(1)
Research Oncology, Guy’s Hospital, London, United Kingdom
Abstract
Genetic studies have demonstrated multiple differences between male and female breast cancer, most noticeably that mutations of BRCA1 play a very small role in MBC whereas those of BRCA2 may be associated with up to 14% of male cases. Mutations of PALB2, partner and localiser of BRCA2, have been found in 16% of MBC cases, with or without a family history. In a large genome wide association study a common variant of RAD51B, a low penetrance gene, was found to be significantly associated with MBC. The EMSY gene is amplified in 13% of sporadic FBC but in 35% of MBC, with low amplification in BRCA2 associated cancers. Mutations of androgen receptor gene and CYP17 are rare in MBC. Bacterial artificial chromosome (BAC) arrays have revealed more genomic gains and fewer genomic losses in MBC, identifying 2 subgroups: male-complex and male-simple, the latter being found only in men. Genetic testing should be considered in men having one first degree relative with MBC and ≥1 with FBC or ovarian cancer since among this group, mutations have been found in 36%.
The great tragedy of Science – the slaying of a beautiful hypothesis by the ugly truth. Thomas Huxley
Because of an appreciation of the need to collaborate in order to obtain meaningful information, geneticists have led the field in the study of MBC. As a result there is a great deal of knowledge on the subject but every new advance brings forth as many questions as answers. In 1975, 39 cases of MBC were reported from Yale New Haven Hospital and these included 2 brothers who were diagnosed aged 52 and 69 [1]. Of 142 MBC cases seen at the Memorial Sloan-Kettering Cancer Center and the Ochsner Clinic, 15% gave a family history of breast cancer but this had no impact on stage at diagnosis or prognosis [2]. From 10 population databases, 320 MBC were identified of whom 75% participated in a case control study with age-matched controls selected by random digit dialing [3]. Among the cases MBC was diagnosed in 3 fathers, 1 brother and 4 maternal uncles but in only one brother of a control. The odds ratio for any male with a relative with MBC was 6.07. In contrast having a relative with female breast cancer (FBC) was associated with an odds ratio of 2.17. In a Swedish study the incidence of cancer among first degree relatives of 153 MBC cases was determined and the standardised morbidity ratio (SMR) was elevated at 1.36 [4]. There were significantly elevated SMRs for FBC and ovarian cancer being 1.80 and 2.27 respectively.
Aneuploidy
Teixera et al. performed cytogenetic analysis of three cases of gynaecomastia and four MBC and found a normal karyotype in two gynecomastias but an abnormality in one who had a prior MBC excised [5]. In this small study there were clonal abnormalities in all 4 MBC cases, suggesting that gain of the chromosome X and 5, together with loss of 5 and Y together with del(18)(q21) were non-randomly present in MBC.
Using a multiplex ligation-dependent probe amplification method Lacle et al. investigated copy number changes on chromosome16q in 135 MBC tumours [6]. There were copy number changes present in 112 (83%). Two recurrent amplicons were found on 17q23.1 in 40% of MBCs compared with 60% of FBCs. This resulted in increased copy numbers of neurogenic differentiation factor 2 NEUROD2. There was a significant correlation between amplification of NEUROD2 and grade of the tumour (p < 0.0001). NEUROD2 copy number gain was associated with a significantly worse survival (p = 0.015).
In a large scale study of aneuploidy Jacobs et al. used X and Y centromere probes on blood smears from 565 MBC and 54 male controls [7]. The results in terms of proportion of aneuploidy and age of participants are summarised in Table 4.1. Aneuploidy was seen in 63% of the cases and 57% of the controls. There was a significant increase in proportion of aneuploid cells with age but this was more marked in the controls 85% versus 71%. The authors concluded that aneuploidy in MBC warranted further investigation in cohort studies.
% Aneuploidy | 0 | <2 | 2–4 | 5–9 | 10–19 | ≥20 | |
|---|---|---|---|---|---|---|---|
<45 years | Cases Controls | 77% 62% | 4% 8% | 15% 31% | 4% 0 | 0 0 | 0 0 |
45–64 years | Cases Controls | 45% 57% | 15% 0 | 33% 24% | 5% 14% | 1% 5% | 0 0 |
65–80 years | Cases Controls | 29% 15% | 12% 5% | 33% 45% | 11% 5% | 10% 5% | 5% 25% |
Total | Cases Controls | 37% 43% | 13% 4% | 32% 33% | 8% 7% | 6% 4% | 3% 9% |
BRCA1/2
In 1994 the identification and localisation of the two major breast cancer susceptibility genes was achieved [8, 9]. BRCA1 is located on 17q21 and BRCA2 on 13q12-13 with the former exerting a more major role in hereditary FBC. It therefore came as a surprise when Stratton et al. reported that in 22 families with at least one affected male there was no linkage between BRCA1 and MBC [10]. Furthermore, in an Icelandic extended pedigree study of 252 males and 229 females there were 4 cases of MBC and 3 of FBC and all had BRCA2 associated cancers with loss of the wild-type allele [11]. Further work by the same group to include 21 families with 9 MBC cases revealed a deletion in exon 9 of BRCA2 in all the cases, indicative of a founder effect [12].
The Cambridge Group analysed 94 British MBC cases looking for BRCA1 and BRCA2 mutations, and calculated breast cancer risk in female relatives using family history data [13]. Nineteen men (20%) had a first-degree relative with breast cancer and of these seven also had a second-degree relative with the disease indicating a 2.4 fold increase in risk of FBC compared with the general population. There were no BRCA1 mutations but five men were BRCA2 mutation carriers.
The Breast Cancer Linkage Consortium collected 164 families with breast/ovarian cancer and germline BRCA2 mutations to evaluate genotype-phenotype correlations [14]. By the age of 80 years, the cumulative risk of breast cancer in male carriers of a BRCA2 mutation was estimated as 7%. BRCA1 and BRCA2 mutation status was also investigated in an Australian cohort of 60 familial MBC cases [15]. Among these there were 28 carriers (3 BRCA1 and 25 BRCA2) and 32 non-carriers with strong family histories. In comparison with FBC there was larger proportion of BRCA2 tumours, (43% versus 8%), and underrepresentation of BRCA1 tumours (5% versus 14%), suggesting significant differences in the genetic aetiology of MBC and FBC. In a study of 261 Israeli MBC cases there were 21 BRCA2 with 6174delT and 8 BRCA1 with 185delAG mutations were found [16]. There was a similar proportion of BRCA1 and BRCA2 mutation carriers were found among Ashkenazi and non-Ashkenazi Jews (12.8% and 9.1%).
Other Susceptibility Genes
BRCA1/2 mutations account for less than 25% of familial FBC cases so alternative susceptibility genes have been sought, with varying degrees of success, These include PALB2, androgen receptor gene, CYP17, CHEK2 and RAD51B.
PALB2
This is the acronym for “partner and localiser of BRCA2” and the PALB2 encodes a protein which maintains the nuclear placement and stability of BRCA2, enabling DNA repair of double strand breaks. Mono-allelic mutations of BRCA2 and PALB2 increase risk of FBC and bi-allic mutations are associated with Fanconi anaemia, Rahman et al. sequenced PALB2 in DNA from 923 individuals with familial breast cancer and found truncating mutations in 10 (1.1%) as compared with none of the 1084 controls [17]. One of these mutations was found in a member of a family with both MBC and FBC cases suggesting that PALB2 mutations might increase the risk of MBC.
Adank et al. investigated PALB2 mutations among 12 MBC and in one case found a truncating PALB2 mutation, c.509_510delGA [18]. Ding et al. screened 115 MBC cases and found BRCA2 mutations in 18 (16%) [19]. Of the 97 without BRCA2 mutations one male had a PALB2 mutation. Because of this the authors recommended screening all MBC for PALB2 mutation regardless of family history. Blanco et al. determined the incidence of PALB2 mutations in 131 Spanish BRCA1/BRCA2-negative breast/ovarian cancer families in which there was ≥1 MBC [20]. In one family there was a PALB2 deletion suggesting that PALB2 germline mutations are not more frequent in families with MBC cases.
Fernandes et al. sequenced DNA from 1478 breast cancer patients who had no BRCA1/2 mutations and divided them into high risk (955) or lower risk (523) [21]. High risk cases had breast cancer before age 50 or a relative developing the disease before age 50, or one MBC or ≥2 relatives with breast cancer aged <50 years. Overall 12 PALB2 mutations were found. In the high risk individuals there were 10, including one MBC, compared with 2 in the low risk group, with no significant difference in prevalence. From a group of 8 MBC patients Vietri et al. identified a truncating mutation of PALB2 designated c.1285_1286delAinsTC [22]. This does suggest that PALB2 should enter the pantheon of MBC susceptibility genes.
Recently, Silvestri et al. used whole-exome sequencing (WES) and targeted gene sequencing to examine the significance of PALB2 in 48 sporadic MBC cases from an Italian multicentre study [23]. They had found a truncating mutation (PALB2) c.419delA carried by the proband, her father, and paternal uncle all of whom had breast cancer and the nonsense mutation N-acetyltransferase 1 (NAT1) c.97C>T in her maternal aunt. Within the series of 48 MBC the c.1984A>T nonsense mutation was present in one case. They went on to conduct a case-control series of 433 BRCA1/2 mutation-negative MBC and FBC cases with 849 male and female controls. NAT1 c.97C>T was not found in any of the participants, suggesting a small but important role for PALB2 in MBC evolution.
RAD51C
The gene RAD51C is essential for homologous recombination repair and biallelic mutations are associated with Fanconi-like anaemia [24], and breast cancer in families not carrying BRCA1 or BRCA2 mutations [25]. RAD51C mutations were highly penetrant and present in 1.3% of families with ovarian and breast cancers. Orr et al. performed a genome wide association study of 823 European MBC cases with 2,795 controls [26]. A subsequent validation study was performed using independent sample with 438 cases and 474 controls. There were 17 SNPs that were significantly associated with MBC but in the validation set 2 emerged as significant, rs1314913 sited on intron 7 of RAD51B gene and rs3803662 which mapped to TOX3 (16q12.1).
EMSY
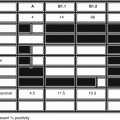
Hughes-Davies et al. identified a protein EMSY, which binds BRCA2 within exon 3, and is deleted in cancer [27]. The first line of the protein sequence reads SISTER so the first author named it after his sibling, Emsy, a Breast Care Nurse. The protein associates with chromatin regulators, and localises to repair foci following DNA damage. The EMSY gene is amplified in 13% sporadic FBC and is associated with worse survival. Navazio et al. sought to determine the role of EMSY amplification in specimens from 75 MBC cases using quantitative real-time PCR [28]. All had been analysed for presence of BRCA1/2 mutations. There was EMSY amplification in 35% of MBCs with a significant association between EMSY copy numbers and BRCA1/2 mutations (p = 0.03). When specimens were subdivided into low and high amplification levels there was low amplification in BRCA2-associated cancers.
BCoRL1
BCL6 corepressor-like 1 (BCoRL1) gene is located on the X chromosome and encodes the protein BCoR-L1 involved in both DNA damage repair and transcription regulation. To investigate the role of BCoRL1, Lose et al. carried out a mutation analysis in 38 Australian families with a breast cancer disposition who did not have BRCA1/2 mutations [29]. Within these families there were 11 MBC and within the coding region little variation was found. There was however a great deal of variation in BCoRL1 in both cases and controls. This suggested that BCoRL1 had very little influence on MBC susceptibility.
PIK3CA
Phosphatidylinositol 3-kinase, catalytic, alpha polypeptide (PIK3CA) encodes the p110 alpha (p110α) protein, a subunit of phosphatidylinositol 3-kinase (PI3K). PI3K signalling is involved in cell growth and division. Deb et al. used high resolution melting analysis and confirmatory signalling to look for somatic mutations in PIK3CA in 57 MBC cases [30]. Mutations were identified in 6 (10.5%) and were more frequent in non BRCA2 patients (17% versus 0%).
Ataxia-Telangectasia Mutated (ATM)
In another study Deb et al. carried out high-throughput somatic sequencing on archival DNA from 48 familial MBCs [31]. Three had BRCA1 mutations, 17 BRCA2 mutations, and 28 were BRCAX (no known mutation). Seeking somatic mutations and copy number changes in 48 genes implicated in cancer susceptibility they found 12 missense mutations included nine PIK3CA mutations (seven in BRCAX patients), two TP53 mutations (both in BRCA2 patients) and one PTEN mutation. Copy number losses of ATM were found in 34%.
Androgen Receptor Gene
In 1992 Wooster et al. reported 2 brothers with MBC, diagnosed at ages 75 and 55 who had androgen deficiency with hypospadias, inguinal canal testes and sparse trunk and limb hair (Reifenstein syndrome) [32]. Leucocyte DNA from both brothers was sequenced and this showed a guanine to adenine substitution in the androgen receptor gene in the DNA-binding domain. This mutation was not present in 100 AR alleles of unrelated individuals nor was it present in their sister. Following this, Lobacarro et al. sequenced leucocyte DNA from 13 French MBC cases and found that one with partial androgen insensitivity syndrome (PAIS) had a guanine-adenine point mutation at nucleotide 2185 [33].
A Swedish study sequenced the complete coding regions of both BRCA2 and the AR gene in 34 MBC cases [34]. Although truncating mutations of BRCA2 were found in 7 men, no AR gene mutations were identified although there was a reduced number of AR polyglutamine repeats among the BRCA2 carriers. Within the AR gene at exon 1 there are variations in CAG repeats so Young et al. examined the lengths of the VCAG repeats in 59 MBC and 78 controls [35]. They found no difference in the distribution of alleles in the cases and controls.
Using a cohort of 32 Finnish MBC patients Syrjäkoski screened the entire coding region of the AR gene for mutations [36]. They found no germ-line mutations and when compared with Scandinavian population controls CAG and GGC repeat lengths were similar. Their conclusion was that the AR gene did not significantly predispose to MBC risk.
CYP17
The rate-limiting step in androgen synthesis is P450c17α hydroxylase coded by the steroid metabolism gene CYP17. A single base change at the 5` promoter was shown to be associated with polycystic ovary syndrome in females and male pattern baldness in men as a result of increased gene expression leading to elevation of androgen synthesis [37]. In a study of 76 MBC cases from South East Scotland, Young et al. examined whether the C allele of CYP17 was associated with increased risk of cancer compared with the general male population [38]. There was a >4-fold increase in frequency of the C allele among MBC cases. In a subsequent case control study of 69 of the cases and 76 controls looking at a tetranucleotide repeat (TTTA) in intron 5 of CYP17 there was no significant difference between the frequency in cases and controls [39].
Gudmunsdottir et al. examined DNA from 39 Icelandic MBC and 309 male controls to determine the role of a T (A1 allele) to C (A2 allele) TC polymorphism in the CYP17 promoter region [40]. Of the cases, 15 (38%) were BRCA2 mutation carriers. There was a higher frequency of the CC genotype among 999del5 carriers compared with non-carriers (33% versus 17%) but this did not achieve statistical significance. Overall, there appeared to be no association between CYP17 and risk of MBC but this has to be interpreted with caution because of the relatively small numbers involved.
CHEK-2
CHEK2 (CHK2) encodes a G2/M checkpoint kinase which is involved with BRCA1 associated DNA repair. The CHEK-2 Breast Cancer Consortium reported that the CHEK2*1100delC mutation which inhibits kinase activity was present in 1.1% of the normal population [41]. In contrast, in MBC families without BRCA1/2 mutations CHEK2*1100delC was carried by 13.5%, leading to a tenfold increase in risk of MBC. This promising insight into a novel abnormality was unfortunately destined to be another false lead.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree