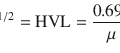(1)
Institute of Oncology “Prof. Ion Chiricuță”, Nuclear Medicine & Endocrine Tumors, Cluj-Napoca, Romania
Look deep into nature, and then you will understand everything better.
Albert Einstein
1.1 Principles and History of Nuclear Medicine
Nuclear medicine is a medical specialty involving the application of radioactive substances in the diagnostic and therapy of diseases. The most important characteristic of nuclear medicine methods is the ability of the substances used as tracers to reflect the function of different organs; these methods can reveal also structure and anatomy.
Nuclear medicine procedures and images show function, physiology, and metabolism and have the capacity to determinate the cause, nature, or manifestations of a disease or condition. This may include monitoring the progression or regression of the disease or injury in response to therapy.
The collaboration between Professor Karl T. Compton, President of the Massachusetts Institute of Technology, and Saul Hertz, the Director of the Thyroid Clinic of Massachusetts General Hospital, Boston, was part of the early history of nuclear medicine. In the letter wrote by Saul Hertz in December 23, 1936, he underlined the fact that if the radioiodine artificially obtained by the team of Professor Compton is selectively taken up by the thyroid gland, this will be a useful method of therapy in cases of overactivity of the thyroid gland. Doctor Saul Hertz was one of the scientists whose pioneering work played an important part in the foundation of nuclear medicine. In 1937 he used radioiodine in studying thyroid physiology, and he was the first who used the “atomic cocktail” (iodine-131) for thyroid treatment, in 1941. Another pioneer scientist that suggested the use of radionuclides to label compounds in biology and medicine was the Hungarian chemist G. Hevesy, awarded with the Nobel Prize in 1943.
Comparing to standard radiography, nuclear medicine images (scans or scintigraphies) always require the administration of radioactive tracers. These radiolabeled drugs, named radiopharmaceuticals, usually reflect the blood flow, the capillary permeability, the specific tissue extraction, or the specific pathways of metabolism and pathophysiologic processes.
One of the most well-known examples regarding the fundamental differences between nuclear scans and standard radiology is the evaluation of brain activity, for example, a computer tomography (CT) performed in a situation of a corpse or a patient being in cerebral death can reveal a normal brain structure, while a nuclear scan confirms the lack of neurological activity and death.
Comparing to X-ray methods, nuclear medicine has specific patterns of activity. A radiograph results when an external beam passes through the patient and is recorded on the other side. This principle is equally true for a simple classic radiography or a computed tomography (CT). Nuclear scans result when a radioactive tracer administered inside the patient allows the external record of the radiation activity. This difference explains the need for specific instruments and equipment in nuclear medicine, comparing to radiology.
All nuclear medicine methods are using radioactive tracers called radiopharmaceuticals. Most of them consist of two parts: a radioactive compound that provides the signal, which will be detected in the exterior, and a ligand that determines the tracer’s distribution in the body.
Most of the nuclear imaging procedures are “in vivo” and involve the administration to the patient of relatively short-lived radionuclides and the use of some specific instruments called gamma cameras or other specialized instrumentation to form the images; these images will be either planar or tomographic ones.
The planar images are static or dynamic, and they are obtained as if the distribution of the radiopharmaceutical within the patient’s body was from a single “plan.”
Tomographic images are those that have been composed and constructed from linear projection data, acquired by placing the radiation detectors 360° around the patient. The tomographic images in nuclear medicine are single-photon emission tomography (SPECT), when the camera rotates around the patient, and positron emission tomography (PET), when the radiation detectors are located in a ring around the patient and their use leads to detection. The idea to combine PET with CT was made in the early 1990s by Townsend, Nutt, and co-workers, and it was to be 7 years before the first prototype combined PET/CT scanner was completed and installed in the University of Pittsburgh Medical Center, USA.
The clinical importance of hybrid imaging has rapidly increased. The images obtained are fused, providing anatomical information from CT or MRI and the functional information from PET. Each system performs a different type of tomography: transmission for CT and emission for PET. Due to this, the detection and acquisition systems of the two modalities are different and highly specialized for the specific requirements of each modality.
1.2 Basics of Nuclear Physics
1.2.1 Atomic Structure
The atom is the basic unit of matter; originally, the sense of the word atom denoted a particle that cannot be divided into smaller particles, but in modern scientific usage, the atom is composed of various subatomic particles. The constituting particles of an atom are the electrons, the protons, and the neutrons. Even if the atomic models described in the last several decades (the quantum models) are more complex, the structure of the three particles is one of the simplest being the first model discovered by E. Rutherford and by the Nobel Prize-awarded Danish physicist Niels Bohr; this model is sufficient to explain the basic physical concepts necessary in nuclear medicine.
Atoms consist of small and very dense nucleus composed of neutrons and protons, surrounded by electrons. The number of electrons in one atom is equal to the number of protons from the nucleus. The number of protons from the nucleus represents the Z number, and it is named atomic number.
The atoms having the same atomic number form a chemical element.
As the heavy particles, protons and neutrons, are gathered in the nucleus, the atomic mass is concentrated within it.
The atomic number Z is typically subscripted preceding the element symbol ( Z X).
Another characteristic element of the atom is the atomic mass. The atomic mass is measured in atomic mass unit, where 1 AMU = 1/12 mass of carbon measured in grams. The atomic mass number is equal to the number of protons plus neutrons and provides the average weight of all isotopes of any given element.
The protons (p or p+) weigh 1 atomic mass unit (AMU) and carry a positive electric charge. Neutrons (n or n0) are minimally heavier and have no electric charge, while electrons (e or e−) have a mass of only 0.000548 AMU and carry a negative charge.
The atomic mass is usually represented as superscript preceding the element symbol ( A X).
The chemical symbol of an element is represented as follows:
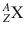
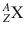
Atoms with the same atomic number, but different atomic mass numbers, are called isotopes. The isotopes of an element have the same chemical properties. The electron’s configuration of an atom determines its chemical properties and reactivity.
A nuclide is a nuclear configuration that contains a specific number of neutrons and protons. Some nuclides are inherently stable, while others are not. Unstable nuclides are called radionuclides. The radionuclides trend to move to stable configuration through the process of radioactive decay. There is a large spectrum in the degree of radioactivity among different nuclides. Some are extremely unstable and decay within microseconds, and others decay over billions of years. The time required for one-half of the original radioactive sample to decay is the physical half-life (T 1/2) of the nuclide. Size is one of the main determinants of nuclear stability; therefore, heavy nuclides, which have large atomic diameters, are less stable than the light- or medium-weight nuclides.
Elements can be grouped into families:
Isotopes: Nuclides having the same atomic number, Z
Isobars: Nuclides with the same mass number, A
Isotones: Nuclides with the same neutron number, N
All isotopes of the elements having the mass numbers above bismuth-209 (atomic number Z = 83) are radioactive. The heaviest stable (nonradioactive) element in the nature is bismuth-209, at least according to nowadays knowledges; in 2003 the researchers from the Institut d’Astrophysique Spatiale were able to calculate the half-life of bismuth-209 as 1.9 × 1019 years, which is about a billion (109) times the age of the universe. If a nuclide does not contain the stable neutron/proton ratio between 1 and 1.5, it is probably radioactive.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree