the presence of a positive test within the family; otherwise the possibility of an unidentified mutation remains and the risk is still dependent on the family history.
Figure 11-1
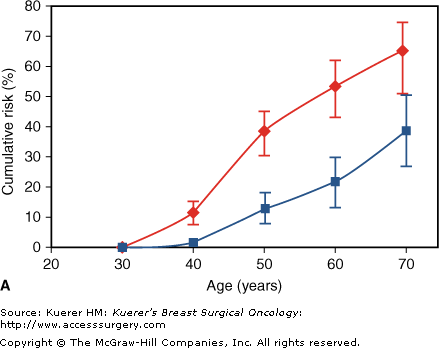
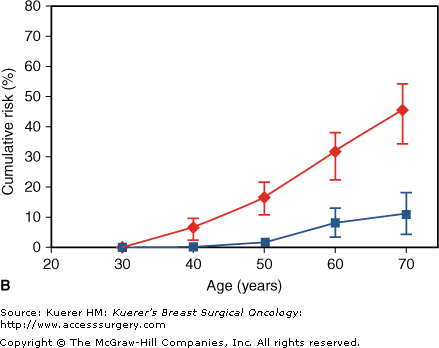
Cumulative risk of breast (♦) and ovarian (▪) cancer in (A) BRCA1 mutation carriers and (B) BRCA2 mutation carriers. (From Antoniou A, Pharoah PD, Narod S, et al. Average risk of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130, with permission.)
A personal history of breast cancer is a long-established risk factor for development of a new primary breast cancer,12 logical since the remainder of that breast and the contralateral breast have been exposed to the same genetic, environmental, and hormonal factors that led to the first breast cancer. Women with stage I and II breast cancer have a contralateral breast cancer risk of approximately 1% per year, with a cumulative risk of 17% at 20 years after first breast cancer diagnosis.13,14 The risk of a second breast cancer is much higher in women with a strong family history of breast cancer—up to 35% by 16 years after the initial diagnosis of breast cancer.15 The presence of a BRCA1 and BRCA2 mutation elevates the risk of contralateral breast cancer even higher, to 12% to 31% at 5 years after the primary cancer diagnosis.16-18 In a longer-term study of BRCA carriers who underwent breast conservation, the risk of contralateral breast cancer was 39% at 15 years.19
In addition to family history,13,20 other factors associated with an increased risk of contralateral breast cancer include multicentric primary breast cancer,21 history of radiation therapy for the first breast cancer,22 lobular neoplasia,23,24 and additional ipsilateral high-risk pathology.25 Patients newly diagnosed with breast cancer who present with these features are at higher risk for developing cancer in the contralateral breast, and CPM is more justifiable in these patients. The relationship of age at diagnosis and risk of contralateral breast cancer is unclear, with two studies demonstrating increased risk in young women23,26 and one study demonstrating the opposite.25
The women who elect bilateral prophylactic mastectomy (BPM) and those who choose to undergo contralateral prophylactic mastectomy (CPM) are different in their characteristics, history of breast cancer, and goals for risk-reduction surgery. These scenarios will be discussed separately.
Several studies confirm that BPM reduces the incidence of breast cancer in BRCA carriers. Meijers-Heijboer and associates27 conducted a prospective study of 139 BRCA1 or BRCA2 mutation carriers. No breast cancers developed in the 76 women who underwent BPM compared to 8 breast cancers in the 63 women who pursued close surveillance with a mean follow-up of 3.0 years (p = 0.003). However, the risk-reduction effect of BPM could not be isolated from the effect of PSO, which also reduces breast cancer risk (see “Risk-Reduction Alternatives to PM” later in the chapter), since premenopausal PSO was more common in the BPM group (58%) compared to the surveillance group (38%).
Similarly, Hartmann and associates at the Mayo Clinic reported on 26 women with BRCA mutations who underwent BPM. At a median follow-up of 13.4 years no breast cancers developed.28 The relative risk reduction attributed to BPM was estimated as 85% to 100% using various statistical models. In the more recent PROSE study of Rebbeck and colleagues, 105 BRCA carriers who underwent BPM were followed prospectively and compared to 378 matched BRCA controls without BPM.29 Breast cancer developed in 2 women who underwent BPM (1.9%) compared to 184 breast cancers (48.7%) in the group who did not undergo BPM, with mean follow-up of 6.4 years. In this study, cases and controls were matched based on PSO. The relative risk reduction for breast cancer was 95% in women with PSO and 90% in those with intact ovaries. Taken together, these studies confirm a 90% to 95% relative risk reduction in breast cancer after BPM in BRCA carriers.
Some women without a known BRCA mutation, but who have other risk factors, consider BPM. For these women there are several studies providing evidence on the efficacy of BPM for high-risk women regardless of BRCA status. Hartmann and coworkers reported on a cohort of 639 high-risk women (based on a positive family history of breast cancer) who underwent bilateral subcutaneous mastectomy from 1960 to 1993 (90% with preservation of the nipple–areolar complex, 10% with removal of the nipple–areolar complex).30 This cohort was divided into high- and moderate-risk groups based on the strength of family history. A control group of the patients’ female siblings who did not undergo BPM was also followed.30 Breast cancer incidence was reduced 90% to 94% in the high-risk group and 90% in the moderate-risk group at a median follow-up of 14 years. Breast cancer incidence was not significantly different between those women with preservation of the nipple–areolar complex and those where it was removed.
Geiger and associates evaluated the efficacy of BPM in community practices.31 In this retrospective population-based study, BPM was found to reduce the risk of breast cancer by 95%. However, the absolute risk of breast cancer in the whole population was low, with 0.4% incidence in the BPM group compared to 4% in the control group. Borgen and colleagues also reported effective risk reduction from BPM, with less than 1% incidence of breast cancer at 14.8 years mean follow-up in 370 women who underwent BPM.32
Although BPM does provide a dramatic decrease in breast cancer incidence, it is important that physicians and patients realize that breast cancers can still develop despite risk-reduction surgery. There are multiple case reports of breast cancer after BPM, with intervals from risk-reduction surgery to the diagnosis of breast cancer ranging from 3 to 42 years.33-38 When counseling women regarding PM, the persistent long-term possibility of developing breast cancer should be discussed.
There are no data confirming an overall survival benefit in patients undergoing BPM for cancer prevention compared to similar-risk individuals who do not. While the studies on BPM in BRCA carriers clearly show reductions in breast cancer occurrence, there is no prospective clinical evidence yet that confirms a statistically significant overall survival advantage attributable to this procedure. Among women with a family history but unknown BRCA status, the study of Hartmann and colleagues demonstrated reductions in disease-specific mortality after BPM, with an 81% to 94% reduction in mortality from breast cancer for the high-risk group and 100% reduction in breast cancer mortality for the moderate-risk group.30
It would be expected that successful prevention of breast cancer would translate into a survival benefit, especially for patients at a high lifetime risk of breast cancer. However, with improvements in detection and treatment of breast cancer it becomes questionable whether breast cancer prevention actually translates into improved survival. Schrag and colleagues used theoretical modeling to estimate survival gains from risk-reduction surgery for BRCA mutation carriers.39 They calculated that on average a 30-year-old BRCA1 or BRCA2 mutation carrier would gain 2.9 to 5.3 years of life expectancy from PM and from 0.3 to 1.7 years from PSO. Another similar analysis also reported survival gains of 3.5 years for PM and 4.9 years with both BPM and PSO.40 In both studies, gains in life expectancy declined with age and were minimal for women over 60 years of age (Fig. 11-2). The preponderance of evidence from the prospective clinical cohorts and theoretical modeling described suggest that in BRCA carriers, BPM reduces the incidence of breast cancer and in younger women likely improves longevity.
Figure 11-2
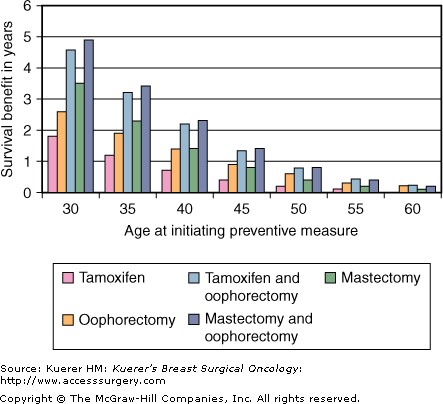
Effects of timing on benefits of preventive measures upon survival. (From Grann VR, Jacobson JS, Thomason D, et al. Effect of prevention strategies on survival and quality-adjusted survival of women with BRCA1/2 mutations: an updated decision analysis. J Clin Oncol. 2002;20:2520-2529, with permission.)
Surgical complication rates after BPM range from 30% to 64% and complications are more common after immediate reconstruction.41-43 The most common complications are pain (35%), infection (17%), and seroma formation (17%).43 Unanticipated reoperations after BPM with immediate reconstruction are common. In one report,44 second operations were required in 49% of patients for the following reasons: implant-related issues (46%), implant removal (37%), aesthetic concerns (32%), and immediate postoperative complications (22%).
Psychosocial outcomes such as satisfaction with the decision, cosmesis, psychological well-being, and issues concerning body image and sexuality have been evaluated in patients choosing BPM. In one study, approximately 5% of women report they were dissatisfied with their decision to undergo BPM,32 and this was more common among women reporting physician’s advice as the primary reason for BPM.32,45 Another survey study of 106 women after BPM and 62 high-risk women without BPM showed that 84% of patients choosing BPM were satisfied with their decision, and the quality of life was not different between the 2 groups.46 Dissatisfaction with cosmetic outcomes has been reported in 16% to 40% of patients, mostly pertaining to dissatisfaction with reconstruction,32,45,47,48 but in one study was also related to a perceived lack of information on expected outcomes.48 Cancer-related anxiety, body image concerns, and sexuality are all associated with satisfaction or regret regarding the surgery itself.32,45,49 On the other hand, women who forego reconstruction following BPM have high levels of satisfaction.45 Overall, the quality of life among women undergoing BPM appears slightly better than the general population based on Quality of Life Index (QLI) scores.50 These unexpected results likely reflect the characteristics of women who choose to undergo this preventive surgery rather than an effect of the surgical procedure. Another study affirms that survey studies of satisfaction after PM need to include open-ended questions, because closed-ended question surveys may overestimate satisfaction.51
Women presenting with an index breast cancer in one breast are at an increased risk of contralateral breast cancer. Similar to BPM, studies have shown a decrease in the incidence of breast cancer after CPM, both in BRCA carriers and non-BRCA carriers.
Van Sprundel and associates reported on 148 BRCA1/2 carriers treated for unilateral invasive breast cancer stages I to IIIA.52 Seventy-nine women underwent CPM and 69 women underwent surveillance. One contralateral breast cancer occurred in the CPM group compared to 6 in the surveillance group (p < 0.001). This 91% risk reduction in contralateral breast cancer was independent of PSO.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree