Sedatives
Benzodiazepams
Diazepam
0.2–0.3 mg/kg (maximum 10 mg) orally 45–60 min before the procedure
Midazolam
0.2–0.4 mg/kg (maximum 15 mg) orally 30–45 min or 0.05 mg/kg IV 3 min before the procedure
Ketamine
1–2 mg/kg given 3 min before procedure
Opioids
Morphine sulfate
0.05–0.1 mg/kg IV over 1–2 min given 5 min before the procedure
Fentanyl
1–2 μg/kg IV 3 min before the procedure
Meperidine
0.5–1 mg/kg IV over 1–2 min given 2–5 min before the procedure
Local anesthetics
Lidocaine 1 % (without epinephrine)
1–3 mg/kg infiltrate skin and periosteum; wait 4–5 min
Chemotherapy Safety
The inadvertent administration of vincristine as IT chemotherapy is a catastrophic event. From 1968 to 2007, this error was reported 55 times in several international settings, resulting in death in nearly all cases. Therefore, every pediatric oncology institution must ensure that maximal structural and procedural safeguards are in place to prevent this error. In 2007, the World Health Organization (WHO) issued an alert on this topic and recommended that syringes should not be used for vincristine administration, that where possible this drug should be prepared by dilution in small volume intravenous bags (“minibags”), and that labeling should include a clear warning [30].
Equipment
The equipment to be collected for procedures are shown in Table 15.2. The operator should ensure that the required equipment is available and properly set up prior to the procedure. For LPs, a large body of literature has focused on determining the ideal needle to be used, mainly from the perspective of reducing the risk of post-dural puncture headache (PDPH), which is the most common complication.
Table 15.2
Equipment required for procedures in pediatric oncology
For all procedures | Sterile tray |
Skin prep (chlorhexidine or iodine) | |
Requisitions, consent forms, procedure notes | |
Labels | |
Specimen bags or containers | |
Mask, gown, sterile gloves, sterile towels or drapes | |
Sharps bin or container | |
For lumbar punctures | LP tray and extra needles |
Chemotherapy, orders, protocol | |
20 mL syringe for chemotherapy | |
Sterile gauze | |
For bone marrow aspirations | Two 18-gauge drawing needles and one 25-gauge injecting needle |
Local anesthetic and heparin vials | |
Many 5 or 10 mL syringes (to draw samples) | |
BMA needle(s) | |
Slides, slide tray, pencil | |
EDTA tubes, heparinized tubes, research tubes if indicated | |
For bone marrow trephine biopsies | Bone marrow biopsy needle(s) |
Formalin specimen jar | |
For thoracentesis and paracentesis | 21-Gauge needle and 50 mL syringe |
Cannula | |
3-Way tap | |
Tubes and containers |
Needle Length
The following needle lengths are widely available: 1.5, 2.5, or 3.5 in. A longer needle is more challenging to use, as it is more likely to bend upon insertion. Therefore, the shortest needle that is likely to reach the target space should be selected. Generally, a 1.5 in. needle can be used for most patients weighing less than 35 kg, a 2.5 in. needle can be used for patients 35–70 kg, and a 3.5 in. needle is usually required for patients weighing more than 70 kg.
Needle Type
Most practitioners are familiar with the traditional cutting tip LP needles (Quincke or bevel-tip) which are commonly available. Nevertheless, the atraumatic needle (Whitacre, Sprotte, or pencil point) has been shown in multiple randomized control trials to reduce the rates of PDPH. Using an atraumatic needle requires some learning for practitioners who are more used to cutting needles. An introducer may be required to pass the atraumatic needle through the skin, and the tactile feedback from subcutaneous tissues is different.
Needle Size
LP needles can be found in 20, 22, and 26 gauge (smallest size). As expected, a smaller sized needle is associated with lower rates of PDPH, likely because the smaller dural hole causes less CSF leakage. Switching to atraumatic needles or smaller gauge needles (or both) for patients who have experienced a moderate to severe PDPH after a prior LP is strongly advised. However, smaller gauge needles can bend upon insertion, decrease the rate of CSF flow, and increase the resistance felt when injecting chemotherapy. Therefore, a 22-gauge needle may be the optimal needle size for most LPs.
During the Procedure
Like most procedures, the LP is a practical skill that is best learned by observation and practice. Institutions should develop best practices to impart education and training on new staff members.
Positioning the Patient
While LPs can be performed in the sitting position, the lateral decubitus position is used when the patient is to be sedated. Furthermore, this position allows better distribution of injected chemotherapy through the CSF. The patient’s legs are flexed toward the chest, and the shoulders and pelvis are square and perpendicular to the bed, as shown in Fig. 15.1 [31]. In this position, an imaginary vertical line drawn between the posterior superior iliac crest would approximately cross the L4 spinous process. LPs can be done in the L3-L4, L4-L5, or L5-S1 interspace. LPs should be avoided at higher interspaces, as this risk injuring the conus medullaris. Conversely, LPs performed at lower interspaces increase the difficulty of palpating the spinous processes.
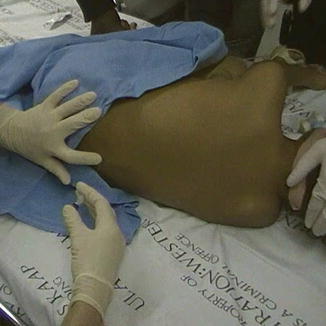

Fig. 15.1
A lumbar puncture needle being inserted
Performing the LP
Wearing sterile gloves and a mask, the operator should cleanse the puncture site with an antiseptic solution. Sterile drapes should be applied, leaving the intended puncture site exposed.
The stylet should be in place within the needle. The needle should only be advanced with the stylet in place, or else the needle bore can become obstructed with a core of tissue and increase the risk of developing an epidermoid cyst.
The tip of the spinous process superior to the chosen interspace should be palpated. The needle should be inserted inferior to this tip, midway between two spinous processes.
If a traditional Quincke or bevel-tip needle is used, the bevel face should be oriented parallel to the long axis of the spine, so as to separate the longitudinal fibers of the dura rather than transect them. Thus, in a child lying in a left lateral decubitus position, the bevel should face up toward the ceiling. Several studies have shown that this bevel orientation helps reduce the rate of PDPH [32, 33].
The needle should be advanced slowly at a slight cephalic angle so as to move parallel to the angle of the spinous processes and avoid bony contact [34]. In most patients, this angle can be approximately achieved by advancing the needle as though aiming for the umbilicus. If bony contact occurs or resistance is felt, the needle should be withdrawn to the subcutaneous position and reinserted at a slightly different angle.
Usually, a pop is felt when the needle penetrates the ligamentum flavum. However, in young children or in patients who have had repeated LPs, the pop may not be a reliable sign. Therefore, once the needle has reached close to the estimated depth of insertion, the stylet should be removed to check for CSF flow. If there is no fluid, reinsert the stylet and advance the needle slightly, withdrawing the stylet and checking for fluid after each movement.
If a bloody tap occurs, it is advisable to remove the needle, obtain a new needle, and reattempt the LP at a different interspace. If the LP fails a second time, a different person should attempt it, especially if a more experienced operator is available. After a bloody tap, however, the presence of a small hematoma may make a successful procedure more difficult in spite of the operator’s proficiency. In such cases, the LP should be attempted again after a few days, allowing time for the hematoma to resolve.
Pressure Measurements
ICP can be measured by connecting a manometer to the needle or via a stopcock, and allowing CSF to flow into the manometer until the fluid level ceases to rise. This procedure is not often used in practice, but may be performed in cases of suspected ICP. There will be normal variation in the fluid level with respiration. After the measurement is obtained, the CSF in the manometer should be released into a collection tube using the stopcock rather than be wasted.
Collecting Samples
CSF should be allowed to drip into the collecting tubes. It should never be aspirated into a syringe as this could result in significant negative pressure. For most LPs performed for delivery of intrathecal chemotherapy, a tube is collected for cytospin and another for biochemistry (protein and glucose). With LPs done for patients with brain tumors or solid tumors, a tube for cytology should also be obtained. In the case of suspected meningitis, additional tubes for bacterial studies (gram stain, culture, and sensitivity) and/or viral studies should be obtained.
Volume of CSF
For a diagnostic LP, only the minimal required CSF volume should be collected. Excess CSF removal will increase the risk of a PDPH. Conversely, where intrathecal chemotherapy is to be administered, it is advisable to remove a CSF volume equal to the volume of drug that will be administered. Theoretically, a large net positive infusion volume may increase the risk of intracranial hypertension. Recognizing the limitations of time in the procedure room, some leukemia protocols recommend that clinicians should aim to remove at least 50 % of the volume of drug to be injected.
Injecting
Once CSF collection is completed, the needle hub should be connected to the chemotherapy syringe and a tight seal obtained. The needle should be stabilized with the non-dominant hand to ensure that its position is not altered during this maneuver, as seen in Fig. 15.2. Slowly inject the chemotherapy. Resistance usually increases as the injection proceeds. However, if excessive resistance is felt, cease administration and remove the syringe to ensure that CSF is still free flowing.
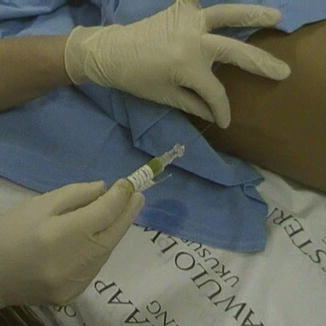

Fig. 15.2
Intrathecal chemotherapy being injected through a lumbar puncture needle
Reinserting the Stylet
One study [37] found that reinserting the stylet after a diagnostic LP was associated with reduced rates of PDPH. The authors hypothesized that strands of dura may enter the needle bore during CSF sampling and be threaded back during removal of the needle, producing a larger dural defect and allowing longer leakage of CSF. Replacing the stylet may allow the strands to be pushed out, reducing the risk of prolonged leakage.
If this postulated mechanism is correct, then it is reasonable to assume that injecting medication through the needle may achieve the same desired effect. Therefore, if IT chemotherapy was injected, the needle and syringe can be removed as a unit without reinserting the stylet. However, if the LP was done for diagnostic sampling only, the stylet should always be reinserted prior to removing the needle [38].
After withdrawing the needle, manual pressure using gauze should be applied for a few minutes, with longer times preferred in those with lower platelet counts or traumatic procedures.
After the Procedure
Bed Rest
In patients who have received intrathecal chemotherapy, lying supine for 1–2 h is advisable as it facilitates distribution of chemotherapy throughout the CSF. However, in patients who have received a diagnostic LP only no specific bed rest is required and patients may ambulate once they have recovered from the effects of sedation [39].
Documentation
As for all procedures, proper documentation of consent, orders, and the procedure details should be carefully documented. The operator should properly document any problems encountered, the number of attempts, and chemotherapy administration. Standardized procedure forms or templates can be useful to allow efficiency in this regard.
Figures 15.3, 15.4, 15.5, and 15.6 show samples of standardized documentation used at our institution for LPs and bone marrow aspirates/biopsies. Figure 15.3 is a standardized consent form; Fig. 15.4 is a preprinted orderset for intrathecal chemotherapy; Fig. 15.5 is a procedure documentation form; and Fig. 15.6 is a wall poster used for “time-out” safety checking. The use of such template documentation instruments can help facilitate the efficiency and quality of care in the procedure room.


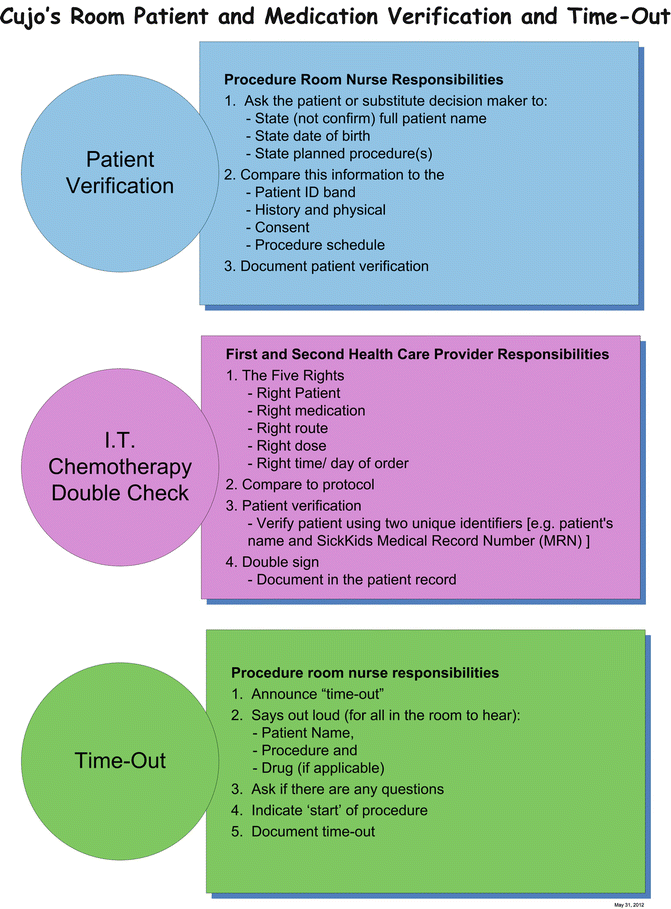

Fig. 15.3
Sample consent to treatment form for lumbar punctures and bone marrow procedures (used with permission from The Hospital for Sick Children © 2012)

Fig. 15.4
Sample preprinted orderset for procedure and intrathecal chemotherapy orders (used with permission from The Hospital for Sick Children © 2012)

Fig. 15.5
Sample procedure documentation form (used with permission from The Hospital for Sick Children © 2012)

Fig. 15.6
Sample wall poster for patient and medication safety reminders
Complications
Headache
The most common complication of an LP is PDPH. It can be defined as any headache after an LP that worsens within 15 min of sitting or standing and is relieved within 15 min of lying down. Most PDPHs occur within 3 days of the procedure. They are believed to be caused by a puncture in the dura that allows CSF leakage. The reported rate of PDPH varies across studies from 2 to 60 %, depending on the setting [2]. As mentioned, the rates of PDPH have been shown to decrease with atraumatic needles, smaller gauge needles, maintaining the needle bevel direction parallel to the dural fibers, and reinserting the stylet in diagnostic LPs. Bed rest and fluid supplementation do not prevent PDPH [40]. For the management of PDPH, the reader is referred to recent Cochrane systematic reviews [41, 42].
Other Complications
The most significant complications include cerebral herniation, spinal hematomas, and inadvertent administration of incorrect IT chemotherapy. Other complications include backache, subdural fluid collections, epidermoid cysts, and infectious complications such as cellulitis, skin abscess, discitis [43], and epidural abscess [44].
Bone Marrow Aspiration and Trephine Biopsy
Indications
Bone marrow aspirations (BMA) are used for the diagnostic investigation or monitoring of patients with abnormal blood counts, hematologic disorders, suspected malignancies, or metabolic, infiltrative, or infectious diseases that can involve the bone marrow. Additionally, bone marrow trephine biopsies (BMB) are performed when an assessment of bone marrow cellularity, architecture, or focal lesions is required, when there is inadequate marrow aspiration, and for the staging of lymphomas and small round blue cell tumors of childhood [45]. The bone marrow aspirate and trephine biopsy are complementary procedures and are usually performed together (BMAT).
Contraindications
When performed by a trained operator, BMATs are generally safe procedures and adverse events are uncommon [46]. The most frequent complications are bleeding and pain. Rare cases of severe bleeding with large hematomas [47], anemia, and death [48] have been reported. Hemorrhagic disorders, severe thrombocytopenia, and anticoagulation may be considered relative contraindications and their correction should be considered before the procedure whenever feasible [49]. Local skin or bone infections should exclude the use of that particular site for the procedure.
Methods
Before the Procedure
Many of the principles of preparing for the BMAT are similar to those outlined above for LPs. A full patient assessment and informed consent should be performed and documented. The patient’s identity, indications, and contraindications for the procedure and allergies should be reviewed. The BMAT is a painful procedure and conscious sedation or general anesthesia should always be used when it is performed on children. In addition, infiltration of local anesthestic such as lidocaine into the periosteum should be used, as it can help reduce post-procedure pain.
Ensure all equipment is prepared (see Table 15.2). BMA needles are generally available in 18 gauge or 15 gauge. BMB or Jamshidi needles are generally available in adult (11 gauge, 4 in.), pediatric (13 gauge, 3.5 in.), or infant (13 gauge, 2 in.) sizes. Ensure that all slides and tubes needed for the various required samples are available.
During the Procedure
The optimal site for the procedure should be palpated and identified. The posterior superior iliac crest is a common site of this procedure for most children [50]. It can be accessed with the patient in the lateral decubitus position. The anterior iliac crest may also be used and, in obese patients, may be easier to palpate. In infants, the tibial tuberosity can also be used. The sternum is generally best avoided.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree