The grafting of skin originated among the tile-maker caste in India approximately 3000 years ago.1 In 1804, an Italian surgeon, Baronio, successfully performed a full-thickness skin autograft of a lamb.2 Since then, numerous pioneers and historical developments have evolved making this procedure common place among almost all surgical disciplines. With proper techniques and instrumentation, and the exercise of appropriate principles, skin grafting has become a reliable and successful operation for wound coverage. In situations in which the wound or defect is not amenable to a skin graft, or in which the defect is better served with more durable coverage, flap coverage would be performed instead. The use of flaps for reconstruction dates back to 600 bc with the earliest recorded application of pedicle flaps for nasal reconstruction, which was attributed to the Sushruta Samhita.3 It was not until the 1960s to 1970s with the advancement in the understanding of anatomy and medical technology (microsurgical instrumentation) that the reconstruction field experienced an explosion in the development of new categories of flaps and ever-increasing named flaps based on blood supply and location of origin. In this chapter, the reader is introduced to the general principles of skin graft and flap surgery. Other chapters demonstrate the applications of various flaps in reconstruction of oncological defects; therefore, clinical application of flaps is not thoroughly discussed in this chapter.
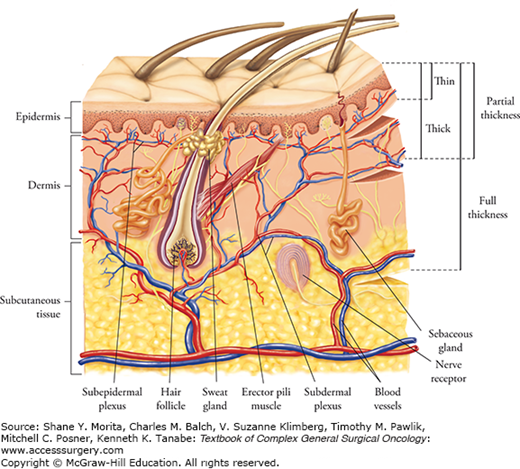
For skin defects which cannot be closed by direct approximation or allowed to heal by secondary intention, either skin grafts or flaps can be employed. Grafts are harvested from a donor site and transferred to the recipient site without its own blood supply. The skin graft relies on regeneration of new blood vessels from the recipient bed. Skin grafts can be categorized as a split-thickness graft or a full-thickness graft. When a graft includes only a portion of the dermis, it is referred to as a split-thickness skin graft (STSG), whereas when the entire dermis is included it is called a full-thickness skin graft (FTSG). The thicker the dermis which is included in the harvest, the less likely the graft will take. A STSG has a higher chance to take, but it will encounter a greater amount of postoperative contracture. A better vascular bed is required for the survival of a FTSG, but it will undergo less contracture upon healing, resist trauma better, and generally look more natural than a STSG. A typical thickness for a split-thickness graft is 0.012 to 0.018 in. (0.30 to 0.45 mm). The donor site generally will heal spontaneously within 7 to 21 days, depending on the thickness of the graft, the donor site skin thickness, and patient factors. Re-epithelialization occurs from adnexal remnants, the hair follicles, and sweat glands. In contrast, with FTSG donor sites, re-epithelialization is generally not feasible. When planning for a FTSG, the surgeon intends to harvest sufficient skin needed but will be able to close the surgical wound primarily. Therefore, FTSGs are generally not used for large wounds. For a large-surface area to be covered with a single piece of a FTSG, the donor site would end up needing a STSG for proper healing.
These grafts can be taken virtually from anywhere on the body. Of course, the donor site should be taken from areas which can be concealed if at all possible. Unless the surgeon is dealing with extensive burns, small areas to be grafted should be harvested from areas hidden by clothing. The thighs, buttocks, and trunk are popular sites to harvest STSG. Keep in mind that the lateral thigh is more favorable than the inner thigh, since the dermis is thicker laterally. In addition, if a limited area is to be harvested, all attempts should be taken to harvest the graft as proximal as possible to minimize exposure of the donor scar when the patient wears short pants. The donor site in most situations heals very well, but the scars can be hypertrophic or discolored. The patient’s innate healing potential and comorbidities, the thickness of the harvest, and whether or not healing was expedient or delayed would all contribute to the final appearance of the donor site scar.
The other important principle is to, if possible, replace the missing skin with skin of similar characteristics as close to the same region as possible. For the face, FTSGs are usually employed, generally for relatively small areas; therefore, grafts can be harvested from the “blush zone.” The blush zone is above the shoulders, including the scalp, neck, postauricular, and supraclavicular areas. This same principle should be applied if a STSG is to be used for the face to allow the best color match. One must be cognizant that unwanted hair follicles may be transferred with a thick skin graft. Therefore, in donor sites of hair-bearing areas, it is important to keep the STSG as thin as feasible without jeopardizing functionality (avoidance of secondary contracture).
In unusual situations with amputations or avulsions, if not severely mangled, the discarded part can be a valuable source of donor site skin. Of course, donor sites with any suspicious lesion must be avoided to prevent transfer of a malignant neoplasm to the recipient site.
If the residual dermal thickness is too thin, re-epithelialization can be delayed, and the secondary scar can become more unsightly (hypertrophy). In infants and the elderly, where the skin is thinner, the split-thickness graft to be harvested needs to be thinner than usual. In addition, a male patient’s skin is typically thicker than a female patient’s skin. Harvest sites can deepen postoperatively due to infection or the patient’s poor-healing potential, such as in the elderly, critically ill patient, and patients with comorbidities (such as diabetes mellitus and steroid use). The deepened donor site would then need to be skin grafted, a complication which must be avoided. Therefore, careful attention needs to be paid to the thickness of the graft relative to the donor site, the patient factors, and the postoperative care of the recipient site.
Full-thickness skin grafts are most commonly used on the face, such as the eyelids and neck. They are also important for flexor surfaces to minimize flexion contractures in areas such as the volar surface of the fingers and wrist. For facial defects, more attention needs to be placed for the consistency, thickness, texture, and color match of the donor skin, unlike defects in other parts of the body. The principle of replacing skin “like with like” is most critical with facial defects. For defects in the eyelid, opposite eyelid skin should be used if defect is small and there is enough laxity present in the opposite side; this is usually only possible in the elderly. Postauricular skin, which is thin with similar color and few glandular elements, can also be employed for the eyelid skin defect. For the nose, where the skin is thicker with more glandular elements, the nasolabial fold, preauricular region, and supraclavicular area would be appropriate donor sources of skin. One should follow the important principle to replace missing skin with like tissue. If the groin skin were used for the nose, the final grafted skin would be darker, leading to more noticeable disfiguration. For extensive defects with inadequate donor areas in the blush zone, skin graft from the inner arm, abdomen, or inguinal crease may be required if no better choice is available. If time permits, for larger defects in the blush zone, tissue expansion can be employed to recruit an adequate volume of FTSG.4 A FTSG undergoes immediate (primary) contraction after harvest, losing 40% of its original area in contrast to a thin STSG which loses only 10% or a medium STSG which loses 20%. However, a FTSG usually will not shrink much as it heals (less secondary contraction), unlike a STSG, which tends to grow minimally with the patient and contract where it can, such as over flexor surfaces.
The inguinal crease is a common site for harvesting skin grafts since primary closure can be accomplished readily in this location, and the donor scar is hidden from regular clothing. However, it is important to stay as lateral as possible to avoid transferring hair-bearing pubic skin. This rule needs to be followed closely in children and infants when a full-thickness graft is harvested in this region, because the hair follicles are not identifiable until puberty. For defects on the hand, the hypothenar area, wrist crease, and elbow crease are excellent donor sites for FTSGs.
A common form of treatment of donor site wounds involves the application of fine mesh gauze impregnated with a lubricant and antibiotic, such as Xeroform (Kendall, division of Covidien, Manfield, MA), which is a sterile, fine-mesh gauze impregnated with a blend of 3% bismuth tribromophenate.5 It is left on until it peels off, and the edges can be trimmed as it rolls up with epithelialization of the underlying superficial wound bed. For smaller defects, a contrasting method of dressing is the use of bio-occlusive dressing, which has been shown to promote faster healing than the semi-open method described above, with better pain control.6 A commonly employed bio-occlusive dressing is a polyurethane material such as OpSite (Smith & Nephew, Memphis, TN).7
The ideal donor site dressing should prevent wound desiccation and at the same time allow oxygen exchange to accelerate re-epithelialization. It should be impermeable to exogenous microorganisms and provide comfort for the patient. Nursing care should be minimized in labor input for wound management. The dressing should be flexible and pliable to permit conformation to irregular wound surfaces. Resistance to linear and shear stress is required, as is good tensile strength to resist fragmentation with body movement.
A multitude of dressing materials are available for donor site dressing, which is beyond the scope of this discussion. Recent literature showed promising result with faster healing using a bioelectric dressing, which is a polyester fabric containing elemental silver and zinc microcells held in position with a biocompatible binder. It produces a microcurrent within a range of 2 to 10 μA.8 Examples of other dressing materials are collagen-alginate dressings,9 synthetic materials such as sterile nonadhesive silicone net dressing without biologic components,10 hydrocolloids, cellulose-based dressings, and tulle gras.11 The reader should understand that there is no standardization at this time as to the universal dressing material to use. Biological materials such as skin substitute are available as well but their use is limited by cost.
In the planning of any FTSG, the intention should be to close the donor site primarily. Therefore, the management of the donor site incision is straightforward. There is the extremely unusual circumstance where the surgeon may choose to use a FTSG for a large defect where the donor site itself needs to be skin grafted with a split-thickness graft, in which case the donor site would be treated similarly as in any STSG recipient site (Fig. 159-1).
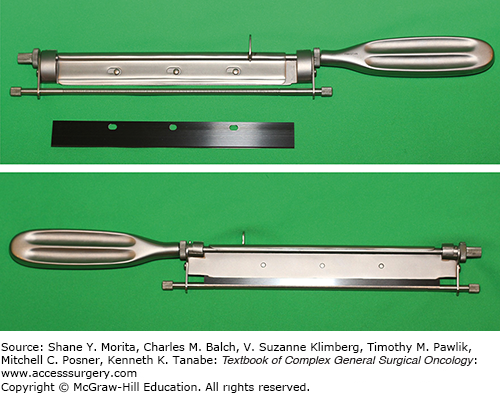
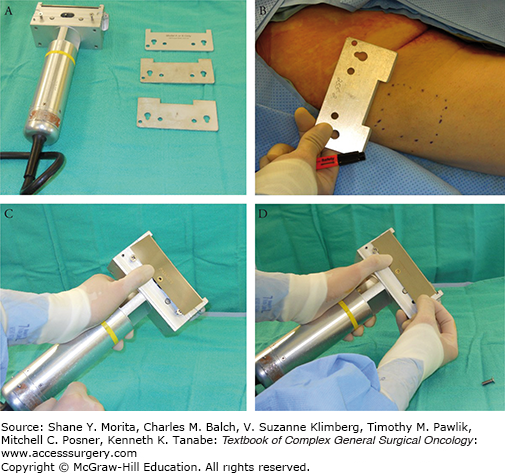
The two methods of harvesting STSGs are the freehand knife and the power-driven dermatome. In modern-day surgery, the freehand method is not popular because the technique is very user-dependent; the thickness is difficult to control and graft edges are irregular. The blades are equipped with a guard, which limits the thickness of the graft to be harvested (Fig. 159-2). These handheld blades come in handy in facilities lacking compressed air or electrical power, or lacking financial resources. The Brown dermatome was the first of the electric dermatomes to be developed and is especially valuable because a large amount of skin can be harvested rapidly.12 The blades are presterilized while the cutting head and rubber-covered cable are autoclaved. Two commonly used motorized dermatomes are the Zimmer (Zimmer, Inc., Warsaw, IN) dermatome (air and electric), and Padgett (Integra Life Sciences Holdings Corp., Plainsboro, NJ) dermatome (Fig. 159-3).
FIGURE 159-3
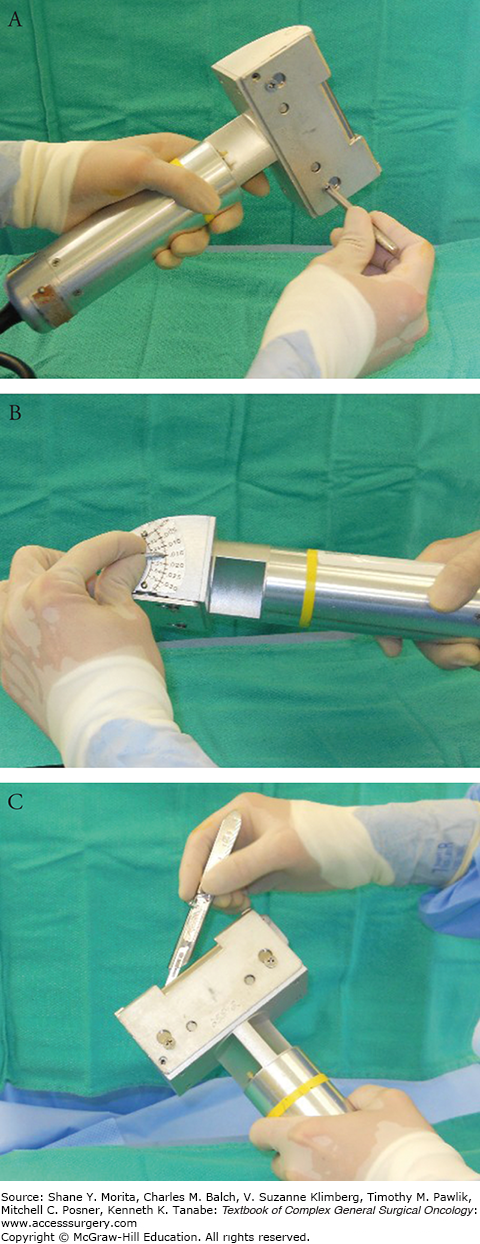
Along with Figures 159-4 and 159-5, this set of photographs demonstrate how to operate the Padgett dermatome. A. Padgett dermatome with assortment of guards. B. Assorted guards control the width of the graft to be harvested. C, D. One of two screws is temporarily removed in order for the blade to negotiate into position.
The air-powered Padgett dermatome is easy to handle and comes with several available guards 3, 4, and 5 in. wide (Fig. 159-3A). The dimension of the graft to be harvested is marked and it should be 15% to 20% larger than the dimensions of the defect. The width of the guard is then chosen based on this width marked (Fig. 159-3B). The key in using these devices is to make sure the surgeon is accustomed to assembling the blade and making sure it is seated properly (Figs. 159-3C,D). The guard should not be overtightened (Fig. 159-4A). The dialing of the depth needs to be double-checked to assure the thickness of harvest (Fig. 159-4B). STSGs are subdivided into thin, medium, and thick STSGs.13
Thin (0.008 to 0.012 in. or 0.2 to 0.3 mm)
Medium (0.012 to 0.018 in. or 0.3 to 0.45 mm)
Thick (0.018 to 0.030 in. or 0.45 to 0.75 mm)
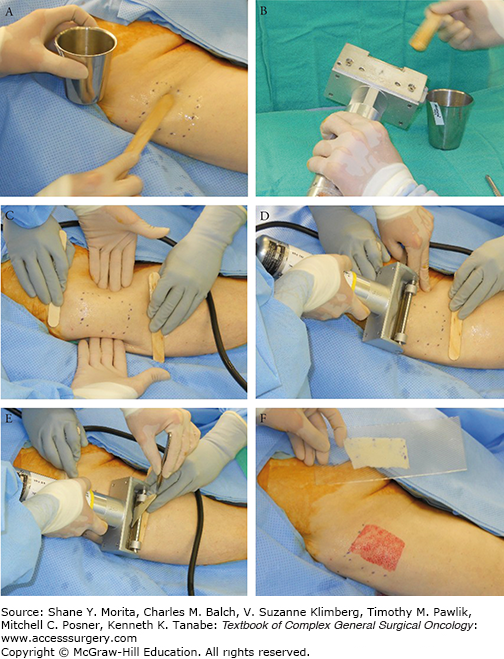
The thickness of commonly used split-thickness graft is between 0.012 and 0.018 in. (0.30 to 0.45 mm). The average STSG is cut at 0.015 in., which can be checked by inserting no. 15 Bard-Parker blade between the blade and guard (Fig. 159-4C).14 Lubrication with mineral oil can be applied to the blade (Fig. 159-5B). The power source is then connected and tested. It is prudent to have a backup dermatome available in case of machine malfunction. The skin can be lubricated with mineral oil, saline, or other sterile agent, such as Vaseline ointment (Fig. 159-5A). Importantly, if Betadine or another sticky antiseptic is used in the prep, this must be washed off before harvesting; otherwise, excessive friction at the dermatome and skin interface will prevent smooth gliding of the blade. An assistant should apply traction on both ends and/or sides using a tongue blade to exert tension during the harvest (Fig. 159-5C). The dermatome is positioned at 30 degrees to the skin surface and the power switch is turned on (Fig. 159-5D). The surgeon exerts gentle pressure with constant forward movement. Excessive pressure against the skin could result in excessive depth of harvest. The skin graft can be lifted with a pair of forceps during the process to assure smooth and adequate harvest of skin (Fig. 159-5E). At the end of the glide, the surgeon should lift up the dermatome to cut the graft off from the donor surface. Modification of technique includes infiltration with lactated Ringer’s solution beneath the donor site to stiffen the skin and make the harvest easier and more predictable, especially over bony prominences. Epinephrine can be added to control bleeding, which probably is not necessary in most oncological cases where the defects are small in contrast to the treatment of major burns.
FIGURE 159-5
A, B. It is important to lubricate the blade and donor skin with mineral oil or agent of choice. C, D. Assistants apply pressure from the sides and counter traction on opposite ends with tongue blades. E. This photograph shows the option of lifting the skin up to assure a proper, smooth glide and harvest. F. The skin graft is transferred to a carrier if meshing is desired.
If the surgeon intends to mesh the graft using the popular Zimmer Meshgraft II system (Zimmer, Inc., Warsaw, IN), the graft can be placed on the carrier (Dermacarrier®) with either side down (Fig. 159-5F). The Dermacarrier, however, must be positioned with its grooves side up against the graft (Fig. 159-6A); otherwise, the graft will not mesh but will be shredded. The carriers come in mesh ratios of 1.5:1, 3:1, 6:1, and 9:1. With the Zimmer Meshgraft II system, the surgeon or assistant must lift the graft off the cylinder if it gets caught on the cutter (Fig. 159-6B). The routine mesh ratio is 1.5, which expands the graft surface area by 50% (Fig. 159-6C,D). Zimmer also offers a mesher which uses a smooth carrier that inserts into the mesher, guided by matching templates on the bottom of the carrier. It is a little wider than the former, at 4 in. instead of 3.1 in. and holds carriers that are 8 in. and 16 in. long. The Brennen mesher (Integra LifeSciences Holdings Corp, Plainsboro, NJ) offers another design in skin graft meshing which does not rely on disposable carriers. It meshes strictly by a noncrushing mechanism of cylindrical cutters with ratios of 1:1, 2:1, 3:1, 4:1, 6:1, and 8:1. Wider graft up to 4.5 in. can be meshed with this mesher, which uses no disposable parts.
FIGURE 159-6
A. The surgeon lines up the carrier parallel to the side stop on mesher, perpendicular to the meshing mechanism. B. The surgeon prepares to lift up the graft so it does not get caught up into the roller. C. The assistant is prepared to catch the carrier so it does not fall off the table. D. An example of 1.5:1 meshed graft is shown.

The only instrument required with full-thickness harvest is the scalpel. One can use the paper from the packaging of sterile gloves to cut a template of the defect, and this template can be applied to the recipient site to estimate the size of graft required. It is generally a wise decision to harvest the graft slightly wider because of primary contraction of the graft. All of the dermis is included in the harvest. Technique can vary as to how much fat is removed with the graft. The fat or subcutaneous tissue needs to be completely trimmed off the graft using curved sharp scissors.
For the STSG, the surgeon has to decide whether or not to mesh the graft. Meshing allows for better drainage from the wound bed and allows for the graft to accommodate to a convoluted wound surface. Meshing is also appropriate for large surface areas in order to minimize the donor site wound burden and scarring. However, the interstices create raw areas which heal by secondary intention; thus, more scar contracture is associated with meshing. The cosmetic deformity is also more pronounced with a net-like appearance. Therefore, if the area to be grafted is not expected to contract much, it is advisable not to mesh the graft. This also applies to areas in which cosmetic deformity should be minimized, such as the face. One must keep in mind that meshing does not guarantee the prevention of hematoma formation; therefore, there is no substitution to meticulous hemostasis. If adequate hemostasis cannot be attained at the time of the wound creation or debridement, a pressure dressing is placed and skin grafting is performed at a second stage.
In applying the graft, the external epithelial side must be assured to be up. The dermal side is usually shiny compared to the duller, epithelial side. The color difference is usually obvious in dark skin grafts but can be difficult to discern in patient with very thin and pale skin.
It is crucial to make sure there is no subcutaneous fat left behind before graft application. As with unmeshed STSG, perforation with a knife (“pie-crusting”) can also be performed to some degree to allow drainage of wound fluid after application.
Before the skin graft is applied, it is essential that the recipient wound be prepared properly. The wound bed must be well vascularized and free of infection. Absolute hemostasis needs to be present. Acute, heavily contaminated wounds are not amenable to immediate skin grafting. All devitalized tissue must be removed sharply. If hemostasis cannot be achieved, a pressure dressing can be applied, and the grafting can be delayed for another day. For chronic wounds, signs of infection need to be ruled out. Chronic unhealthy-appearing (gray, tan, dull red, hypertrophic) granulation tissue should be debrided. Ideally, the chronic wound should have healthy bright red granulation tissue with signs of epithelialization at the margins. If the wound has questionable infection or excessive colonization, a wound biopsy can be performed for quantitative analysis. If there are more than 105 organisms per gram of tissue, the wound should not be grafted.15
Immediately before dressing application, the wound should be inspected for hematoma. For unmeshed STSG and FTSG, the blood made is hidden under the graft, so careful attention to details is important; an angiocath can be placed under the graft to flush out the blood which may have accumulated.
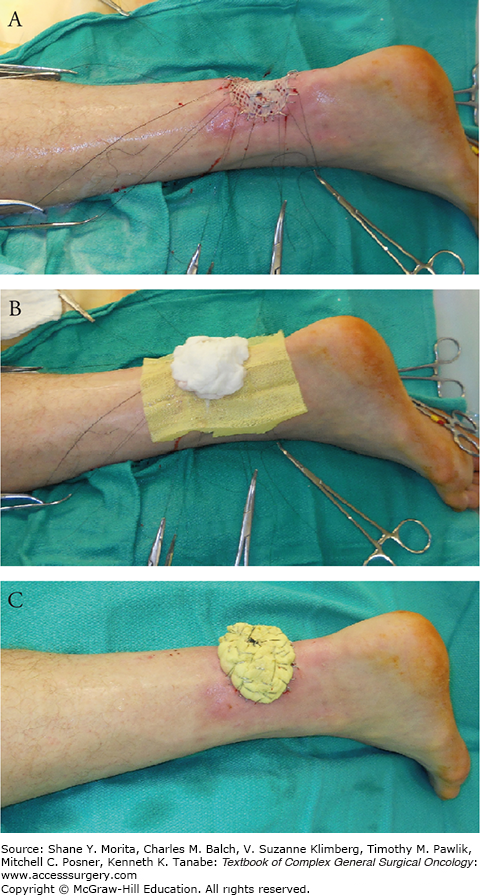
The dressing is as vital as the execution of the grafting. If the graft is not properly stabilized, the operation is likely to fail. The first step entails the application of a nonadherent gauze, such as Xeroform, to prevent graft adherence during removal at the first dressing change. Moistened cotton balls or fluffed gauze using normal saline, mineral oil, or other physiologic solution is then applied over the nonadherent gauze to conform to the wound bed, ensuring contact between graft and wound bed. Sutures at the edges, left long, can be tied to create a tie-over bolus dressing (Fig. 159-7). Figure 159-8 shows skin graft healed at 3 months. Alternatively, the nonadherent dressing, Adaptic (Johnson & Johnson, New Brunswick, NJ), can be stapled to native skin at the periphery of the defect; then the wings of the nonadherent dressing can be wrapped over the moistened cotton balls and stabled together to provide a snug bolster. Various materials, including foam, such as Reston (3M, St. Paul, MN), have been described for such bolster dressings.
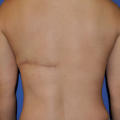
FIGURE 159-8
Postoperative photo at 3 months showing complete take of skin graft.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree