MALT lymphoma is the most common PGL in Western countries.
9 The stomach is also the most common organ involved by MALT lymphoma (
Table 10-2). The concept of MALT lymphoma was first proposed by Isaacson and Wright in 1983.
12 Its connection with
H. pylori infection was later demonstrated by Wotherspoon et al.
13 Since the progression of chronic gastritis to MALT lymphoma is a continuous process, morphological overlap exists between gastritis and early MALT lymphoma.
13 Although there is no consensus on the morphologic criteria for differentiating
gastritis and MALT lymphoma, Wotherspoon et al.
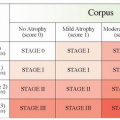
14 have proposed a scoring system for this purpose (
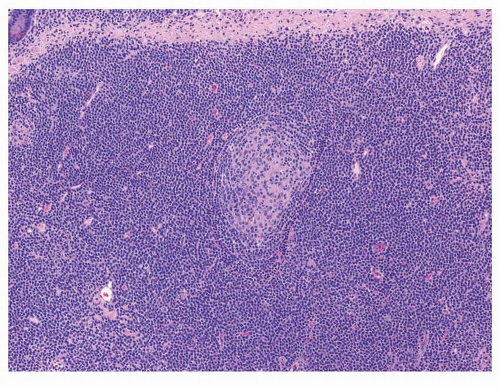
Table 10-3). Generally speaking, expansion of the marginal zone of the hyperplastic lymphoid follicles (
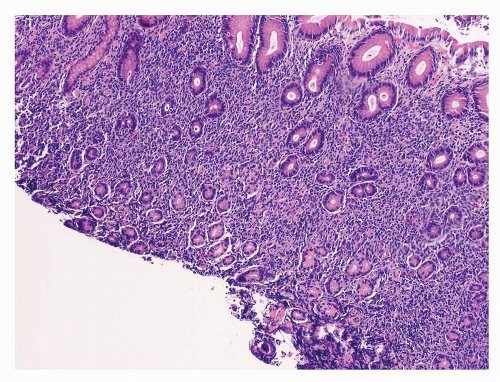
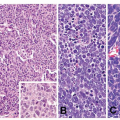
Fig. 10-2), dense lymphoid infiltrate composed of predominantly small B cells (
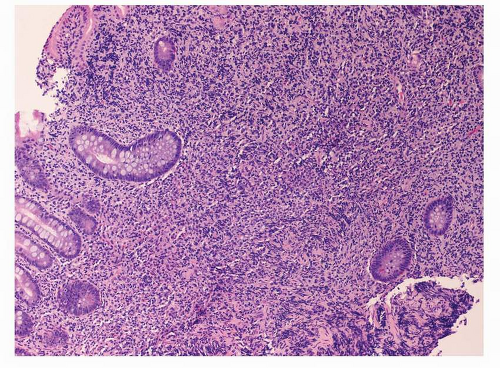
Fig. 10-3), increase in plasmacytoid lymphocytes, dropping out of gastric glands (
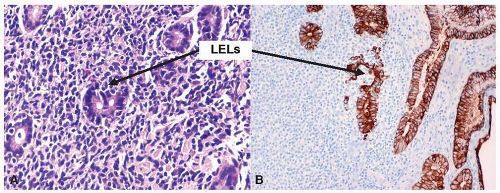
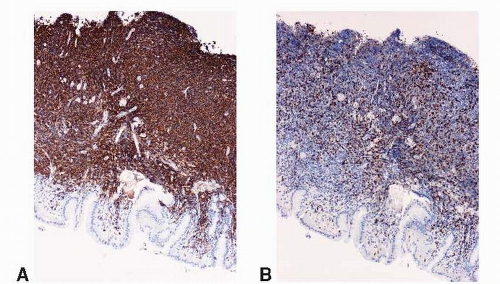
Fig. 10-4), and conspicuous lymphoepithelial lesions (LELs) (
Fig. 10-5) are all morphological features that favor MALT lymphoma. However, these features also can be seen in rare cases of severe gastritis. When in doubt, molecular study to detect VDJ rearrangement should be performed to rule out a B-cell clonal process.
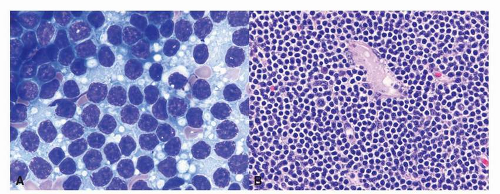
15Microscopically, MALT lymphoma is morphologically heterogenous. The characteristic lymphoma cells are small to medium in size and have a bland appearance with relatively increased cytoplasm, slightly irregular nuclei, moderately dispersed chromatin, and inconspicuous nucleoli, resembling centrocytes. The relative abundant, pale cytoplasm allows the cells to exhibit a “monocytoid” morphology
16 (
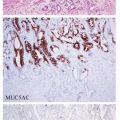
Fig. 10-6). The neoplastic cells can also infiltrate the gastric glands and form clusters within the glandular epithelium, fostering “lymphoepithelial lesions” (
Fig. 10-5A). The characteristic LELs can be highlighted by staining for pancytokeratin (
Fig. 10-5B).
The cells are almost always positive for CD19, CD20 (
Fig. 10-7), CD22, and CD79a and show surface immunoglobulin light chain kappa or lambda restriction. They are frequently positive for CD43. Typically negative for CD5 and CD10, an immunophenotype differs from those of most other small B-cell lymphomas (chronic lymphocytic leukemia/small lymphocytic lymphoma, follicular lymphoma, and mantle cell lymphoma [MCL]). The neoplastic B cells often are accompanied by plasmacytic differentiation as well as monoclonal plasma cells. Although primary lymphoplasmacytic lymphoma in the stomach is much rarer than MALT lymphoma, it has been reported.
17 Because of the overlapping features, differentiating between these two diseases is extremely difficult. In addition to Waldenstrom macroglobulinemia, which is often
associated with lymphoplasmacytic lymphoma, cytogenetic analysis is helpful in differentiating these two lymphomas.
18