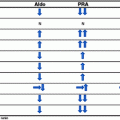
Fig. 5.1
Pathway of adrenal steroid biosynthesis. The enzymes responsible for each biosynthetic step are above or adjacent to arrows. Each box represents a respective zone of the adrenal cortex. Three steps in aldosterone synthesis are mediated by a single gene, CYP11B2. 11β(beta)-hydroxylase and some 18-hydroxylase activity are mediated by CYP11B1. 3β 3β(beta)-hydroxysteroid dehydrogenase; 21-OH 21-hydroxylase; 11-OH 11β(beta)-hydroxylase; 18-OH 18-hydroxylase; 17-OH 17α(alpha)-hydroxylase; DOC deoxycorticosterone; B corticosterone; 18-OH-DOC 18-hydroxydeoxycorticosterone; 18-OH-B 18-hydroxycorticosterone
Table 5.1
Comparison of congenital adrenal hyperplasia disorders associated with hypertension
11β(beta)-hydroxylase deficiency | 17α(alpha)-hydroxylase deficiency | 21-Hydroxylase deficiency | |
|---|---|---|---|
Gene | CYP11B1 | CYP17A1 | CYP21A2 |
Etiology of hypertension | Mineralocorticoid excess | Mineralocorticoid excess | Multifactorial: treatment-related, obesity-related, unknown hormonal imbalances |
Laboratory findings | ↑ DOC, ↑ 11-deoxycortisol DOC | ↑ DOC | 17-OH-progesterone |
↓↓ B | ↑ B | ||
↓↓ 18-OH derivatives | ↑ 18-OH derivatives | ||
↓↓ Aldosterone | ↓↓ Aldosterone | ↓ or Nl Aldosterone | |
Clinical features at diagnosis | Genital ambiguity (XX) | Delayed puberty in phenotypic female (XX, XY) | Genital ambiguity (XX) |
Precocious virilization (XY) | Possible genital ambiguity (XY) | Precocious virilization (XY) ± salt losing adrenal crisis |
Clinical Presentation
Increased androgen production in utero causes virilization resulting in ambiguous genitalia evident at birth in genetic females. Internal structures are normal for female. Males do not have obvious signs at birth but present with precocious virilization in the first few years of life. In both genders, excess androgen production postnatally may lead to premature secondary sexual characteristics including early appearance of pubic, axillary, and facial hair, acne, deepening of voice, and rapid skeletal growth. Although 11β(beta)-hydroxylase deficiency results in mineralocorticoid excess, there have been reports of transient salt wasting in newborns [14], presumably due to the unique physiology of the newborn which includes mineralocorticoid resistance. Males diagnosed as toddlers, usually have hypertension at presentation, in addition to the signs and symptoms of androgen excess. This initial hypertension usually resolves with glucocorticoid therapy.
Chronic low renin hypertension occurs in approximately 2/3 of patients with 11β(beta)-hydroxylase deficiency. Onset of hypertension typically occurs in later childhood and although it has been reported, it occurs rarely in infancy [15]. The degree of hypertension is often mild to moderate, but can become malignant as there are reports of retinopathy, retinal vein occlusion, cardiomyopathy, and left ventricular hypertrophy [7, 16, 17]. Pediatric patients may have elevated blood pressure primarily during acute illnesses, when the adrenal is stimulated, but then remain normotensive when well (personal experience DPM).
The incidence of non-classic 11β(beta)-hydroxylase deficiency is quite rare. Blood pressure may be normal or mildly elevated in these patients [18].
Physiology of Hypertension in 11β (beta)-Hydroxylase Deficiency
DOC is a weak mineralocorticoid; however, its level can rise up to 60 times normal in patients with 11β(beta)-hydroxylase deficiency [19]. Accumulation of DOC, or other mineralocorticoid hormones, causes a net sodium retaining effect and extracellular fluid volume expansion which eventually leads to an increase in total peripheral vascular resistance and hypertension. In addition, mineralocorticoid hormones have a positive inotropic effect resulting in increased stroke volume and cardiac output which also contribute to the development of hypertension [13]. Suppression of the renin-angiotensin system due to mineralocorticoid excess results in low plasma renin activity and aldosterone levels although the ability to synthesize aldosterone is unimpaired.
Intuitively, it would seem that the accumulation of DOC could be the sole cause of HTN in patients with 11β(beta)-hydroxylase deficiency. However, there are several factors that argue against this: (1) elevations in blood pressure, suppressed plasma renin activity and hypokalemia, present variably in this patient population; [7, 10, 14] (2) suppression of DOC does not always result in resolution of hypertension; [14] and (3) elevation in blood pressure does not correlate with DOC levels [12, 14, 20]. It is hypothesized that there are unidentified mineralocorticoid metabolites contributing to the development of hypertension in patients with this type of CAH [3, 14, 15].
Diagnosis of 11β (beta)-Hydroxylase Deficiency
Diagnosis of 11β(beta)-hydroxylase deficiency is evident by the steroid pattern produced in response to chronic elevation of ACTH and is based primarily on elevation of 11-deoxycortisol. In general, the following hormones are elevated in 11β(beta)-hydroxylase deficiency: (1) non-11β(beta)-hydroxylated products including 11-deoxycortisol, DOC, 17-hydroxyprogesterone and androstenedione; (2) tetrahydro-11-deoxycortisol (THS) and tetrahydrodeoxycorticosterone (THDOC), the principal urinary metabolites of serum 11-deoxycortisol and DOC, respectively; and (3) urinary 17-ketosteroids (reflecting elevated androgen levels) [10].
α(alpha)-Hydroxylase Deficiency
Epidemiology
Classic 17α(alpha)-hydroxylase deficiency is an autosomal recessive disorder that is very rare. However, 17α(alpha)-hydroxylase deficiency, as opposed to 11β(beta)-hydroxylase deficiency, is the second most common form of CAH in Brazil, where a founder effect likely accounts for the majority of cases [13, 21].
Genetics
The CYP17A1 gene consists of eight exons and is located on chromosome 10 (10q24.3) [22]. It encodes the cytochrome P450c17 α enzyme which is present in both the adrenal glands and the gonads. Over 70 mutations have been identified in the CYP17A1 gene [23]. Frequently reoccurring mutations are seen in four populations: (1) Brazilians; (2) Canadian Mennonites; (3) Japanese; and (4) East Asians. However, mutational hot spots have not been identified in most other large populations [23].
Pathophysiology
Unlike other steroidogenic enzymes, P450c17α is responsible for catalyzing two reactions in the steroidogenic pathway. First, as 17α(alpha)-hydroxylase, it 17-hydroxylates pregnenolone and progesterone, reactions that are necessary for cortisol production. Second, as 17, 20 lyase, it transforms 17-hydroxypregnenolone to DHEA and 17-hydroxyprogesterone to androstenedione which are reactions necessary for sex steroid production (Fig. 5.1).
17α(alpha)-hydroxylase and 17, 20 lyase are expressed in the zona fasciculata, zona reticularis, testicular leydig cells, and ovarian theca cells [24]. They are not found in the zona glomerulosa. Mutations in the CYP17A1 gene can result in one of three types of deficiencies: (1) isolated 17α(alpha)-hydroxylase deficiency; (2) combined 17α(alpha)-hydroxylase and 17, 20 lyase deficiencies; or (3) isolated 17, 20 lyase deficiency. It is difficult to differentiate isolated 17α(alpha)-hydroxylase deficiency from combined 17α(alpha)-hydroxylase and 17, 20 lyase deficiencies and isolated 17, 20 lyase deficiency is rare [25, 26]. Classic “17α(alpha)-hydroxylase deficiency” refers to the combined 17α(alpha)-hydroxylase and 17, 20 lyase deficiencies resulting in loss of glucocorticoid and sex steroid production [27].
Blockage of 17α(alpha)-hydroxylase activity shunts steroid production to the 17-deoxy pathway resulting in an increase of DOC, corticosterone (B), 18-hydroxycorticosterone (18-OH-B), and 18-hydroxydeoxycorticosterone (18-OH-DOC). Corticosterone is a weak glucocorticoid and can substitute cortisol action. However, because corticosterone is not as potent as cortisol, high serum levels are required before negative feedback inhibition will reduce pituitary gland production of ACTH [13]. This leads to an ACTH driven increased production of mineralocorticoid hormones causing hypertension in patients with 17α(alpha)-hydroxylase deficiency (Table 5.1).
Clinical Presentation
CAH patients with classic 17α(alpha)-hydroxylase deficiency suffer from lack of glucocorticoid and sex steroid production. However, patients do not suffer from adrenal insufficiency because overproduction of corticosterone allows for normal endogenous glucocorticoid activity. Therefore, the enzyme deficiency is often not detected until adolescence when patients present as females with delayed puberty.
Hypergonadotropic hypogonadism is characteristic in this patient population. 46XX patients do not enter puberty and present with primary amenorrhea and sexual infantilism. 46XY patients may present early in life with ambiguous genitalia or sexual infantilism, but often are phenotypic females at birth and may not be identified as 46XY until adolescence [28]. In contrast to CAH patients with 21- and 11β(beta)-hydroxylase deficiencies, these patients do not suffer from restricted growth, but are often tall presenting with eunuchoid proportions [26].
Significant variations in sexual development have been described in patients with 17α(alpha)-hydroxylase deficiency indicating some sex steroid production. A wide range in the degree of virilization of 46XY patients and, the presence of menses and breast development in 46XX patients have been described [26, 29, 30].
While elevations in mineralocorticoid hormone levels are present since birth, hypertension and hypokalemia are not typically the presenting symptoms in these patients. Hypertension has been reported as early as infancy and as late as the fifth decade of life, but is most often detected at the time of diagnosis when patients present with delayed puberty. There is considerable variation in the severity of hypertension. Blood pressure can range from normotensive to malignant hypertension associated with end-organ disease [26, 28, 31]. The same variable presentation is true for hypokalemia.
The variability in sexual development and the wide range of effects on blood pressure and potassium are possibly due to distinct genetic mutations resulting in differing degrees of enzyme activity or other unknown environmental or genetic factors [29, 32]. Interestingly, the severity and degree of hypertension does not correlate with the degree of hypogonadism [26].
Physiology of Hypertension in 17α(alpha)-Hydroxylase Deficiency
Similar to patients with 11β(beta)-hydroxylase deficiency, patients with17α(alpha)-hydroxylase deficiency have low renin hypertension. Mineralocorticoid excess leads to suppression of plasma renin activity and decreased aldosterone production. In 17α(alpha)-hydroxylase deficiency, DOC undergoes both 18-hydroxylation and 19-hydroxylation [33, 34] leading to production of 18-OH-B, 18-OH-DOC, and 19-nor-deoxycorticosterone (19-nor-DOC). This is distinct from 11β(beta)-hydroxylase deficiency, where DOC is not significantly hydroxylated at the 11 or 18 positions. Thus, DOC levels are much greater in 11β(beta)-hydroxylase deficiency than in 17α(alpha)-hydroxylase deficiency [29]. The metabolite, 19-nor-DOC, is thought to be a more potent mineralocorticoid than DOC and contributes significantly to the development of hypertension [13]. Interestingly, there are reported cases of patients with 17α(alpha)-hydroxylase deficiency who have normal or even elevated aldosterone levels [26, 29]. The cause of this physiological paradox is unclear.
Diagnosis of 17α(alpha)-Hydroxylase Deficiency
Diagnosis of 17α(alpha)-hydroxylase deficiency is made by detecting markedly elevated levels of the 17-deoxysteroids including progesterone, DOC, and corticosterone at baseline and also upon ACTH stimulation testing [21]. Plasma 17-hydroxyprogesterone and androgen levels and urinary pregnanetriol and 17-ketosteroid levels are low to undetectable. Distinguishing 17 from 11β(beta)-hydroxylase deficiency can be done by calculating the corticosterone to DOC ratio which is negligible in the latter.
-Hydroxylase Deficiency
Epidemiology
CAH due to 21-hydroxylase deficiency is typically divided into three phenotypic categories: classic severe (associated with mineralocorticoid deficiency, “salt losing”), simple virilizing (“non-salt losing”), and mild or non-classic (NC). Data pooled from 13 countries shows an overall incidence of 1/15,000 live births where approximately 67% are salt losing and 33% are non-salt losing [35]. Similarly, the incidence in the United States amongst Caucasians is 1/15,500, but is lower in the African-American population (1/42,000) [36]. Newborn screening does not accurately detect NC CAH; however, the limited data available suggest an incidence of approximately 1/1,000 in the Caucasian population [37, 38].
Two isolated populations are known to have an increased incidence of 21-hydroxylase deficiency: the Yupik Eskimos of Alaska (1/280) [39] and the people of the French island of La Réunion (1/2,100). Brazil and the Philippines are also areas of increased frequency [40]. NC CAH is more frequent in Hispanic, Yugoslavic, and eastern European Jewish populations [38].
Genetics
The gene encoding 21-hydroxylase is located on the short arm of chromosome 6p21.3 within the HLA histocompatibility complex class III region [41]. There are two highly homologous 21-hydroxylase genes that sit approximately 30 kb apart: CYP21A2 (CYP21B) is the active gene and CYP21A1P (CYP21A, CYP21P) is the inactive pseudogene [42]. Recombination events between the active and inactive gene occur frequently accounting for up to 95% of the mutations associated with this enzyme [43–45].
There are several different mutations in the 21-hydroxylase gene resulting in a wide range of genotypes and associated phenotypes. The majority of patients are compound heterozygotes meaning that they have different mutations on the two alleles. The clinical phenotype results from the less severely mutated allele and is thus dictated by the residual 21-hydroxylase activity [35].
Pathophysiology
CAH due to 21-hydroxylase deficiency is characterized by cortisol deficiency with or without mineralocorticoid deficiency. Similar to 11β(beta)-hydroxylase deficiency, low or absent cortisol levels drive increased ACTH production which, in turn, stimulates adrenal androgen steroidogenesis. Impairment of cortisol biosynthesis allows steroid precursors to be shunted into the unimpeded androgen production pathways resulting in androgen excess (Fig. 5.1, Table 5.1).
Clinical Presentation
The classic form of 21-hydroxylase deficiency presents in early childhood. Females with classic salt losing CAH or classic non-salt losing CAH present with ambiguous genitalia at birth due to excess androgen exposure in utero. Similar to 11β(beta)-hydroxylase deficiency, the internal female structures are normal. Male infants do not have signs of CAH at birth except possible mild hyperpigmentation and penile enlargement. Prior to the advent of neonatal screening programs, the diagnosis was dependent on the severity of the mineralocorticoid deficiency with severe deficiency presenting at approximately 2 weeks of life with weight loss, dehydration, hyponatremia, vomiting, and possibly shock. Males with the simple virilizing, non-salt losing classic form, if not detected by neonatal screening, present with signs of early virilization at approximately age 2–4 years.
NC CAH patients typically present with signs of androgen excess in school-aged children, adolescence, or early adulthood. Males with NC CAH are often asymptomatic. Females may present with hirsutism, oligo- or amenorrhea with polycystic ovaries and acne alone or in combination [35, 46]. These patients do not have cortisol deficiency.
Hypertension is not pathognomonic of CAH due to 21-hydroxylase deficiency. Mineralocorticoid deficiency or normal mineralocorticoid production is the norm, unlike the mineralocorticoid excess characteristic of 11β(beta)-hydroxylase and 17α(alpha)-hydroxylase deficiency. In 2003, Roche et al. performed a study evaluating 24-h ambulatory blood pressure and obesity in 38 children (range 6.1–18.2 years) with classic salt losing 21-hydroxylase deficiency. Mean daytime SBPs were significantly higher than those of the reference population and 58% of patients, both males and females, had systolic hypertension. Mean DBP was also elevated and 24% had diastolic hypertension. In this study, CAH patients also had a higher BMI than the reference population and BMI was significantly correlated with SBP, especially in females [47].
Since then, several small studies have looked at the incidence and prevalence of hypertension in this population including salt losers and non-salt losers. Nebesio et al. found 5/91 (5.5%; two female infants, one 6-year-old female, one 12-year-old male, and one unknown age female) patients with hypertension that required anti-hypertensive treatment [48]. In a German study of 55 children, Volkl et al. found approximately 11% of patients to have blood pressures in the hypertensive range and a significantly greater percentage of CAH patients to have blood pressures above the 50th percentile compared to a reference population [49]. One small study of 11 children reported 1 patient with daytime systolic hypertension and 6 with nocturnal hypertension [50].
Conversely, there are several studies of pediatric and adult patients that did not observe an increased prevalence of hypertension in patients with classic CAH due to 21-hydroxylase deficiency [51–55]. In addition, data collection methods for the reference population in the study by Roche et al. had limitations in that single blood pressure measurements were used as opposed to the more accurate 24-h ambulatory monitoring [49].
Pathophysiology of Hypertension in 21-Hydroxylase Deficiency
The discrepant findings regarding hypertension in patients with classic 21-hydroxylase deficiency likely result from the multiple potential causes of increased blood pressure in these patients. Several hypotheses have been proposed.
Supraphysiologic glucocorticoid treatment may lead to elevation in blood pressure. Glucocorticoids activate the mineralocorticoid receptor at high doses and the affinity of cortisol for the mineralocorticoid receptor is at least equal to or greater than that of aldosterone [56]. However, metabolism of cortisol by 11β-hydroxysteroid dehydrogenase to inactive metabolites protects against excessive binding of glucocorticoid to mineralocorticoid receptors [57, 58]. Multiple studies have shown no correlation between glucocorticoid dose and blood pressure [47–50, 54, 55]. While the dose itself may play an indirect and/or minor role in the development of hypertension in these patients, other factors related to glucocorticoid treatment may contribute, including the type of glucocorticoid (i.e., long-acting vs. short-acting), the fact that current replacement regimens cannot replace the normal physiological rhythm of cortisol [59], and patient variability in glucocorticoid sensitivity and metabolism. Moreover, the dose, regimen, and non-physiologic nature of the mineralocorticoid replacement therapy could be a contributing factor.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree