Principles of surgical oncology
Mark Bloomston, MD, FACS  Kenneth K. Tanabe, MD, FACS
Kenneth K. Tanabe, MD, FACS  Raphael E. Pollock, MD, PhD
Raphael E. Pollock, MD, PhD  FACS and Donald L. Morton, MD, FACS (deceased)
FACS and Donald L. Morton, MD, FACS (deceased)
Overview
The discipline of surgical oncology describes a surgical super specialty to which a board certification process is now attached in the United States. A cognitive as well as technical surgical focus, the surgical oncologist is an oncology specialist who uses surgery as his or her mainstay therapeutic modality in treating tumor problems. As such, the surgical oncologist has a thorough grounding in the natural history of solid malignancy, extensive experience in tumor biopsy and staging approaches, the knowledge needed to orchestrate a multidisciplinary solid tumor treatment program, and the commitment, via vigorous personal participation, in the many relevant research opportunities by which we will advance comprehensive care of the cancer patient.
In spite of significant advances in various systemic approaches to the care of the cancer patient, surgical therapy remains the mainstay of treatment for most solid malignancies and plays a role in various components of the cancer care continuum, from prevention to diagnosis, curative therapy, survival prolongation, and palliation. To be maximally effective, the cancer surgeon must function as a member of the oncology team and is frequently the first oncology specialist that a patient will consult. The cancer surgeon is commonly charged with the responsibility to establish a tissue diagnosis for a suspicious lesion; this may require either an operative procedure or an image-directed or other biopsy approach. The cancer surgeon will usually bear the responsibility for communicating the biopsy findings to the patient, completing the procedures needed to stage the cancer, and initiating subsequent interaction between the patient and other members of the multimodality oncology team. Because of these responsibilities, it is most often the cancer surgeon who initially explains to the patient the sequence and rationale of the various treatment components that will be used to manage the specific malignancy. To be maximally effective, the cancer surgeon must therefore be aware of the different therapeutic options, the natural history of a given malignancy, and how these factors will be integrated into a well-conceived and appropriate multimodality treatment algorithm. It is also usually the surgical oncologist’s responsibility to provide initial information about prognosis and to make decisions about follow-up care and surveillance to detect tumor recurrence. In these aspects, the surgical oncologist is unlike almost any other surgical specialist in that the commitment to a given patient is for both the acute and the long-term components of the patient’s disease process.
Over the years, the practice of surgical oncology has come full circle. Originally, surgeons attempted to treat cancer conservatively by removing only the gross lesion. Unfortunately, this led to extremely high rates of local recurrence and subsequent patient mortality. In the late nineteenth century, surgeons began to undertake radical en bloc resections and amputations to treat patients with malignant disease. These techniques yielded improved results, but the procedures were often mutilating. With the advent of other complementary and effective treatment modalities, notably radiation therapy in the 1920s and chemotherapy after the 1940s, the orientation of surgical resection is once again becoming conservative with a focus on organ preservation and restoring the comorbid state when possible.
Adjuvant chemotherapy, alone or in combination with radiation therapy, has improved disease free survival and prolonged quality of life for patients who have been rendered free of gross disease by surgery but who still have a high likelihood of recurrence as a consequence of microscopic residual metastases. Randomized clinical trials have demonstrated the benefit of adjuvant chemotherapy in a variety of tumors, including breast cancer, colorectal cancer, pancreatic cancer, osteosarcoma, testicular cancer, ovarian cancer, and certain lung cancers.
Surgery is most effective in the treatment of apparently locoregionally confined primary disease. The principles of surgical resection include en bloc resection of the primary tumor that attempts to encompass gross and microscopic tumor in all contiguous and adjacent anatomic locations. For some tumor types, concomitant resection of regional lymph nodes comprises an important component of the initial surgical management. In many cases, when disease is diagnosed and removed at an early stage, resection is the single therapeutic modality, often associated with a high rate of long-term success. Intuitively, it appears logical that surgery should have little role in disease management once a neoplasm has spread from the primary location to a distant site. However, surgical therapy is being applied with increasing frequency for metastatic disease as well. Prolonged survival can be seen in selected patients following resection of various metastatic sites, including in the liver, lung, or brain. In particular, complete resection of hepatic colorectal metastases results in 5-year survival rates in excess of 50% in most contemporary series. As more active systemic cytotoxic and targeted therapies are prolonging survival in patients with various tumor types, resection or ablation of residual metastatic sites are being utilized with increasing frequency.
Surgery operates by zero-order kinetics, in which 100% of excised cells are destroyed. In contrast, chemotherapy and radiation therapy operate by first-order kinetics, where only a fraction of tumor cells are killed by each treatment. It is for this reason that these therapies can be considered complementary. Surgical resection reduces the tumor burden, which hopefully increases the efficacy of nonsurgical adjuvant therapies intended to eliminate microscopic residual disease, thereby decreasing the risk of recurrence and prolonging survival.
During the past several decades, a significant reduction has been seen in the morbidity and mortality associated with many complex cancer operations. These results, in part, can be attributed to improvements in surgical technique, patient selection, and regionalization to high-volume centers. For example, both perioperative risk and long-term survival after pancreaticoduodenectomy have been shown to be strongly influenced by hospital volume.1 In addition, trends toward more limited cancer resections are being seen with comparable of improved oncologic outcome. Specifically, breast-conserving surgery has become an alternative to mastectomy in patients with breast carcinoma, limb salvage is often possible in patients with bone and soft-tissue sarcomas, and sphincter function and sexual potency can frequently be preserved for patients with rectal cancer. Because surgery is increasingly combined with other treatment modalities, it is essential that most patients with solid neoplasms have their treatment planned by a multidisciplinary team, which includes radiation and medical oncologists as well as surgical oncologists. To retain a primary role in the management of the cancer patient, the successful surgical oncologist must be able to coordinate and integrate the efforts of the entire oncologic team while maintaining a patient-centered focus on dignity and quality of life.
Table 1 Landmark advances in surgical oncology
| Year | Event | Surgeon |
| 1775 | Etiologic basis of cancer | Percival Pott |
| 1809 | Elective oophorectomy | Ephraim McDowell |
| 1829 | Metastatic process | Joseph Recamier |
| 1846 | Ether as anesthesia | John Collins Warren |
| 1867 | Carbolic acid as antisepsis | Joseph Lister |
| 1873 | Laryngectomy | Albert Theodore Billroth |
| 1878 | Resection of rectal tumor | Richard von Volkman |
| 1880 | Esophagectomy | Albert Theodore Billroth |
| 1881 | Gastrectomy | Albert Theodore Billroth |
| 1890 | Radical mastectomy | William Stewart Halsted |
| 1896 | Oophorectomy for breast cancer | G.T. Beatson |
| 1904 | Radical prostatectomy | Hugh H. Young |
| 1906 | Radical hysterectomy | Ernest Wertheim |
| 1908 | Abdominoperineal resection | W. Ernest Miles |
| 1909 | Thyroid surgery (Nobel Prize) | Theodore Emil Kocher |
| 1910 | Craniotomy | Harvey Cushing |
| 1912 | Cordotomy for the treatment of pain | E. Martin |
| 1913 | Thoracic esophagectomy | Franz Torek |
| 1927 | Resection of pulmonary metastases | George Divis |
| 1933 | Pneumonectomy | Evarts Graham |
| 1935 | Pancreaticoduodenectomy | Allen O. Whipple |
| 1945 | Adrenalectomy for prostate cancer | Charles B. Huggins |
| 1957 | Isolated limb perfusion | Oliver Creech |
| 1958 | Organization of National Adjuvant Breast and Bowel Project (NSABP) to conduct prospective randomized trials | Bernard Fisher |
| 1965 | Hormonal therapy of cancer | Charles Huggins |
| 1971 | Free tissue transfer with microvascular anastomosis | Harry Buncke |
The history of surgical oncology
Oncology (from the Greek words onkos, meaning mass or tumor, and logos, meaning study) is the study of neoplastic diseases. Early authors suggested that certain families, races, and working classes were predisposed to neoplastic transformations. In 1862, Edwin Smith, an American Egyptologist, discovered the apparently earliest recordings of the surgical treatment of cancer.2 Written in Egypt circa 1600 BC, this treatise was based on teachings possibly dating back to 3000 BC. The Egyptian author advised surgeons to contend with tumors that might be cured by surgery but not to treat those lesions that might be fatal.
Hippocrates (460–375 BC) was the first to describe the clinical symptoms associated with cancer. He advised against treating terminal patients, who would enjoy a better quality of life without surgical intervention.3 He also coined the terms carcinoma (crab legs tumor) and sarcoma (fleshy mass). In the second century ad, Galen published his classification of tumors, describing cancer as a systemic disease caused by an excess of black bile.4 Galen cautioned that as a systemic disease, cancer was not amenable to cure by surgery, which was often promptly followed by patient death. This strong admonition against surgery for cancer persisted for more than 1500 years until eighteenth-century pathologists discovered that cancer often grew locally before spreading to other anatomic sites. Before the advent of safe general anesthetics, surgery was used primarily to manage trauma or severe infectious problems such as abscess drainage. In that era, cancer surgery consisted primarily of amputation or cauterization of surface tumors of the trunk or extremities. Patients were usually unwilling to submit to the pain of tumor surgery, when there was little likelihood of improved survival.
During the eighteenth and nineteenth centuries, advances in anatomic pathology led to an increase in autopsies, which in turn resulted in a better understanding of human anatomy and physiology. The early work of Morgagni, Le Dran, and Da Salva established that there was an initial period of local tumor growth before distant dissemination. This led to the understanding that not all tumors spread systemically and that certain malignancies cause death solely by local invasive growth. Percival Pott (1714–1788) was the first to describe a specific etiologic factor associated with cancer development. In 1775, Pott demonstrated a high incidence of cancer of the scrotum in chimney sweeps who had reached puberty and recommended wide local resection for cure. In 1829, the French Surgeon Joseph Recamier (1774–1852) first described the complicated process of tumor dissemination. The first recorded elective tumor resection was performed in 1809 by Ephraim McDowell, an American surgeon. He successfully removed a 22-pound ovarian tumor from a patient, who subsequently survived 30 years. McDowell’s work, which included 12 more ovarian resections, stimulated greater interest in elective surgery for cancer patients.
Surgeons were initially hindered by the extreme discomfort that patients experienced during surgical procedures as well as the lack of agents that could reduce the incidence of infection. Crawford Long (1815–1878) was the first to use ether for general anesthesia in 1842, but it was the reported work of John Collins Warren (1778–1856) and William T.G. Morton (1819–1868) that brought the potential of anesthesia to public attention. The surgical procedure in Warren’s first published account of ether anesthesia (1846) was the elective removal of a tongue carcinoma for which submaxillary gland resection and partial glossectomy were performed. Warren was also responsible for the first American-authored textbook of tumor surgery, Surgical Observations on Tumors, published in 1838. Joseph Lister (1827–1912) was the first to report the successful use of antisepsis during elective surgery. In 1867, Lister applied Pasteur’s concept that bacteria caused infection, when he introduced the use of carbolic acid as an antiseptic agent in conjunction with heat sterilization of surgical instruments. Lister is also credited with the introduction of absorbable ligatures as well as the placement of drainage tubes to control secretions and dead space in surgical wounds.
Even with the advent of antisepsis and general anesthesia, surgical oncology in the second half of the nineteenth and early twentieth centuries was still associated with high mortality rates. Cancer was rarely diagnosed in the early stages; consequently, few patients were considered candidates for curative surgery. Those surgeons who did attempt surgical excision of malignant lesions were hindered by rudimentary anesthesia, which was also independently associated with high patient mortality. Antibiotics were not yet available, and surgical instruments were crude. The importance of the microscope to evaluate frozen tissues for surgical margins was not yet appreciated, and surgeons had great faith in their own unaided gross visual assessment of the tumor perimeter. However, several important developments in this era led to rapid advancements in surgical oncology. Emphasizing meticulous surgical technique, gentle tissue handling, and applications of Listerian principles, pioneers such as Albert Theodore Billroth (first gastrectomy, laryngectomy, and esophagectomy), William Stewart Halsted (en bloc resection, radical mastectomy), and many other more contemporary surgeons defined and advanced the boundaries of surgical oncology (Table 1).3
Ongoing innovations to advance effective surgical primary tumor control have improved surgical outcomes and quality of life. Advances in microvascular surgery now permit the free transfer of complex autologous tissues, such as free jejunal grafts to reconstitute the upper aerodigestive system or osteomyocutaneous flaps to reconstruct extremities and other mobile body parts such as the jaw. Automatic stapling devices as well as laparoscopic/robotic instrumentation coupled with high-resolution optics have remarkably advanced minimally invasive cancer surgery resulting in less-morbid procedures that require significantly less patient discomfort and recuperation time (Figure 1). The rapid deployment of robotic technologies is changing traditional surgical interventional approaches. Among the potential advantages, robotic surgery is performed as a direct extension of the operator’s prehensile hand replete with multiplanar articulating robotic “wrists,” thereby avoiding the crossed rigid armature impediments of laparoscopic surgical maneuvers. Optoelectronic visualization systems incorporated into robotic display enables the appearance of three-dimensional surgical fields with supra-normal visual acuity. Gating the displayed images coupled with the ability to scale up or down discrete surgical actions (e.g., suture placement and cannulation) enables damping out of tremor effects while facilitating operations performed on moving anatomic structures, especially on the microscopic level. Robotic procedures can be performed over great distances between the robot and console display systems, which will facilitate telesurgical applications in the future. The development of molecular radiologic probes for imaging tumor cellular components possessing more ominous genetic character portends a future in which interventional onco-radiologists and minimally invasive surgical oncologists will work together in the operating room to laser capture micro-dissect these less favorable tumor subsections, perhaps in conjunction with intraoperative navigation systems and various visual interfaces such as direct retinal display systems.
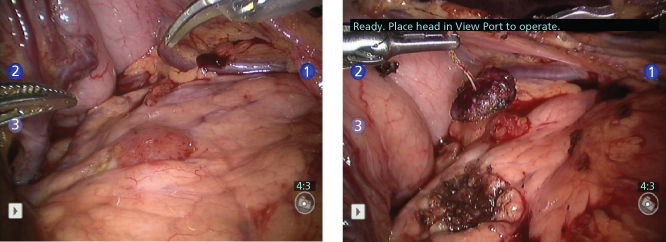
Figure 1 Robotic enucleation of insulinoma in the body of the pancreas.
Improvements in preoperative optimization of comorbid disease and advances in perioperative critical care have made it possible to safely undertake increasingly complicated surgical procedures. A more sophisticated awareness of the patterns of tumor spread has also resulted in increasing opportunities for less-invasive surgical approaches. One example is the use of lymphatic mapping and sentinel node biopsy instead of formal lymphadenectomy in early-stage melanoma and breast carcinoma. In other cases, this better understanding of recurrence risk has led to more, not less, extensive surgical resections. An example of this includes the selected use of total hepatectomy and orthotopic liver transplantation for early-stage hepatocellular carcinoma.
Surgical oncology in the modern era
Surgical oncologists are surgeons who devote most of their time to the study and treatment of malignant neoplastic disease. They must possess the necessary knowledge, skills, and clinical experience to perform both the standard and extraordinary surgical procedures required for patients with cancer. Surgical oncologists must be able to diagnose tumors accurately and differentiate aggressive neoplastic lesions from benign reactive processes. In addition, surgical oncologists should have a firm understanding of radiation oncology, medical oncology, and diagnostic and interventional radiology. They must also be capable of organizing interdisciplinary studies of cancer. Surgical oncologists should also be trained in pathology because they will be called on to excise appropriate tumor samples for pathologists and make decisions about adequacy of surgical margins. Surgical oncologists should have a shared role with medical oncologists as the “primary care physicians” of cancer treatment. Almost all cancer patients will initially be managed by one of these two specialists, who will bear the ultimate responsibility for coordinating appropriate multimodality care for the individual patient.
Given the complexity of contemporary multidisciplinary approaches to the cancer patient, cancer centers have developed facilities to provide the needed planning expertise, clinical care, patient support services, and access points to clinical trials. Comprehensive cancer centers are often affiliated with academic medical institutions and offer the complete spectrum of oncology therapies, clinical trials, rehabilitation, and social services as well as basic and translational research programs to move new knowledge from the laboratory bench to the patient bedside. In this contemporary understanding of the continuum of care of the cancer patient, the role of the surgical oncologist is taking on an ever-increasing importance.
Surgical oncology is more of a cognitive than a technical surgical specialty. With the exception of a small cluster of index operations, such as pancreaticoduodenectomy, limb salvage, retroperitoneal sarcoma surgery, isolated limb perfusion, and complex liver resection, most of the surgical procedures that are performed by surgical oncologists are similar to those performed by surgeons who are not oncologically trained. What frequently differentiates these two types of surgeons is not mere knowledge about how to do a specific operation but an awareness of how and when to do that operation; that is, the cognitive knowledge of contemporary multimodality cancer care. A broad knowledge of cancer in its presenting and recurring forms as well as an awareness of the mechanisms driving tumor proliferation and dissemination is an integral part of the special cognitive database of the surgical oncologist.
As cancer management continues to march forward in the age of genomics, proteomics, and metabolomics, there is an ever-increasing need to study human tumor tissue. At a minimum, the surgical oncologist can contribute to cancer science by helping to secure access to these precious tissues. In reality, the surgical oncologist can do much more than passively provide tumor tissue access. An unparalleled understanding of the pathophysiology of solid tumors coupled with intimate knowledge of anatomy and the workflow in the operating room and pathology department places the cancer surgeon in the central role of organizing, maintaining, optimizing, and overseeing effective tissue procurement and tumor banking; thus making the surgical oncologist a vital member of a translational science team. In addition, the cancer surgeon, working with pathologists and researchers, has the opportunity to provide meaningful clinical information, which can be used to annotate archival tissue repositories and aid in the creation of tissue microarrays. These are valuable tools whose utility can range from explorative, hypothesis-generating retrospective studies to confirmation of specific laboratory findings.
As part of the larger surgical community, the surgical oncologist is a critical conduit for the dissemination of cancer information to colleagues in general surgery and other surgical specialties. This individual makes academic presentations at large surgical meetings, directs hospital-based tumor boards, and consults on behalf of individual cancer patients. Because of their leading role in the initial diagnosis of cancer, it is not surprising that surgical oncologists are also frequently in leadership roles in cancer prevention and screening programs. Nationally based multimodality clinical trial groups also depend on surgical oncology expertise in helping with trial design; establishing the criteria of surgical quality control; educating trial participants regarding standards of surgical care (including indications for procedures); assuring safe acquisition of research grade tumor and autologous normal tissues for correlative studies, and assisting in accurate data collection, analysis, and presentation of trial results.
Multidisciplinary management
Multidisciplinary management of solid tumors requires surgeons to play a key role in decisions concerning sequencing of treatment modalities. For example, a patient with rectal cancer and resectable liver metastases may ultimately be treated with a liver resection, rectal resection, rectal radiation therapy, and systemic chemotherapy. Traditionally, the sequence for these treatments was preoperative chemoradiation therapy, followed by rectal resection (e.g., abdominoperineal resection or low anterior resection), subsequent liver resection, and then adjuvant chemotherapy—an aggressive approach but one that produces long-term survival in a subset of patients. However, in recognition that the greatest risk for mortality in these patients comes from systemic relapse, there is a more recent trend toward starting with chemotherapy, rather than leaving it to the end. Another benefit of this approach is that the nature of response to neoadjuvant treatment serves as an important prognostic marker. Moreover, tumor shrinkage may lead to a less difficult liver resection or rectal resection. While liver and bowel operations were rarely performed simultaneously, these are now more commonly performed together based on data showing safety of this approach in select patients. More often, in cases in which the two operations are performed separately, liver resection is now often performed first. The basis for this approach is that while preoperative chemotherapy rarely has any adverse impact on colon or rectal operations, accumulation of chemotherapy treatments is known to increase the risk of chemotherapy-induced liver pathology leading to complications from liver surgery. Use of short course adjuvant radiation therapy such as 25 Gy in five fractions followed by surgery 1 week later rather than the traditional 5-week course of radiation shortens the time required for trimodality treatment and reduces the length of time off of chemotherapy. Fundamental principles that influence the sequence of multimodality treatment apply to most other solid tumors as well, and are requisite knowledge for surgical oncologists.
Pediatric oncologists pioneered the use of combined modality therapy (radiation in combination with chemotherapy and surgery) as effective management of childhood neoplasms. Control of localized retinoblastoma in children has been dramatically increased using multimodality therapy. The cure rate for patients with Wilms tumor is 75% and if surgical therapy is followed by chemotherapy and, in some cases, radiation, by an increase of 40% over operation alone. Embryonal rhabdomyosarcoma responds best to combinations of radiation, chemotherapy, and operation. Until recently, the effectiveness of multimodality therapy was only occasionally demonstrable for adult neoplasms. A striking example is the approach to skeletal and soft-tissue sarcomas. Surgical therapy, the accepted method for local management of most skeletal and soft-tissue sarcomas of the extremities, is associated with frequent treatment failure if used alone. In the past, approximately 50% of patients with soft-tissue sarcomas and 80% of those with bone sarcomas eventually succumbed to distant metastases, even after amputation of the extremity bearing the primary tumor. Consequently, multimodality treatment regimens were developed to improve these results. Preoperative chemotherapy with intra-arterial doxorubicin followed by radiation resulted in extensive tumor cell necrosis in as many as 75% of patients.4 The effectiveness of this preoperative therapy permitted local resection of the sarcoma and salvage of a viable functional extremity. Local recurrence rates were as low as with amputation, and long-term results were functionally and psychologically superior. In addition, there was no decrease in overall or disease-free survival rates. Multimodality therapy is also effective for other solid malignancies, including colorectal cancer. Specifically, clinical trials have demonstrated improved efficacy and higher sphincter-preservation rates with the use of neoadjuvant chemoradiation therapy for stage II or III rectal cancer. Multimodality therapy has also been demonstrated to improve resectability rates and long-term survival in patients with hepatic colorectal metastases.5
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








