(1)
Research Oncology, Guy’s Hospital, London, United Kingdom
Abstract
When properly matched for stage age and co-morbidity the prognosis of MBC and FBC is similar. Because MBC patients are older and often have more co-morbidity than FBC cases, overall survival may be worse but cancer-specific survival is similar. Ethnic differences in outcome are cultural and economic but not genetic. Hierarchical clustering indicates that best survival is exhibited by luminal B1.1 group and the worst by luminal A. BRCA2 mutation carriers have a worse prognosis than that of sporadic cases. Oncotype Dx™ may be useful in determining recurrence risk and selected of appropriate adjuvant systemic therapy. Over-expression of both HIF-1a and plasminogen activator inhibitor 1 (PAI-1) have been shown on separate multivariate analyses to be significant prognostic variables. In contrast MBC tumours over-expressing TAZ/CTGF and YAP/CTGF carried a worse prognosis. Prognostic models developed for FBC cases can also be of value in MBC.
Prediction is very difficult, especially if it’s about the future. Nils Bohr
Males Versus Females
Considerable energy has been devoted to the question “Is male breast cancer inherently more aggressive than the female disease?” After all this frenetic activity it emerges that there is not an inherent disadvantage of being a male with breast cancer. The major problem was the lack of good matching, particularly for stage because of the male habit of presenting with more advanced cancers. The studies which have compared 5 year survival of MBC and FBC are summarised in Table 11.1.
Table 11.1
Comparative studies of overall survival in male and female breast cancer
Author | Country | MBC No | MBC 5y OS | FBC No | FBC 5y OS |
|---|---|---|---|---|---|
Mausner 1969 [1] | USA | Stage 1 34 Stage II 24 | 65% 43% | Stage I 442 Stage II 339 | 76% 48% |
Levi 1992 [2] | Switzerland | 39 | 75% | 4199 | 71% |
Willsher 1997 [3] | England | 41 | 55% | 123 | 65% |
Scott-Conner 1999 [4] | USA | Stage I 442 Stage II 536 | 78% 68% | Stage I 358 Stage II 411 | 85% 70% |
Giordano 2004 [5] | USA | Stage I 394 Stage II 516 | 76% 67% | Stage 1 80,657 Stage II 63.988 | 88% 75% |
El-Tamer 2004 [6] | USA | 53 | 74% | 53 | 74% |
Anan 2004 [7] | Japan | 14 | 92% | 140 | 86% |
Macdonald 2005 [8] | Canada | RT 34 No RT 26 | RT 939 No RT 3242 | ||
Nahleh 2007 [9] | USA | Stage I 138 Stage II 241 | 40% 40% | Stage I 745 Stage II 703 | 60% 54% |
Marchal 2009 [10] | France | 58 | 59% | 116 | 68% |
Anderson 2009 [11] | USA | 5496 | 1976–1985 79% 1986–1995 85% 1996–2005 90% | 835,803 | 1976–1985 75% 1986–1995 85% 1996–1905 90% |
Foerster 2011 [12] | Germany | 108 | 71% | 108 | 70% |
Nilsson 2011 [13] | Sweden | 99 | 54% | 396 | 80% |
Shaaban 2012 [14] | UK | 251 | 87% | 263 | 75% |
Chen 2013 [15] | China | 150 | 66% | 300 | 75% |
Kwong 2014 [16] | Hong Kong | 132 | 79% | 8118 | 78% |
Iorfida 2014 [17] | Italy | 99 | 89% | 198 | 92% |
Yu 2015 [18] | China | 91 | 80% | Postmeno 182 Premeno 182 | 80% 75% |
Yu 2015 [19] | Canada | 37 38 | Node –ve 95% Node +ve 79% | 580 733 | Node –ve 92% Node +ve 73% |
Choi 2016 [20] | Korea | 260 | 91% | 1300 | 93% |
Examining the breast cancer registry of the Philadelphia County Medical Society Mausner et al. reported that between 1951 and 1964, 9003 patients were recorded [1]. They took a 10% random sample of 830 FBC cases and compared their characteristics with 72 MBC cases. The males were significantly older with a mean age of 64, compared with 56 for the females. There was also a substantial increase in male delay being more than a year in one third of MBC and one fifth of FBC. There was a slightly worse 5-year observed survival for males with stage I and II disease, but no gender difference in relative 5-year survival. Using data from the Cancer Registry of the Swiss Canton of Vaud Levi et al. compared crude and relative survival rates for 39 MBC and 4.199 FBC cases [2]. The relative survival rates were 0.95 and 0.94 respectively. For males, relative survival was not significantly affected by age.
Wilsher et al. compared 41 MBC with 123 FBC, matched for age, tumour size, grade and nodal status [3]. The matching for the latter very important variable could not be achieved since the axillary nodal status was unknown for 23 (56%) of the MBCs. In this study there was a worse 5 year survival among the male cases. Scott-Conner et al. examined the National Cancer Data Base with 4755 MBC and 624,174 FBC diagnosed between 1985 and 1994 [4]. An attempt was made to select for each MBC an FBC case matched for age, ethnicity, economic status and tumour stage and this was successful in identifying 3627 matched pairs. Surgery involved mastectomy in 65% of males and 55% of females. Post-mastectomy radiotherapy was given to 29% of men and 11% of women. For patients with stages I/II 5 year survivals were similar but there was worse outcome for males with stage III and IV disease.
Giordano et al. interrogated the SEER register of breast cancer cases identified between 1973 and 1998 database were used [5]. There were 910 MBC and 144,645 FBC. In terms of adverse prognostic factors, males had a higher incidence of advanced stages and axillary nodal involvement. When matched for stage the relative survival was similar for males and females. Using the Columbia/ Presbyterian Medical Center database, El-Tamer et al. identified 53 MBC cases and compared their outcome with 53 FBC who had been matched for both age and date of diagnosis, together with stage and histology [6]. The 5-year overall survival for both males and females was 77%. When however the cancer-specific survival curves of the two gender groups were compared, at 5 years it was 81% for females and 90% for males and the respective figures after 10 years were 70% and 90%.
In a relatively small study from Japan, Anan et al. examined the 5-year OS of 14 MBC with that of 140 age and stage-matched FBC [7]. There was no significant difference in OS which was 92% for the males and 86% in the females. Disease-free and overall survival did not differ significantly between the sexes. Five-year disease-free survival was 77% for the men and 75% for the women. There was however an increased mortality in males from other causes probably reflecting the male preponderance of comorbidity.
Approaching the problem from a clinical oncological perspective, Macdonald et al. from the British Columbia Cancer Agency sought to determine the prognostic significance of post-mastectomy radiotherapy (PMRT) in MBC and FBC [8]. The study included all cases of invasive breast cancer between 1989 and 1998 and there were 60 MBC and 4181 FBC. PMRT was more likely to be given to those with larger cancers, involved margins, nodal involvement and male gender. Using the Veterans Affairs Central Cancer Registry (VACCR) Nahleh et al. examined the outcome for 612 MBC patients and 2413 FBC cases [9]. The males were on average 10 years older than the females (67 versus 57 P < .005). The median overall survival was 7 and 9.8 years respectively (P < .005). For node-negative patients, the median survival rates were 6.1 and 14.6 years (P < .005). In contrast, the overall survival of node positive showed no gender difference.
Marchal et al. conducted a case-control study with 58 MBC and 116 FBC, matching for age, stage and year of diagnosis [10]. For MBC the 5 and 10-year OS was 59% and 34% compared with 68% and 52% in females. Although males had an increased likelihood of dying from other diseases, the disease-specific survival of both genders was similar. Anderson et al. analysed SEER data from 1973 to 2005 which included 5494 MBC and 835,805 FBC cases [11]. When cases diagnosed between 1976–1985 were compared with those treated from 1996–2005, after adjusting for age, stage, and grade there was a decline in cause-specific mortality of 28% in males and 42% in females.
In a matched pair analysis of German MBC and FBC, 108 cases were compared after controlling for year of diagnosis, age, stage, nodes, grade, and ER/PR/HER2 status [12]. The 5-year OS was 71% for males and 70% in females. Nilsson et al. carried out another case-control study with 99 MBC cases and 396 FBC controls, matched for age and year of diagnosis [13]. There was inferior OS in males (41% versus. 55%) and also worse relative survival (74% versus 88%, p = 0.015)
Shaaban et al. performed a large scale biomarker study and compared survival of 251 MBC and 263 FBC with matching for age, grade, and nodal status [14]. There were no significant gender differences in OS at 10 years. Chen et al. compared 150 MBC patients with 300 stage-matched FBC cases [15]. The mean ages at diagnosis were 59 years for males and 57 for females. The 10-year overall survival rates were 54% and 69% respectively (P = 0.002).
Kwong et al. reported different findings in a Chinese case-control study of 132 MBC and 396 FBC cases [16]. Mean ages at diagnosis were 65 (male) and 53 (female). Because of lack of matching MBC were of lower grade, stage, size, and more likely to be ER+ve. The 5 year OS for males was 79% and 78% for FBC. Males were however more likely to die of other causes. Males had better disease-specific mortality rates at all ages (p < 0.01).
Reporting from the European Institute of Oncology Iorfida et al. examined the outcomes of 99 MBC cases and 198 FBC, matched for age, stage, grade, year of surgery, and ER/HER2/Ki67 status [17]. The 10-year OS was 71% (MBC) and 84% (FBC). There was a significantly increased risk of non-cancer related deaths among the males but the 10-year disease-specific survival of the two gender groups was similar (82% versus 88%). In another Chinese case control study, Yu et al. compared the survival of 91 operable MBC cases with 182 pre/perimenopausal FBC and another 182 postmenopausal cases who had been treated at Zhejiang Provincial Cancer Hospital [18]. After a median follow-up of 112 months, the 10-year OS rates were 79% for MBC, 79% in the pre-perimenopausal FBC and 88% in postmenopausal FBC cases. It was concluded that MBC had a similar survival to premenopausal FBC but a worse outcome compared with the post-menopausal.
FBC. However, the DFS and OS values of MBC were similar to those of pre/peri-menopausal FBC and were worse than were those of post-menopausal FBC.
A Canadian study compared 75 operable MBC cases with 1313 FBC [19]. They used propensity score matching (PSM) in an attempt to estimate the effect of a treatment after accounting for the covariates that predict receiving the treatment and reduce the inherent biases in non-randomised studies. The median follow-up was 90 months and after PSM there was reasonable balancing of prognostic variables in the MBC and FBC cases. The 10-year survival for node negative MBC and FBC was 39% and 85% respectively. Among the node positive cases the respective 10-year survival rates were 34% and 49%. The 10-year cancer specific survival rates for node negative cases were 54% and 85% and for the node positive patients 55% and 56%. Recently Choi et al. examined OS in 400 Korean MBC cases and compared this with that of matched FBC patients [20]. Each MBC had 5 FBC controls and after matching for stage and ER status, there remained 260 MBC and 1300 FBC. The respective 5-year OS rates were 91% and 93%. On multivariate analysis the only significant prognostic variables in MBC were age >60 and tumour.
Age
In a joint Scandinavian project, 1429 MBC cases were studied with regression analysis of annual relative survival rates [21]. What emerged was a lower relative survival rate in older patients. During the first 5 years of follow-up the relative excess risk of death from breast cancer increased more than threefold compared with those aged <40 years at diagnosis. There were significantly higher mortality rates in Denmark and Finland, compared with Sweden which may have been due to later presentation.
Between 1933 and 1983, 124 MBC cases were treated at the Mayo Clinic [22]. Median follow-up was 6.7 years. There was a positive family history of breast cancer in 30 (27%) and 9 (7%) had previous chest wall irradiation. The 5-year disease-free survival (DFS) was 64%. Overall survival was 57% at 5 years and 31% at 10 years. Apart from the usual adverse factors: tumour size, nodal status and tumour grade, pain and increasing age were significantly associated with decreased survival.
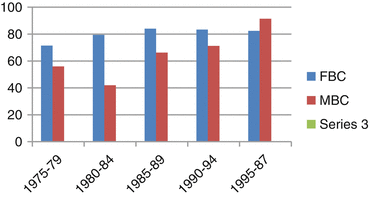
Ioka et al. used the Osaka Cancer Registry data to examine 5-year survival of 97 MBC and 19,772 FBC cases diagnosed between 1975 and 1997 in Osaka Prefecture or between 1993 and 1997 in Osaka City [23]. MBC comprised approximately 0.5% of all breast cancer cases. The relative survival for males and females were 82% and 71% respectively. Figure 11.1 shows the relative survival of males and females according to date of diagnosis. Although there was a fairly steady state in FBC there was a substantial improvement in survival of MBC with time. Survival in older males was worse and this was associated with an increased proportion of advanced cases.
Tural et al. reported outcomes for 99 MBC patients treated in Istanbul between 1972 and 2011 [24]. They split the cases into two age groups: younger (<65) and older (≥65).
Although the older patients had larger tumours these were more likely to be ER and PR+ve. The 10-year OS was 56% in younger men compared with 49% for older patients. On multivariate analysis tumour size and axillary nodal involvement were significant indicators of prognosis.
Using the National Cancer Database, Sineshaw et al. investigated outcome in males aged 18–64 and ≥65 at the time of diagnosis [25]. The study group consisted of 5247 white and 725 black patients with operable MBC. In both age groups treatment was given similarly to both ethnic groups but among those aged ≥65, chemotherapy was given less frequently. For white men the treated proportions were 79% and 42% as compared with 77% and 39% in black MBC cases. Mortality in younger black men was 76% higher than in the comparable white men when adjustment was made for clinical variables but fell to 37% becoming non-significant after adjustment for socio-economic factors.
Because of the problems of an excessive number of potential prognostic variables, Shahraki et al. used the LASSO method (Least Absolute Shrinkage and Selection Operator) to examine prognosis [26]. This constrains the absolute value of the regression coefficients, so that many diminish and some fall to zero. Such an approach is particularly applicable to MBC where the number of variables exceeds the sample size. They analysed 50 Iranian MBC cases with both Cox proportional hazard and LASSO-Cox models, fitted for 20 variables. Because the relative efficiency of LASSO-Cox was 22.29 times greater than the Cox model this eliminated 8 low strength variables. The most important variables to emerge after 19 years follow-up were age, alcohol consumption, laterality, nipple discharge, tumour grade and symptom duration. The finding that tumour laterality was a significant prognostic variable does appear somewhat counter-intuitive but the worse outcome for men with left sided cancers may have resulted from cardiac irradiation.
Breast cancer is exceedingly rare in male adolescent and young adults (aged between 15 and 39 years). Flaherty et al. examined the National Cancer Data Base for years 1998–2010 and identified 677 MBC cases of whom 122 (18%) had DCIS and 555 (82%) were diagnosed with invasive disease [27]. Factors associated with reduction in overall survival were age, ethnicity and socio-economic status. Those aged ≤25, of black race and who were uninsured all had significantly worse survival. As well as the established TNM variables, not having any surgery, or omission of nodal evaluation impacted significantly on survival. On multivariate analysis the two significant variables which emerged were age ≤25 years (HR 3.064, 95% CI 1.216, 7.720) and lack of evaluation of nodal status (HR 3.070, 95% CI 1.423, 6.626). The lesson to be learnt is that the very young do badly but age per se should not be an excuse for under-treatment.
Marital Status
Leone et al. investigated 2992 MBC cases diagnosed between 2003 and 2012 and registered on the SEER database [28]. In a multivariate analysis, the factors adversely affecting OS were older age, higher tumour grade, ER−ve cancer stage IV disease, being unmarried and not receiving surgery or radiotherapy. These findings suggest that vulnerability to worse outcome for MBC is increased by age and possibly as a result of isolation from living without a significant other.
In another SEER derived study (1990–2011), Adekolujo et al. examined the effect of marital status on stage at diagnosis and outcome in 3761 MBC cases [29]. Only those aged ≥18 years were included with marital status dichotomised to married or unmarried (single, divorced, separated and widowed). Kaplan-Meier method was used to estimate the 5-year cancer-specific survival. Multivariate regression analyses were done to determine the effect of MS on presence of Stage IV disease at diagnosis and on cancer-specific mortality. The study included men; 2647 (70.4%) were married. Unmarried men were more often diagnosed with Stage IV MBC compared with married (10.7% vs. 5.5%, p < .001). Unmarried men (compared with married) were significantly less likely to undergo surgery (92.4% vs. 96.7%, p < .001). Overall unmarried males with Stages II, III, and IV MBC had significantly worse 5-year cancer-specific survival compared with married men. On multivariate analysis, being unmarried was associated with increased hazard of death (HR = 1.43, p < .001) and increased likelihood of Stage IV disease at diagnosis (OR = 1.96, p < .001). Unmarried males with breast cancer are at greater risk for Stage IV disease at diagnosis and poorer outcomes compared with married males.
Ethnicity
Keller from the US Veterans Administration collected 181 biopsy-confirmed MBC cases and two groups of male controls selected by terminal claim digits from a 1961 hospital discharge claim-number listing [30]. Controls were matched for age within 5 years, and either for place of residence or type of hospital. One control group (non-cancer) had non-malignant diagnoses and the other cancer control group had been treated for bladder or kidney cancer. When the 7 year overall survival rates of white and black males were compared they were 35% and 46% respectively. This suggested that, among those having access to good medical care through the Veterans Administration, there was no major difference in outcome based on ethnicity.
Brenner et al. collected 131 MBC cases diagnosed at two medical centres on Israel between 1960 and 2000, together with a further 470 reported to the Israel Cancer Registry from 1980 to 1997 [31]. Of the 131 Jewish patients seen at Rambam and Rabin hospitals, 102 (78%) were Ashkenazi and 29 (22%) Sephardic. Although both groups had similar clinical characteristics there was significant more comorbidity in the Sephardim with a trend towards younger age and higher stage at the time of diagnosis. This was associated with significantly worse prognosis. Analysis of the Cancer Registry cases showed an 80% increase in the risk of developing MBC among the Ashkenazim. There was however a worse prognosis for the Sephardim with estimated 5-year survival rates of 62% versus 64% in the Ashkenazim.
O’Malley et al. used SEER data to examine survival rates in 1979 MBC cases reported between 1973 and 1997 in relation to ethnic origin: non-Hispanic white, non-Hispanic black, and other (mostly Asian/Pacific Islander and Hispanic) [32]. They used two endpoints: all-cause and breast cancer specific mortality. Because there were significant differences in survival of each ethnic group, subsequently individual group analysis was performed. The overall 5-year survival rate was 66% for whites, 57% for blacks, and 75% for men of other ethnicity. Black men were more likely to be diagnosed with more advanced disease. Within tumour stages there was worse survival for both black and white men compared with others. Other prognostic factors such as age, use of surgery or radiotherapy were apparent but not always significant in all ethnic groups.
In another SEER study Crew et al. found 510 MBC cases aged ≥ 65 years with stage I-III disease diagnosed between 1991 and 2002 [33]. Of the cases 456 (89%) were white and 34 (7%) black, with 479 (94%) undergoing mastectomy, 143 (28%) being given adjuvant chemotherapy, and 148 (29%) having radiotherapy. Black men were half as likely to have consultation with an oncologist and receive adjuvant chemotherapy; however, the results did not reach statistical significance. Their outcome was worse with a cancer–specific mortality hazard ratio of 3.29 compared with white men.
O’Brien et al. were interested in differences in survival of MBC cases in relation to sociodemographic status and examined the outcome of 1589 males registered with the Florida Cancer Data System between 1996 and 2007 [34]. The 5-year overall survival was 66% with a mean survival time of 7.7 years. For white males the mean survival was 7.8 years, compared with 5.9 in black men. Non-Hispanic males fared worse than Hispanic men with mean survival of 7.7 and 8.5 years respectively. Those with the lowest socioeconomic status survived for an average of only 5.9 years compared with 8.2 years in the highest SES. Patients with low SES presented with more advanced cancers, 57% versus 48% for middle-high SES and 51% for the highest SES. In univariate analysis middle high and highest SES had better survival than those with lowest SES but this advantage disappeared on multivariate analysis. Significant prognostic variables for survival were marital status, age, smoking, tumour stage, treatments, and comorbidity. This indicates that any apparent ethnic differences in survival are largely economic, not genetic (Table 11.2).
Further evidence of the impact of socioeconomic status on prognosis of MBC came from the work of Shi et al. who examined the survival of 8828 American patients in relation to their payer status [35]. The patients were registered on the National Cancer Data Base and diagnosed between 1998 and 2006 with follow-up to 2011. Payer status was categorised as private 48%, Medicare 43%, Medicaid 3%, unknown 3%, and uninsured 3%. Median overall survival (MOS) for all patients was 10.6 years. In multivariate analysis, Direct adjusted MOS was 12.46, 11.89, 9.99, 9.02, and 8.29 years for private, “unknown,” Medicare, uninsured, and Medicaid payer’s status, respectively. Patients with private and “unknown” payer’s status showed a significant difference in survival compared with uninsured patients, while Medicaid and Medicare patients did not. Age, race, stage, grade, income, comorbidity, distance travelled, and diagnosing/treating facility were also significant predictors of survival. Treatment delay and cancer program did not have a significant influence on survival.
Molecular Profile
In order to achieve more accurate classification and prognosis of MBC cases the techniques of molecular profiling used for FBC have been applied with varying degrees of success and agreement. At the Mayo Clinic, 111 MBC cases were treated between 1950 and 1992 and of these 77 had material suitable for immunohistochemical analysis [36]. The pattern of expression of estrogen, progesterone, androgen receptors (ER, PR, AR), together with bcl-2, p53, HER-2/neu, cyclin D1, and MIB-1 were determined. The majority of tumours were hormone receptor positive, ER (91%), PR (96%), AR (95%). The apoptosis inhibitor bcl-2 was expressed in 94% of cases. The proliferation marker MIB-1 was expressed in 38% and associated with a worsening of 5-year disease-free survival, DFS (43% versus 83%) as shown in Table 11.3. In contrast, expression of the cell cycle regulator cyclin-D was associated with a better 5-year DFS. There was positive staining for p53 in 21% and a strong association with cyclin D positivity.
Marker | 5 year disease-free survival | ||
|---|---|---|---|
MIB-1 | +ve | 38% | 47% |
−ve | 62% | 83% | |
Cyclin-D | +ve | 58% | 77% |
−ve | 42% | 53% | |
Wang-Rodriguez et al. examined the expression of ER, PR, MiB-1, Her-2, and p53 in tumours from 65 MBC cases derived from the Veterans Administration Cancer Registry [37]. They conducted a case-control study with 17 age-matched male controls with gynaecomastia for each MBC case. The most important prognostic variable was the clinical stage irrespective of tumour size or lymph node status. MiB-1 and PR positivity were unrelated to prognosis but both HER-2 and p53 were associated with reduction in DFS.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree