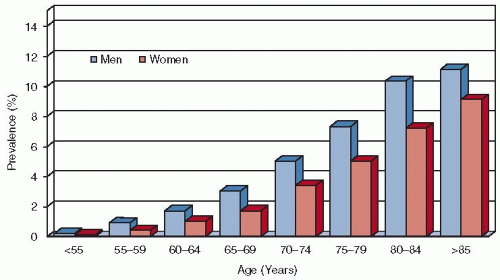
Atrial fibrillation (AF) is the most common sustained cardiac rhythm disturbance and an important independent risk factor for ischemic stroke. AF affects approximately 2.5 million individuals in the United States
1,2 and millions more worldwide. Prevalence is greater in men than in women
3,4,5 and increases with age, rising rapidly beyond the 6th decade to approximately 10% among those older than 80 years (see
FIGURE 92.1).
2,3,4,5,6 The median age of patients with AF is approximately 72 years. As the population ages, the number of individuals with AF is likely to increase substantially in the coming decades.
2
Risk of Stroke Associated with AF
As a result of disorganized and ineffective atrial systole, the fibrillating atria become passive conduits of blood from the systemic and pulmonary venous systems to the ventricles. Impaired atrial emptying leads to stasis and increases the risk of thrombus formation, particularly in the left-atrial appendage (LAA).
8 The factors that promote embolism of intracardiac thrombus and subsequent ischemic events (including stroke and peripheral arterial occlusion) are incompletely understood.
9,10 In addition to cardiogenic embolism, patients with AF commonly have other cardiovascular disease states, such as hypertension and atherosclerosis, which raise the risk of ischemic events.
Approximately 15% of all strokes are attributed to AF.
11 The rate of ischemic stroke among patients with AF not given antithrombotic therapy averages 4.5% per year,
12,13 approximately fivefold greater than in age- and gender-matched individuals with NSR. In the Framingham Heart Study, the risk of stroke attributable to AF rose from 1.5% in patients aged 50 to 59 years to 23.5% in those aged 80 to 89 years.
14 Because both the prevalence of AF and the risk of stroke increase with patient age, the problem of stroke prevention is amplified among the elderly; AF is the most common cause of stroke in women older than 75 years.
15 When compared to ischemic stroke due to other causes, ischemic stroke due to AF is typically more severe and carries a significantly higher mortality rate.
16 Several hypotheses have been advanced to explain the pathogenesis of stroke in patients with AF and the poor clinical outcomes associated with these events. Embolism of thrombus from the LAA may cause complete occlusion of relatively large cerebral arteries, consistent with the relatively frequent finding of large cortical infarcts on brain imaging and less frequent lacunar infarcts.
17 Chronically reduced cerebral blood flow, due either to the arrhythmia itself or to more extensive cerebrovascular disease associated with AF due to hypertension or atherosclerosis, may compromise collateral perfusion when acute infarction develops and contribute to greater stroke severity.
Atrial Flutter
Sustained atrial flutter is uncommon because the rhythm typically either degenerates to AF or reverts to NSR. Patients with persistent atrial flutter may have periods of AF and
vice versa. There are scant data from longitudinal studies assessing the thromboembolic risk associated with isolated, sustained atrial flutter. Echo-Doppler examinations of patients with atrial flutter demonstrate more organized atrial mechanical function and greater LAA flow velocities than are typical in patients with AF.
18 Despite this functional distinction, intra-atrial thrombus and stroke have been documented in patients with atrial flutter.
6,19 In a transesophageal echocardiographic (TEE) study before cardioversion, 25% of patients with atrial flutter of slightly longer than 6 months mean duration had spontaneous echocardiographic contrast, a marker of stasis thought to represent a prethrombotic state, and 7% had LAA thrombus.
20 In patients with prior cerebral ischemic events, the prevalence of intraatrial thrombus associated with atrial flutter was even higher.
21 A retrospective study of 100 patients with persistent atrial flutter found a higher than anticipated risk of stroke.
22 Although the role of anticoagulant therapy for patients with atrial flutter has not been evaluated in clinical trials, current treatment guidelines recommend that antithrombotic therapy follows the same risk-stratification approach applied to patients with AF.
Stroke Risk Stratification in Patients with AF
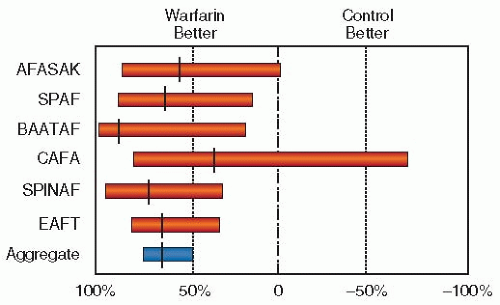
A combined analysis of untreated control groups in five primary prevention trials found a stroke rate of over 8% per year in patients with AF older than 75 years who had one additional clinical risk factor (hypertension, diabetes mellitus, heart failure (HF), or prior stroke or transient ischemic attack [TIA]). For patients of any age with a history of thromboembolism, the annual risk of stroke was 12%.
12,23 In a study of elderly nursing home patients who were not anticoagulated, the 3-year stroke rate exceeded 50%.
24Risk factors that increase the risk of stroke among patients with AF not treated with anticoagulants have been determined from randomized trials of antithrombotic therapy.
23,24,25,26,27,28 The most commonly cited risk-stratification strategies were generated from pooled analyses of five trials by the Atrial Fibrillation Investigators (AFI)
12 and from the Stroke Prevention in Atrial Fibrillation (SPAF) cohorts.
25,28 The AFI group
12 found the following independent risk factors: age (relative risk [RR] 1.4 per decade), prior stroke or TIA (RR 2.5), a history of hypertension (RR 1.6), and diabetes mellitus (RR 1.7). Analysis of 854 patients assigned to aspirin from the first two SPAF trials
29 identified three independent risk factors for stroke: women older than 75 years (RR 3.7), systolic hypertension >160 mm Hg (RR 2.2), and impaired left ventricular (LV) function defined as recent (within 3 months) HF, moderate-to-severe systolic dysfunction as assessed by two-dimensional echocardiography, or fractional shortening <0.25 by M-mode echocardiography (RR 1.8). Extending the analysis to 2,012 patients allocated to the aspirin or combination therapy arms of the SPAF-I to SPAF-III studies
30 identified five characteristics significantly associated with an increased risk of stroke: prior thromboembolism (RR 2.9), age (RR 1.8 per decade), female gender (RR 1.6), history of hypertension (RR 2.0), and systolic blood pressure >160 mm Hg (RR 2.3).
Although prior stroke or TIA, older age, and hypertension were identified as risk factors for stroke in patients with AF in both the AFI and SPAF risk-stratification schemes, there was a differential impact of age in these cohorts. The AFI scheme classified all patients with AF 65 years or older as at elevated risk, whereas the SPAF criteria classified women 75 years or younger and men of any age without other risk factors as at low risk. Uncertainty about risk in either gender aged 65 to 75 years and in men of any age without other risk factors applies to approximately 20% of the population with nonvalvular AF.
31Current guidelines recommend a risk-based approach to selection of patients with AF for oral anticoagulation to prevent stroke.
7 The main clinical risk factors are presented
in
Table 92.1. A modified scale, CHADS
2 (Cardiac failure, Hypertension history, Age >75 years, Diabetes, and Stroke or TIA [doubled]), integrates elements from the AFI and SPAF schemes in assessing the annual risk of stroke. This index allots two points for a history of stroke or TIA and one point each for age over 75 years, history of hypertension (not further defined), diabetes mellitus, recent clinical HF, or impaired LV systolic function (generally taken as an ejection fraction ≤35%) (
Table 92.2). The scheme was initially evaluated in 1,733 Medicare beneficiaries with nonvalvular AF aged 65 to 95 years who were discharged from hospital off warfarin
32 and has been validated in several cohorts.
The CHADS
2 index has the advantage of simplicity, but classifies a relatively large proportion of patients with AF as at intermediate risk and has imperfect predictive capacity overall (c-statistic ˜0.6). Additionally, many of those designated as low risk carry as high as a 1.3% annual risk of stroke. Additional risk factors, albeit less well validated than those that comprise the CHADS
2 index, should be considered in the stratification of stroke risk in patients with AF. Women with AF appear at higher risk than men with otherwise comparable risk profiles.
33,34,35 Vascular disease, including peripheral arterial disease, coronary disease (specifically prior myocardial infarction [MI]), and morphologically complex atheromatous plaque involving the thoracic aorta, is also associated with an increased risk of stroke, but its independent predictive value is controversial. An updated risk-stratification scheme, the CHA
2DS
2VASc score (
Table 92.3), incorporates vascular disease (V) and female sex (S) into the risk-stratification schematic, assigning each of these factors one point, and distinguishes two age categories to account for the continuous relationship of risk to age, allocating one point to patients with AF beyond age 65 and two points to those over 75 years.
36 The CHA
2DS
2VASc score was validated in the Euro Heart Survey cohort, anticoagulated clinical trial cohorts, and a national patient registry of patients with AF who were not anticoagulated.
37 When compared with the CHADS
2 risk score, CHA
2DS
2VASc had greater negative predictive value for stroke for patients classified in the low-risk category and classified fewer patients as intermediate risk, but the c-statistics still indicated imperfect capture of all attributable risks.
36The controlled clinical trials of warfarin included mainly patients with chronic, persistent, and permanent AF and smaller proportions of patients with paroxysmal AF.
7 For the purpose of determining stroke risk, the distinction between paroxysmal, persistent, and permanent AF proved immaterial. Patients with paroxysmal AF typically have a lower risk of stroke than those with persistent or permanent AF, as they are often younger and have fewer associated risk factors,
38,39 but multivariate analyses of prospectively followed cohorts found that the pattern of AF was not a significant predictor of stroke risk.
12,40 Current recommendations for anticoagulation, therefore, do not distinguish patients on the basis of the pattern of AF, though the available evidence does not address transient self-limited AF caused by acute medical illness or recent cardiac or noncardiac surgery.
7
Postoperative Atrial Fibrillation
Patients who develop AF after undergoing cardiothoracic surgery are also at an increased risk of thromboembolic stroke. While there is a small but significant increased risk of in-hospital stroke in patients who develop AF after surgery involving cardiopulmonary bypass, the risk of stroke seems related to underlying comorbidities rather than to AF itself. This was demonstrated in a series of close to 4,000 patients who had cardiac surgery on cardiopulmonary bypass. The rate of AF after surgery was reduced with prophylactic therapy, but the incidence of stroke was not reduced.
41The decision to anticoagulate patients with AF in the postoperative period is confounded by the risk of bleeding and its associated sequelae (cardiac tamponade, hematoma, hemothorax, etc.). Additionally, a significant percentage of patients who develop AF postoperatively convert back to sinus rhythm. The duration of AF postoperatively, the associated risk of thromboembolic stroke, and the decision of when to anticoagulate are unclear. Practice guidelines differ on when to start anticoagulation for postoperative AF. At the time of this writing, the American College of Chest Physicians (ACCP) guidelines recommend initiating anticoagulation with vitamin K antagonist (VKA) 48 hours after a patient has developed AF. The American Heart Association/American College of Cardiology (AHA/ACC) guidelines recommend starting a VKA after 24 hours of developing AF. Both guidelines agree that anticoagulation should continue for 4 weeks following reversion to and maintenance of sinus rhythm, but the level of evidence supporting this duration of therapy is low.
Echocardiographic Markers of Stroke Risk
Although left-atrial size and LV systolic function can be assessed by transthoracic (precordial) echocardiography, TEE is necessary to consistently visualize abnormalities of the LAA and aortic arch linked with thromboembolism. This approach is used not only as an adjunct to elective cardioversion (see below) but sometimes in patients with persistent AF to assist in thromboembolic risk assessment.
42,43 Visible thrombus or dense spontaneous echo-contrast in the left atrium (LA), and particularly in the LAA, is associated with a two- to fourfold increased risk of stroke.
7,8 In the SPAF-III study, patients with atheromatous plaque in the thoracic segments of the aorta that had complex morphology (mobility, pedunculation, ulceration, or thickness >4 mm) had high stroke rates. Because many of these abnormalities were observed in the descending aorta beyond the origins of the cerebral vessels, the association with stroke may reflect either associated cerebrovascular disease or the pathogenic role of risk factors such as hypertension that are common both to atherosclerosis and to conditions of atrial stasis in patients with AF.
43
Other Potential Stroke Risk Factors in AF
Other characteristics that may augment stroke risk include genetic polymorphisms, abnormalities of hemostatic and thrombotic function, increased platelet activation and aggregation, endothelial dysfunction,
44,45 and serum levels of troponin and B-type natriuretic protein, but none have yet proven sufficiently robust for routine clinical use. Thyrotoxicosis is a poorly understood risk factor for stroke among patients with AF, because patients with thyrotoxicosis were excluded from the randomized trials of antithrombotic therapy. AF develops in 10% to 15% of patients with hyperthyroidism, and thyrotoxicosis is evident in 2% to 5% of patients with AF.
46 A high frequency of stroke and systemic embolism has been reported in some
47,48,49,50,51 but not all studies.
52 The association, if real, may be mediated partly by concomitant HF.