Measurement
Surgery recommended in all who meet the following guideline
Age
<50 years old
Bone mineral density
T-score <−2.5 at any site and/or prior fragility fracture
Creatinine clearance (calculated)
<60 mL/min
Serum calcium
>1 mg/dL above the upper limit of normal
Localization studies to identify possible parathyroid adenomas are not necessary for the diagnosis of primary hyperparathyroidism, but are often done to assist with a planned surgery. The most commonly used localization studies are sestamibi scans and cervical ultrasound [6]. Improvement in such preoperative localization techniques, and the development of rapid intraoperative PTH assays have contributed to a shift from conventional four gland parathyroid exploration to minimally invasive parathyroidectomy procedures [6]. Minimally invasive parathyroidectomy procedures have estimated cure rates of 95–98%, with complication rates of 1–3% in initial explorations [6].
Cardiovascular Manifestations of Primary Hyperparathyroidism
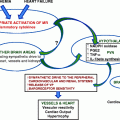
The cardiovascular manifestations of primary hyperparathyroidism are a frequently discussed and evolving topic. Studies on the cardiovascular manifestations of the disorder have assessed a variety of endpoints, including cardiovascular mortality, hypertension, coronary artery disease, presence and extent of valvular and myocardial calcifications, left ventricular hypertrophy, cardiac conduction system disease, and carotid plaque [7–16]. Much of the discrepant data in the literature can be reconciled by the wide variations in disease severity studied. Reports on cohorts with symptomatic disease and marked hypercalcemia cannot be compared with those on populations in whom there are minimal calcium and PTH elevations in the absence of overt symptomatology.
While a full discussion of the cardiovascular manifestations of primary hyperparathyroidism is beyond the scope of this chapter, a brief review of the evolution of cardiovascular mortality is instructive, as it demonstrates the change in disease presentation and manifestation over time. Increased cardiovascular mortality is well described in patients with severe and moderately severe primary hyperparathyroidism [17–19]. The increase in mortality was reported to decrease with time following parathyroidectomy [19, 20].
Mortality is not clearly increased in patients with mild hyperparathyroidism. Analysis of 1,052 patients who underwent surgical treatment for primary hyperparathyroidism (median preoperative calcium 11.2 mg/dL) found no increase in mortality compared to the general population after a median follow-up of 12 years [21]. Indeed, the only population-based study from the United States did not find an increase in cardiovascular mortality in a mildly hypercalcemic cohort (relative risk: 0.6) [22]. A report by Nilsson et al. may explain the shift in mortality rates over time. Their study of over 10,000 Swedish patients treated for primary hyperparathyroidism between 1958 through 1997 found increased mortality in the overall cohort compared to the normal population. However, there was no increase in mortality in the subgroup of patients operated on in more recent years (1985–1997) [7]. One possible explanation for this observation is that the patients seen more recently had lower serum calcium levels. Another study suggesting that the decline in mortality may be associated with a decline in disease severity finds improved survival in cases from recent years when patients had lower preoperative serum calcium levels [20]. Only one population-based study in patients with mild untreated primary hyperparathyroidism reported an increased risk of mortality [23]. However, criteria for the diagnosis of primary hyperparathyroidism in this study raise the possibility that patients with other disorders may have been included in this cohort, making it difficult to interpret these findings. In summary, while increased mortality was seen in more severe primary hyperparathyroidism, the majority of existing studies do not demonstrate strong evidence for increased cardiovascular mortality in milder disease. This change may be due to the decline in calcium levels, although other factors, such as advances in treatment for cardiovascular disease, may also be responsible [15].
Hypertension
Hypertension is often seen in patients with primary hyperparathyroidism. The reported prevalence of hypertension in primary hyperparathyroidism varies widely among different studies, ranging from around 20 to 80% [8, 24–28]. There has been significant debate regarding the nature of this association as well as the impact of surgical cure. To further explore this relationship, we will focus on potential mechanisms linking hypertension and primary hyperparathyroidism and review the current data on the reversibility of hypertension with surgical cure of primary hyperparathyroidism. If surgical cure were shown to improve hypertension, this would have important implications for management of the parathyroid disease.
Mechanisms of Hypertension in Primary Hyperparathyroidism
Multiple mechanisms have been proposed to explain the possible link between hyperparathyroidism and hypertension. Several hypotheses attribute the association to one or both of the cardinal biochemical abnormalities seen in primary hyperparathyroidism, the elevations in serum calcium and PTH levels. Other theories link hypertension to associated findings, such as renal insufficiency, hypomagnesemia, and PTH effects on the renin-aldosterone system.
Hypercalcemia has been proposed to underlie the association between hypertension and primary hyperparathyroidism. Suggestive data comes from several studies in nonhyperparathyroid subjects. In one, the acute hypercalcemia resulting from an infusion of calcium in healthy volunteers resulted in increased systolic blood pressure [29]. In this study, hypercalcemia led to a dose-dependent impairment in endothelial vasodilatory function, suggesting that alterations in vasodilatory response could explain the resultant rise in blood pressure. Alternatively, calcium may be related to hypertension via its role in vascular smooth muscle cells contraction [30]. Similar results linking calcium infusion and rise in blood pressure were seen in another study investigating calcium infusion in both normotensive and hypertensive subjects [31]. This study also showed the rise in blood pressure could be prevented by administration of a calcium channel blocker. In primary hyperparathyroidism, a study of ambulatory blood pressure in 53 patients, of whom 47% were hypertensive, found that 24 h and daytime diastolic blood pressure values were significantly associated with serum ionized calcium in the hypertensive patients [8]. No associations were found between the blood pressure measurements and PTH. However, even if calcium is important in this association, data suggest that this relationship is likely to be complex. For example, when acute hypercalcemia was induced in patients with undetectable PTH levels (due to postoperative hypoparathyroidism) no rise in blood pressure was seen, while a euparathyroid control group did have a significant rise in systolic blood pressure. This suggests a possible role for PTH as a mediator of this relationship [32].
Similarly, data on a possible role for PTH in the association of primary hyperparathyroidism with hypertension is not straightforward. PTH causes dose-dependent transient vasodilation while chronic continuous infusion of PTH in normotensive subjects results in hypercalcemia and hypertension [33, 34]. PTH may be indirectly involved in the mechanism causing hypertension via stimulation of renin as discussed below, or more directly via a prostimulatory effect (mediated by calcium or by PTH activation of the renal 1-alpha hydroxylase enzyme) or prosclerotic effect on vascular smooth muscle cells [35, 36]. Population-based studies also suggest a link between PTH and hypertension in nonhyperparathyroid subjects. For example, a study of 1,205 older men and women found a positive association between blood pressure and serum PTH levels [37]. It is not clear whether this could be mediated by another factor, and whether those with higher PTH levels might have secondary hyperparathyroidism and an alternate reason for both PTH elevation and increased blood pressure. Thus in primary hyperparathyroidism data on the association of blood pressure with either PTH or calcium are conflicting, with studies suggesting one or the other may be responsible, and others revealing no relationship between PTH or calcium and blood pressure [38].
Impaired renal function has also been implicated as the possible link between primary hyperparathyroidism and hypertension. A review of 115 patients with primary hyperparathyroidism found that 54.8% were hypertensive. The patients with both primary hyperparathyroidism and hypertension had significantly higher serum urea and creatinine than the patients with primary hyperparathyroidism who were normotensive [39]. In addition, serum urea was associated with systolic blood pressure among all patients with primary hyperparathyroidism, while no correlations were found between blood pressure and either calcium or PTH. A similar association between renal dysfunction and hypertension in primary hyperparathyroidism has been reported in other studies, but it remains unclear if renal dysfunction is caused by hypertension in these patients, or if it is the underlying link between hypertension and primary hyperparathyroidism [40]. Additionally, renal dysfunction is not a consistent finding in studies investigating these associations [26, 38]. The study of 194 patients with primary hyperparathyroidism in which calcium and PTH did not correlate to blood pressure, found no difference in renal function between those with and without hypertension [38].
Another hypothesis regarding the cause of hypertension in patients with primary hyperparathyroidism proposes a potential role for hypomagnesemia in this association [41, 42]. Both experimental and epidemiologic data have suggested a link between hypomagnesemia and cardiovascular pathology, including hypertension [43, 44]. Hypomagnesemia leads to increased intracellular calcium levels, which may alter vascular smooth muscle contraction. In addition, magnesium efflux itself may be linked to calcium signaling pathways [44]. In an experiment on isolated coronary arteries, removal of magnesium from the surrounding organ chamber solution was associated with impaired endothelium-dependent vasodilation. This effect was reversed by replacement of magnesium [45]. This relationship has also been shown in human studies in vivo. In a study involving healthy young adults, infusion of intra-arterial magnesium increased endothelium-dependent vasodilation, although there was no change in systemic blood pressure [46].
The possible role of hypomagnesemia in mediating hypertension in the setting of hypercalcemia was first reported in studies using animal models. In a study involving hypercalcemic dogs, lowering magnesium levels was associated with increased peripheral resistance [42]. Primary hyperparathyroidism may cause hypomagnesemia because hypercalcemia results in decreased renal magnesium reabsorption. There is epidemiologic data that may support this theory. A retrospective study of 89 patients with primary hyperparathyroidism who underwent parathyroidectomy found no difference between the hypertensive (N = 43) and normotensive (N = 46) patients in terms of calcium, creatinine clearance, or phosphorus levels. However, the hypertensive patients had significantly lower mean serum magnesium levels [41]. Other than this limited observational data, it remains unclear whether hypomagnesemia actually plays a role in an association between hypertension and primary hyperparathyroidism.
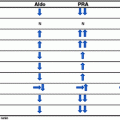
The renin-stimulating ability of PTH, which is thought to be mediated through the inhibition of calcium influx, has also been mentioned as the possible link between primary hyperparathyroidism and hypertension [47]. PTH stimulates renin release in the proglomerular arterioles of rat kidneys [47, 48]. However, most studies investigating the role of the renin-aldosterone system in patients with primary hyperparathyroidism involved only very small groups of patients, and many were done over 30 years ago. In one of the earliest studies, 4 of 7 patients with primary hyperparathyroidism had elevated plasma renin activity (PRA) and hypertension, which both normalized following parathyroidectomy [49]. However, in another study in which patients with primary hyperparathyroidism and hypertension with elevated baseline PRA were given saralasin, an angiotensin inhibitor, no effect on blood pressure was found [50]. Similarly negative results were found in another small study (N = 11) [51]. Studies examining changes in the renin-angiotensin-aldosterone system pre- and post-parathyroidectomy also have conflicting results. In a study of 16 normotensive patients with primary hyperparathyroidism, basal PRA and stimulated PRA and aldosterone levels were shown to be elevated. Although these levels normalized following parathyroidectomy, there was no clinically significant change in blood pressure [52]. Another study found no difference in PRA and aldosterone levels between patients with primary hyperparathyroidism and those with essential hypertension or normotensive controls [53]. The same study found no change in PRA, aldosterone levels, or blood pressure 1 and 6 months post-parathyroidectomy. Finally, among 34 patients with primary hyperparathyroidism, the PRA and aldosterone levels were higher in the patients with hypertension (10 patients) compared to the levels in patients who were normotensive [54]. Additionally, in 8 of 10 of the patients with primary hyperparathyroidism and hypertension, the renin and aldosterone levels normalized, as did their blood pressure, following parathyroidectomy. With such contradictory results and underpowered studies, no firm support currently exists for the association between the renin-aldosterone system and hypertension in primary hyperparathyroidism.
Multiple Endocrine Neoplasia
While there remains considerable controversy regarding the presence of a true association between hypertension and primary hyperparathyroidism in sporadic disease, the same is not true for patients with primary hyperparathyroidism in the setting of MEN. Hypertension in this subgroup of patients can be caused by the presence of an MEN-related pheochromocytoma. The most common clinical sign of pheochromocytomas is hypertension, which is seen in 85–90% of cases [55].
Hyperparathyroidism is a feature seen in both MEN type 1 and 2A. MEN1 is a hereditary syndrome associated with hyperparathyroidism, pituitary adenomas, and pancreatic islet cell tumors. Primary hyperparathyroidism is the most common and often the first feature of MEN1 and there is almost 100% penetrance by age 50 [56]. Another common feature of MEN1 is the presence of entero-pancreatic islet tumors, which occur in 30–75% of MEN1 patients, and include gastrinomas in 40% of MEN1 patients [56]. The Zollinger-Ellison syndrome (ZES) features a gastrinoma, recurrent peptic ulcers, and elevated gastrin levels [57]. When primary hyperparathyroidism and ZES are simultaneously present, the hypercalcemia may worsen ZES because it increases gastrin secretion and also decreases responsiveness to antisecretory agents [56, 58]. Parathyroidectomy improves ZES by normalizing calcium levels, leading to decreased gastrin production. Pheochromocytomas are usually not present in MEN1, but there are case reports of some families having MEN1-related pheochromocytomas, which may then present with hypertension [55]. However, pheochromocytomas are a more common feature in MEN type 2A, which is an autosomal dominant genetic syndrome characterized by the presence of hyperparathyroidism, medullary thyroid cancer, and pheochromocytoma [59]. Primary hyperparathyroidism is seen in 15–20% of patients with MEN2A, while approximately 50% develop pheochromocytomas. Overall, the clinical features of primary hyperparathyroidism in MEN2A are similar to as in sporadic cases. Interestingly, among MEN2 patients with pheochromocytomas, only about 50% have associated hypertension [55]. In addition, in these patients the hypertension is more often paroxysmal and not sustained.
The presence of a pheochromocytoma in patients with MEN-related primary hyperparathyroidism likely explains the link between primary hyperparathyroidism and hypertension in this subgroup. Hypertension in these cases is often attributable to catecholamine excess from the pheochromocytoma and would be expected to resolve following pheochromocytoma resection. After successful removal of a pheochromocytoma, only 25% of patients have persistent hypertension, which is then probably due to coexistent essential hypertension [55]. Unlike the link between improvement in ZES and treatment of primary hyperparathyroidism, there are no data to suggest that parathyroidectomy affects hypertension related to an underlying pheochromocytoma in MEN patients. The subject of hypertension and pheochromocytoma will be covered in much greater detail elsewhere, in Chap. 10.
Reversibility of Hypertension by Parathyroidectomy
If a direct link exists between primary hyperparathyroidism and hypertension, successful parathyroidectomy with resultant cure would be expected to reverse the elevated blood pressure. In several older studies from the 1970s and 1980s, hypertension was reported to improve following parathyroidectomy in up to half of all hypertensive patients [26, 27, 40]. However, in these studies, mean serum calcium levels were markedly higher than we see today, as high as 12.75 mg/dL [40]. Even in older studies with higher mean serum calcium levels, improvement in hypertension following parathyroidectomy was not always seen. For example, in a retrospective review from 1982 (preoperative calcium level 11.7 mg/dL), hypertension reversed in only 2 of the 15 patients following surgery [25].
Furthermore, some observational studies raised the possibility that hypertension could actually worsen following surgery [60, 61]. Fifteen year follow-up of a cohort enrolled in 1969 found higher systolic blood pressure, and a greater rise in blood pressure over time, in the patients who underwent parathyroidectomy as compared to those with persistent untreated hyperparathyroidism, although the number of patients included in each group at the 15 year follow-up time-point was very small [61]. Another observational study of 62 patients who underwent parathyroidectomy and were followed for up to a mean of 9 years postoperatively, found no improvement in the patients with known hypertension (N = 18), and 45% of those without a history of hypertension developed hypertension post-parathyroidectomy [60].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree