Such chronic diseases are mainly atherosclerosis, diabetes mellitus, and obesity. Thus, in view of the multifactorial origin of aging symptoms, effects of LOH may not clearly be detectable. However, evidence from hypogonadism in younger men suggests a range of androgen-dependent functions, for which an adverse affection by LOH can be assumed. Especially the beneficial effects of testosterone substitution on these morbidities provide evidence for a significant role of androgens within the symptomatology of elderly men with LOH. These are discussed in the following.
Diagnostic Approaches to Male Hypogonadism
LOH is a diagnosis made by exclusion of other morbidities resulting in testosterone deficiency.
Hypogonadism manifests itself with a variety of symptoms, which can be of psychological, cognitive, sexual as well as somatic nature. The time of onset is significant in manifestation patterns of hypogonadism as well as the actual serum concentration of testosterone, leading to a stepwise increase of symptoms with decreasing testosterone levels. Older hypogonadal men usually exhibit characteristics similar to younger patients, but possibly to a lower degree. The pattern of complaints in older men may be caused at least partly by various other chronic illnesses related to the aging process (Table 11.1).
Classic symptoms and signs | Nonspecific symptoms and signs |
|---|---|
Reduced libido and sexual activity | Decreased energy, self-confidence |
Decreased spontaneous (morning) erections | Depressed mood or dysthymia |
Gynecomastia | Poor concentration and memory |
Loss of body (axillary and pubic) hair | Sleep disturbance, increased sleepiness |
Small (especially <5 mL) or shrinking testes | Mild anemia |
Infertility, low sperm count | Reduced muscle bulk and strength |
Low trauma fracture, low bone mineral density | Increased body fat |
Hot flushes, sweats | Diminished physical or work performance |
Once a patient presents with symptoms causing clinical suspicion of testosterone deficiency, standardized patterns of diagnostics and therapy should be followed. Underlying causes of hypogonadism are disorders located at the testicular source of testosterone, the Leydig cells (primary hypogonadism) or at the central regulation unit, consisting of the hypothalamus and the pituitary gland (secondary hypogonadism), the latter secreting luteinizing hormone (LH), which stimulates Leydig cells.
Hypogonadal symptoms also occur in cases of target organ resistance, mostly due to inherited alterations of the androgen receptor; in this case, elevated concentrations of both testosterone and LH are found and the androgen-sensitivity index points to androgen resistance (see below, pharmacogenetic implications and ref. [24]).
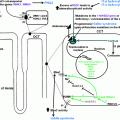
For diagnostic purposes in men suspected of having male hypogonadism, assessment of total testosterone, luteinizing hormone, follicle stimulation hormone (FSH), prolactin, and estradiol is helpful, as well as calculation of free testosterone from total testosterone and sex hormone binding globulin (SHBG) (see Fig. 11.2); this complete overview of hormones classifies the clinical picture in regard to fertility and estrogen-related features of the phenotype (e.g., fat distribution, gynecomastia, bone density). Determination of androgen receptor genotype and concentrations of serum dihydrotestosterone should be performed in special cases of suspected androgen resistance.
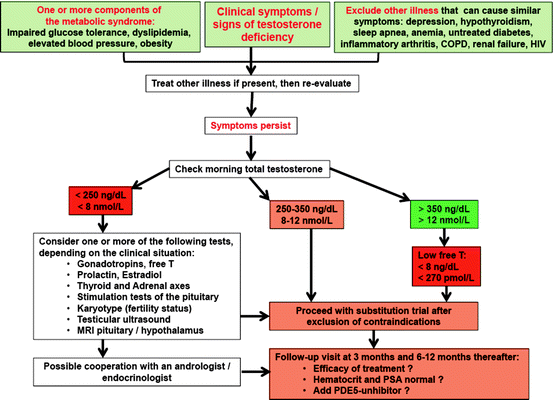

Fig. 11.2
Diagnostic algorithm for evaluating men with possible testosterone deficiency syndrome (TDS). Modified from ref. [8]
Questionnaires related to possible androgen deficits have been utilized for screening purposes and may be useful for monitoring symptoms during testosterone substitution therapy. The androgen deficiency in aging male (ADAM) questionnaire, developed at St. Louis University and the aging male symptom scale (AMS) developed in Germany are two widely used clinical tools to assess clinical symptoms of androgen deficiency. Both of them have high sensitivities (88% and 83%, respectively) but they have very low specificities (60% and 39%, respectively) increasing the chance of false positive results [25–27].
In addition, the effects of decreasing testosterone levels are enhanced by an age-dependent increase of levels of SHBG, further lowering the amount of bioavailable testosterone. Possibly reduced levels of growth hormone and insulin-like growth factor 1, which inhibit SHBG production in hepatocytes, are responsible for this phenomenon.
In cases of borderline total testosterone and existing symptoms (see below), low concentrations of bioavailable testosterone might be taken into consideration for an initiation of treatment. Notwithstanding, normal limits for bioavailable testosterone have not been defined.
Generally, there is no age-limit concerning the substitution of testosterone. Especially the short-acting, dose-titrable transdermal preparations provide the physician with the ability to treat men with LOH (as defined by low testosterone levels in combination with the major symptoms of hypogonadism such as depression, loss of vigor and libido, anemia). Testosterone levels in the low normal to mid-normal range should be aimed at. Reference ranges for healthy nonobese young men have recently been established [28]. In a study including 3,672 men from age 20 to age 98 years, mean age 59.7 years, it was shown that a total testosterone level should exceed 350 ng/dL to reliably predict normal free testosterone and that total testosterone levels have low specificity for the biochemical diagnosis of hypogonadism unless they are less than 150 ng/dL [29].
Body Composition as a Key Component in Understanding Testosterone Action on Metabolism And Blood Pressure
Muscle Tissue
In addition to the effects of T deficiency on body fat content, a loss of fat free mass and decrease in muscle protein synthesis is observed in hypogonadal men [30]. In agreement, when androgens are substituted such patients, fat-free mass increases significantly, which can be attributed to muscle growth [31, 32]. The growth of muscle tissue seems to follow a linear dose–response relationship over a wide range of T levels, as was demonstrated in healthy young men receiving androgen ablation by a GnRH agonist and subsequent T administration in various doses to achieve low to supraphysiological levels. As fat-free mass increased with the increasing T dose, so did volumes of thigh muscles [33]. Muscle biopsies in these men demonstrated a homogenous increase in cross-sectional areas of both type I and II muscle fibers, which maintained their proportion. As muscle fiber hypertrophy involves the addition of newly formed myonuclei via the fusion of myogenic cells, the myonuclear number increased in direct relation to the increase in muscle fiber diameter. Muscle cell hyperplasia did not play a significant role [34].
Body Fat
Cross-sectional investigations in healthy, eugonadal have demonstrated a negative relationship between body fat content and levels of total testosterone [35, 36], this applies in particular to abdominal fat tissue [37, 38]. In healthy obese men, the issue is complicated by simultaneously decreasing levels of SHBG, thus levels of free or bioavailable T are often maintained [39].
In hypogonadal men, an increased total BMI is regularly observed and as well a reduced lean BMI, measured with dual X-ray absorptiometry (DEXA) or bioimpedance, is found when compared to age-matched healthy controls, suggesting higher body weight due to increased fat mass in the presence of lower muscle mass [40, 41]. Testosterone treatment can significantly reduce body fat content in hypogonadal men and vice versa it can increase lean body mass, an observation which is not only due to shifts in proportions but also to growth of muscle tissue (see below) [32, 42–49].
This is exerted via a redistribution of body fat during which mainly visceral and intermuscular fat depots are affected, but subcutaneous tissue seems to be spared [50]; it is likely that fat cell size itself is modulated by androgens [51]. The process seems to follow a linear dose–response relationship to T [50], but the exact mechanism, by which fat cells are subject to androgen influence is not known. It can be speculated that this is mediated via increased insulin sensitivity and improved glucose utilization, resulting in lower insulin levels and, thus, a lower amount of lipid storage.
Visceral fat tissue plays a central role within the metabolic syndrome, which is mentioned in detail further below, acting as a source of inflammatory, anti-insulinergic and atherogenically relevant cytokines such as TNF-α and IL-6 [7, 52–55].
Fat tissue also functions as an endocrine organ: its product adiponectin plays an important role in metabolism and is related to cardiovascular risk factors. It is produced by fat cells in large quantities, yet its levels are inversely associated with total body fat mass, which is most likely caused by auto-/paracrine downregulation via inflammatory cytokines. Improvement of insulin sensitivity and inhibition of various atherogenic processes within the vessel wall are direct effects of adiponectin [55, 56].
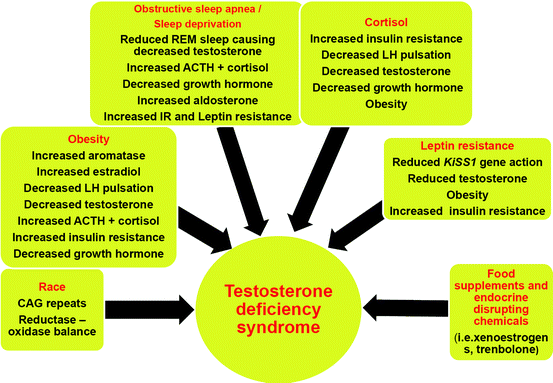
Leptin is another hormone secreted by fat cells. Hypophagia to reduce fat mass is supported by leptin signals, but adipose tissue fosters further food intake by facilitating leptin resistance at the hypothalamic level via afferent nerve signals [57]. A vicious circle is thus induced as leptin resistance increases further adipocyte-related production of this hormone. There are indications that high levels of leptin can mitigate testosterone secretion, for instance via downregulating the action of kisspeptin through the Kiss1 gene [58, 59].
Testosterone seems to have various effects on fat cells and insulin resistance. A study in mouse pluripotent stem cells indicates that testosterone regulates body composition by promoting the commitment of these mesenchymal cells into the myogenic lineage and inhibiting their differentiation into the adipogenic lineage. This provides a unifying explanation for the reciprocal effects of androgens on muscle and fat mass in men [60]. A inhibiting effect of testosterone has also been described concerning the differentiation of preadipocytes: in 3T3-L1 cells that differentiate to form fat cells in adipogenic medium, testosterone inhibits adipocyte differentiation in vitro through an androgen receptor-mediated nuclear translocation of beta-catenin and activation of downstream Wnt signaling (such Wnt signals direct distinct fates of differentiation in precursor cell types) [60]. In addition, testosterone increases lipolysis and the number of adrenoreceptors in male rat adipocytes [61].
Testosterone may facilitate insulin sensitivity both in fat and muscle cells by upregulating the expression of insulin-induced downstream protein expression. Respective dose-dependent effects of testosterone on insulin receptor substrate-1 and glucose transporter 4 expression were seen in cells models [62]. Recent models of insulin resistance also suggest a pivotal role of mitochondrial function with the decreased transcription of oxidative phosphorylation genes in skeletal muscle of insulin-resistant subjects. This leads to decreased oxidative phosphorylation, decreased lipid oxidation, intracellular accumulation of triglycerides in skeletal muscle, and ultimately insulin resistance [63]. A study in 60 men demonstrated testosterone levels to correlate positively with mitochondrial capacity assessed by measuring maximal aerobic capacity and also expression of oxidative phosphorylation genes [64].
As discussed above, leptin resistance and consequently upregulated adipocyte leptin secretion play a pivotal role in obesity. Testosterone substitution in hypogonadal men is able to reduce leptin secretion of fat cells, probably by an androgen receptor-mediated pathway (also see below ref. [65]), thus breaking the vicious cycle of leptin resistance and obesity [47, 66–68].
Metabolic Aspects and Glucose Metabolism
Within the last century, life circumstances have changed in developed countries as physical activity has decreased and, simultaneously, an oversupply of food is present. This results in an increasing prevalence of overweight and obesity, particularly over the past two decades. As a consequence, a complex disorder consisting of visceral obesity, dyslipidemia, insulin resistance, and hypertension emerges with increasing incidence: the so-called metabolic syndrome contributes to a symptomatology, which progressively may lead to the manifestation of type 2 diabetes in genetically predisposed subjects and CVD in all. While the pathogenesis of the metabolic syndrome and each of its components is complex and not well understood, central obesity and insulin resistance are acknowledged as important causative factors [69–72]. Persons affected are twice as likely to die from and three times as likely to suffer a heart attack or stroke compared to those free of the metabolic syndrome [73]. They also have a fivefold greater risk of developing type 2 diabetes mellitus, if not already present [74].
The International Diabetes Federation and the National Cholesterol Education Panel have recently updated and harmonized the criteria for diagnosis of the metabolic syndrome (Table 11.2).
Table 11.2
The International Diabetes Federation: updated criteria for the diagnosis of the metabolic syndrome (ref. [75])
New Criteria for the Definition of the Metabolic Syndrome |
1. Waist circumference >94 cm (European Men) |
2. Triglycerides >150 mg/dL or treatment |
3. HDL-Cholesterol <40 mg/dL or treatment |
4. Arterial blood pressure >130 mmHg systolic and/or >85 mmHg diastolic or treatment |
5. Fasting glucose >100 mg/dL or known type 2 diabetes mellitus |
3 of 5 criteria have to be met (Consensus IDF and NCEP ATP III) |
In men, obesity as the central component of the metabolic syndrome is associated with low testosterone concentrations [37, 38, 65, 76].
On the other hand, in men, testosterone deficiency may contribute to the development of the metabolic syndrome. In turn, states of hyperinsulinemia and obesity lead to a reduction of testicular testosterone production. Testosterone has reciprocal effects on the generation of muscle and visceral adipose tissue by influencing the commitment of pluripotent stem cells and by inhibiting the development of preadipocytes. Insulin sensitivity of muscle cells is increased by augmenting mitochondrial capacity and fostering expression of oxidative phosphorylation genes. Testosterone has a protective effect on pancreatic β cells, which is possibly exerted by androgen receptor-mediated mechanisms and influence on inflammatory cytokines [77].
As some, but not all, epidemiological and interventional studies indicate, testosterone substitution might be helpful in preventing or attenuating the metabolic syndrome in aging men with LOH and in hypogonadal patients with type 2 diabetes mellitus, but larger controlled trials are needed to confirm such hypotheses [6, 7]. Meta-analyzing prospective (longitudinal, interventional) and cross-sectional studies of testosterone replacement therapy (TRT) in men with and without type 2 diabetes revealed that TRT significantly reduced fasting plasma glucose, hemoglobin A1C, fat mass, and triglyceride levels without significantly changing blood pressure [78]. Heterogeneous community-based studies showed low endogenous testosterone to be associated with an increased risk of all-cause and cardiovascular death [79, 80]. In men age 69–81 years, high serum testosterone levels predicted a reduced 5-year risk of cardiovascular events [81]. Men with normal testosterone concentrations appear to have lower rates of atherosclerosis and coronary artery disease. Men with congestive heart failure were more able to walk on testosterone therapy than such men on placebo [82–84].
Obesity is often associated with fatigue, excessive day time sleepiness and sleep apnea, although these manifestations may also occur in lean individuals. Pro-inflammatory cytokines, such as interleukin-6 and tumor necrosis factor alpha, are elevated in patients suffering from sleep apnea. Therapy with continuous positive airway pressure can improve sleepiness and blood pressure, as well as increase testosterone levels in men [5, 85–89].
General Metabolic Perspectives for Men
In an animal model using androgen receptor knock out (ARKO) mice, male ARKO mice were less dynamic and less oxygen consuming with increased secretion of adiponectin (exerting insulin sensitizing effects) and decreased lipolysis, partly explaining their adiposity [90]. Considering that burning calories mainly depends on the resting metabolism which is determined by muscle mass, physical activity to build/exercise muscle, and testosterone availability, this is not surprising. Additional apolipoprotein E-deficiency in ARKO mice accelerated atherosclerosis that could be reduced by testosterone treatment [91].
Looking at a human model of testosterone deficiency and its effects on metabolism, one could analyze individuals with androgen (complete) resistance syndrome. Women with complete androgen insensitivity syndrome are obese and have elevated total and LDL cholesterol levels as well as increased HOMA-IR indexes [92].
Hypogonadism in men with Klinefelter’s syndrome (KFS) causes an unfavorable change in body composition leading to metabolic syndrome [93, 94]. Insulin resistance and metabolic syndrome in men with KFS are four times higher than in age-matched controls. In untreated KFS men, total fat mass and LDL cholesterol, triglyceride, CRP, and leptin levels are significantly increased, while HDL cholesterol levels are significantly decreased. KFS is associated with a significant increase in mortality risk of 40% (hazard ratio, 1.40; 95% confidence interval, 1.13–1.74), corresponding to a significantly reduced median survival of 2.1 years [95].
Special efforts to detect this underdiagnosed chromosome disorder and mitigate the increased mortality of these men due to complications of diabetes mellitus and cardiovascular events are necessary [93]. Although approaches examining the effects of testosterone on sub-parameters of the metabolic syndrome have been made (see above), prospective studies investigating its incidence in hypogonadal men receiving testosterone substitution therapy are needed. Such studies should take the modulatory effect of the androgen receptor into account to fully elucidate the putative potential of testosterone to attenuate or prevent the metabolic syndrome in men.
A Clinical Example to Elucidate the Andrologist’s Approach to Metabolic Disorders in Hypogonadism
Key Question: Is There a Role for Testosterone Testing and Therapy in Obese Hypertensive Men?
Bob is a 52-year-old man complaining from mild erectile dysfunction (ED) and diminished libido. This may be an indicator of atherosclerosis or cardiovascular risk. Bob is 5 ft, 11 in. tall (180 cm) and gained 30 lb over the past 5 years. The physical examination showed that he is mildly hypertensive, with a blood pressure of 166/98 mm Hg, and weighs 217 lb (98.5 kg). You calculate his BMI to be 30.3 kg/m2; and measure his waist circumference at 43 in. (109 cm), all signs indicating metabolic syndrome with late-onset obesity.
Laboratory values showed a serum total testosterone of 310 ng/dL (normal reference range, 350–1,030 ng/dL); bioavailable testosterone 90 ng/dL (normal reference range Males 50–69 years old, 95–285 ng/dL); Luteinizing hormone 4.3 mIU/mL (normal, 1.5–9.3); fasting blood sugar (FBS) 112 mg/dL (normal, 65–99 mg/dL); fasting insulin level: 19 μIU/mL (normal, <2); and a PSA of 1.1 ng/mL.
Based on the signs and symptoms exhibited by Bob, coupled with his blood laboratory values, is there any role for testosterone supplementation in his therapy, in addition to lifestyle changes including diet and exercise?
Rationale
A larger (2,314 men, aged 40–79 years), longer (average 7-year follow-up) nested case-control study found that every 173 ng/dL (6 nmol/L) increase in serum T was associated with a 21% lower risk of all-cause death (OR 0.79, 95% CI, 0.69–0.91, P < 0.01) and CV death (OR 0.79, 95% CI, 0.64–0.97, P = 0.02) after excluding deaths within the first 2 years and controlling for multiple variables (age, BMI, systolic blood pressure, cholesterol, cigarette smoking, diabetes, alcohol intake, physical activity, social class, education, and SHBG). These men had no CVD at baseline [96].
The Rancho Bernardo study (N = 794 men, aged 50–91 years, average follow-up, 11.8 years, but up to 20 years) also found that total and bioavailable T were inversely related to risk of death. Men in the lowest quartile of total T (<241 ng/dL [8.4 nmol/L]) were 44% more likely to die during the follow-up period compared to those in highest quartile (>370 ng/dL [12.8 nmol/L]), independent of age, BMI, waist-to-hip ratio, alcohol use, current smoking, and exercise (HR 1.44, 95% CI, 1.12–1.84). Additional adjustment for prevalent diabetes, CVD, or the metabolic syndrome had minimal effect on the strength of this association. Low total T also predicted increased risk of death due to CV (HR 1.38, 95% CI 1.02–1.85) and respiratory disease (HR 2.29, 95% CI 1.25–4.20) [97–99].
Yet the association of T with death does not appear to operate through many risk factors and comorbidities, as the link between low T and shorter survival remains significant after adjustment for such covariates in multiple studies.
Possible explanations might include a separate association of the development of inflammatory cytokines and insulin resistance with the induction of hypogonadism (e.g., withdrawing testosterone therapy in men with known hypogonadism).
The Guidelines
The relations between testosterone deficiency syndrome (TDS; hypogonadism) and various health risk factors including metabolic syndrome, obesity, diabetes, vascular disease, frailty, and increased mortality in men has received renewed interest over the past decade.
In view of the growing interest from practitioners in TDS and improving men’s health, several clinical guidelines on investigation, treatment, and monitoring of hypogonadism in men have been published by the Endocrine Society, American Association of Clinical Endocrinologists (AAEC), International Society of the Study of the Aging Male (ISSAM), International Society of Andrology (ISA), European Association of Urology (EAU), European Academy of Andrology (EAA), and the American Society of Andrology (ASA) [23, 100].
For maximum dissemination of this valuable information, the guidelines and recommendations were published in several journals to reach larger audiences.
The key recommendations of these guidelines were focused on:
1.
Definition of TDS
2.
Clinical diagnosis
3.
Laboratory diagnosis
4.
Assessment of treatment outcome and decision to continue therapy
5.
Adverse effects and close monitoring
The guidelines recommended making a diagnosis of TDS in men consistent with signs and symptoms (e.g., low libido, erectile dysfunction, decreased muscle mass, decreased strength, increased body fat, decreased bone mineral density, decreased vitality, depressed mood) associated with low testosterone (less than 350 ng/dL; or 12 nmol/L according to the EAU Guidelines and less than 300 ng/dL or 10.3 nmol/L according to the Endocrine Society Guidelines) and some guidelines had provided an algorithm for diagnosis and treatment of men with TDS.
The recommendations also emphasized the need to discuss the benefit and risks of testosterone therapy with the patient and considering potential risks, including prostate cancer.
Further, the recommendations pointed strongly to close monitoring and assessment of the response to testosterone treatment. In the event lack of improvement in signs and symptoms are noted, the treatment is discontinued and other possible causes for the clinical presentation should be investigated.
One of the key areas of controversy in the recommendations and guidelines is the reliability and utility of the methods utilized in the measurements of total, bioavailable, free, and calculated free testosterone.
Also, there is disagreement on the lower limits of testosterone values that can be used in biochemically diagnosing TDS.
Thus, the uncertainty regarding which fraction of testosterone should be measured, and what the values mean clinically, remains the most urgent issue to be resolved.
Nevertheless, these recommendations and guidelines have provided many practitioners with imputes to diagnose and treat patients with TDS.
The guidelines also summarize the large amount of uncertainty that exists in male health care concerning symptoms of hypogonadism, and thresholds of testosterone concentrations that cause these symptoms.
From clinical experience in endocrinology, it is rather obvious that a universal, clear-cut threshold of testosterone concentrations cannot exist for all symptoms and in all men. Rather, there are strong indications that symptom-specificity exists concerning levels of testosterone. In general, mental problems seem to precede somatic/metabolic disorders, while erectile function seems to be maintained longest [101].
In addition, also the genetic background relating to the receptiveness of androgens, hence, androgen receptor polymorphisms are likely to play an interindividual role [12, 13].
Furthermore, a huge area of uncertainty exists regarding (a) the accuracy of testosterone assays, (b) the application of total or free testosterone, and (c) the subject of age stratification.
Such considerations have to be taken into account when taking care of patients like Bob, who definitely needs diagnosis and treatment: physicians have to use their clinical judgement and experience in such cases and handle each individual patient individually (instead of practicing “algorithm” or “cookbook” medicine). For a general practitioner, we suggest to approach men with symptoms of testosterone deficiency as outlined in Fig. 11.2, while considering factors mentioned in Fig. 11.3.
Conclusion of the Case
According to generally accepted knowledge in medicine and cardiology, and quite apart from his testosterone levels, patient Bob has an increased risk for metabolic disorders/type 2 diabetes mellitus, arterial hypertension and is likely to die earlier from cardiovascular events than healthier men of his age. Indeed, he exhibits a sign of vascular compromise by the presence of erectile dysfunction, which may serve as a portal to his health: this is the reason why he came to consult the doctor!
In addition to that, we found low testosterone concentrations in his blood, as is quite typical for men of his age with his clinical profile. His complaints and the laboratory analysis allow the diagnosis: Bob has a TDS concomitant (and possibly causative) with/for his general clinical condition. There is enough evidence to start him on monitored testosterone substitution therapy, to improve both his mental problems, as well as to reduce the state of metabolic dyshomeostasis, including emerging glucose intolerance.
General Summary
1.
Screening for testosterone levels in men of Bob’s age with his matrix of mental and metabolic complaints is a state-of-the-art procedure and is strongly recommended.
2.
TRT in hypogonadal men, following the guidelines, will most certainly improve mental and sexual problems and is likely to ameliorate metabolic dysfunction/insulin resistance. A variety of testosterone therapies exist (see ref. [23].
3.
Evidence is lacking that TRT prolongs the life span of hypogonadal men. Nevertheless, there is evidence that TRT is able to improve quality in men’s life.
Contraindications to Testosterone Therapy According to ref. [23]
Untreated severe obstructive sleep apnea (OSA)
Hematocrit greater than 50%
Uncontrolled or poorly controlled heart failure
Patients with history of breast or prostate cancer
Presence of palpable prostate nodule or induration
Prostate-specific antigen greater than 4 ng/mL or greater than 3 ng/mL in men at high risk for prostate cancer such as African Americans, or men with first-degree relatives with prostate cancer, without further urological evaluation
Severe lower urinary tract symptoms with International Prostate Symptom Score (IPSS) above 19
Benefits of Testosterone Replacement Therapy
As stated previously, symptoms that commonly improve with testosterone replacement include sex drive, erectile dysfunction, mood, energy, quality of life, muscle mass and strength, cognitive function, and bone mineral density [102].
There is a threshold level of testosterone above which there may be no further benefit in symptomatic improvement [46]. An adequate time period for changes in energy, mood, and sexual function on testosterone therapy seems to be 3 months [103]. Effects on body composition including waist circumference and serum lipid profiles likely require a longer period [103, 104]. Such effects depend on individual factors such as the degree of being above ideal body weight, individual body composition/adiposity, resting metabolism energy expenditure/calorie burning, and individual conditioning/oxygen consumption. In normal men, testosterone administration can increase IGF-1 levels [105].
Someone with a history of physical inactivity for a long period of time who has insidiously developed atherosclerotic plaques that are unstable may then experience harm as opposed to someone with rather stable or no atherosclerotic plaques. Therefore, it is advisable to have men (including diabetics) at increased risk for cardiovascular events consult a cardiologist, especially when having a history of being deconditioned, to design an individual stepwise physical activity program. This also accounts for sexual activity. A recent scientific statement from the American Heart Association outlines which steps should be taken and mentions that sexual activity with a person’s usual partner is comparable to moderate physical activity in the range of four metabolic equivalents, i.e., the equivalent of climbing two flights of stairs or walking briskly [106]. Of note, this data and evidence level/recommendation derived from young married men and it is unknown how heart rate and blood pressure are responding in older individuals and men, especially when there is difficulty reaching an orgasm. Interestingly, this position paper from the AHA deals with sexual function and activity but does not mention testosterone in its therapeutic arsenal, although androgens can improve the response to slidenafil in men with erectile dysfunction [107].
When replacing sex steroids one has to consider that alteration of the clearance of thyroxine-binding globulin can occur in patients with thyroid disease, necessitating a dose change of patients on thyroid hormone replacement therapy. Therapy with sex steroids including testosterone might also unmask thyroid illness in previously undiagnosed individuals [108].
Risks and Barriers of Testosterone Replacement
Physicians (non-andrologists) are not generally comfortable starting TRT even if their patient meets all the criteria for TDS. Contrary to one common misconception that testosterone therapy in men causes cardiovascular problems, a systematic review and meta-analysis of randomized placebo-controlled trials found this not to be the case [109].
Another common misconception regarding risk of TRT concerns the perception that it will cause BPH or prostate cancer [100]. This is untrue, although TRT is absolutely contraindicated in patients who have been diagnosed with prostate cancer (as it is a androgen-sensitive disease). The revised Endocrine Society guidelines in 2010 lowered the PSA exclusion criteria to >3 ng/mL in men with higher risk of prostate cancer such as African Americans or men with first-degree relatives who had prostate cancer. Men who have been treated successfully for prostate cancer and are diagnosed later with “hypogonadism”/testosterone deficiency, may still be candidates for TRT after a prudent interval if no clinical or laboratory evidence of residual cancer can be found after a thorough urological evaluation [110, 111].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree