INTRODUCTION
SUMMARY
The numbers of platelet transfusions administered in the United States increased dramatically during the 1980s, and has continued to grow as increasingly aggressive medical and surgical treatments have been developed and become more widely available. In particular, the growth of more intensive treatments for hematologic and other malignancies has spurred demands for platelet transfusion support and put pressure on platelet inventories nationwide.
The response to a platelet transfusion is affected by platelet recovery and platelet survival and includes the random loss of platelets in maintaining endothelial integrity. In a normal individual, weighing 70 kg, approximately 4.8 × 1010 platelets per day will be consumed maintaining the endothelium, less than the number of platelets in a single concentrate. However, many clinical conditions can adversely affect platelet recovery and platelet survival in the circulation. Prophylactic platelet transfusion is an important part of supportive care of patients with hypoproliferative thrombocytopenia because of hematologic malignancy and the effects of its treatment with cytotoxic drugs. A morning blood platelet count of less than 10 × 109/L appears to be an appropriate threshold for prophylactic transfusion. Although higher thresholds may be indicated for patients at high risk or who have active bleeding, little or no data support specific platelet count goals and a transfusion plan should be guided by the clinical setting. Larger doses of platelets do not confer additional protection against bleeding in this patient population, although they do result in higher increments and a longer interval until the next platelet transfusion, a potential benefit in the outpatient setting. The platelet count at which an invasive procedure or major surgery can be safely performed is not supported by randomized control studies and practice has been governed by retrospective data and case reports. Most minor invasive procedures and even major surgery can be safely performed at platelet counts of 20 to 50 × 109/L whereas high-risk procedures or severe bleeding may require platelet counts above 100 × 109/L. Patients may become refractory to platelet transfusion and fail to respond for many reasons. Patients alloimmunized to platelets may respond to platelet transfusions from class I human leukocyte antigen (HLA)-matched donors.
Platelets can be collected by apheresis or obtained from whole blood and pooled for transfusion. Both preparations have comparable effectiveness in the prevention of bleeding in patients with hematologic malignancy. Platelets should remain at room temperature and so are approved for only 5 days of storage because of risks of bacterial contamination and may lose viability within days after that. Leukocyte reduction of platelet components, either upon apheresis collection or by filtration, reduces HLA alloimmunization, prevents cytomegalovirus transmission by transfusion, and reduces febrile transfusion reactions. Pathogen reduction of platelets prevents replication of RNA and DNA in contaminating organisms and leukocytes and may prevent alloimmunization and decrease transfusion reactions. Prospective clinical trials are needed to better define indications for platelet transfusion and improve upon the effectiveness of transfusion therapy.
Acronyms and Abbreviations
AML, acute myeloid leukemia; AP, apheresis platelet; BC, buffy coat; CCI, corrected count increment; GVHD, graft-versus-host disease; HIT, heparin-induced thrombocytopenia; HLA, human leukocyte antigen; HSCT, hematopoietic stem cell transplant; ITP, immune thrombocytopenia; LP, lumbar puncture; PLADO, Platelet Dose study; PRP, platelet-rich plasma; PROPPR, Pragmatic Randomized Optimal Platelet and Plasma Ratios study; rdWBP, random-donor whole-blood platelet; TOPPS, Therapeutic or Prophylactic Platelet Transfusion Study; TPO, thrombopoietin; TTP, thrombotic thrombocytopenic purpura; TRAP, Trial to Reduce Alloimmunization to Platelets; WHO, World Health Organization.
CLINICAL PLATELET TRANSFUSION THERAPY
The number of platelet units transfused in the United States increased by 33 percent between the years 1997 and 2008 (data available at the time of this writing).1 Of more than 2,000,000 doses of platelets transfused in the United States in 2008, hematology-oncology patients used 32 percent, compared to the next highest usage of 15 percent in general medicine and 12 percent in cardiac surgery patients. Platelet transfusion therapy is associated with multiple adverse effects, including transfusion reactions, infection, alloimmunization, and immune modulation. The cost of platelets, their short storage time, and inventory pressures have made appropriate use of platelet transfusions a high priority for the management of thrombocytopenic patients to either prevent or control bleeding. Improved methods of collecting, processing, and storing platelets will be paramount in maintaining platelet inventories in the coming decade.
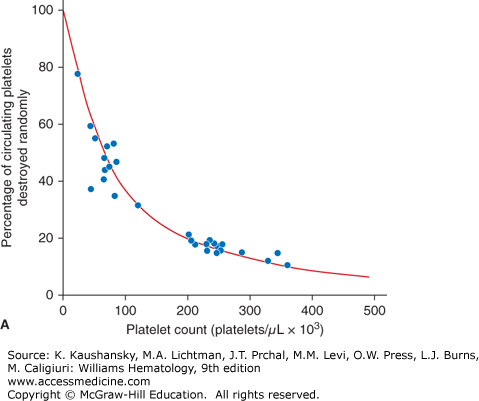
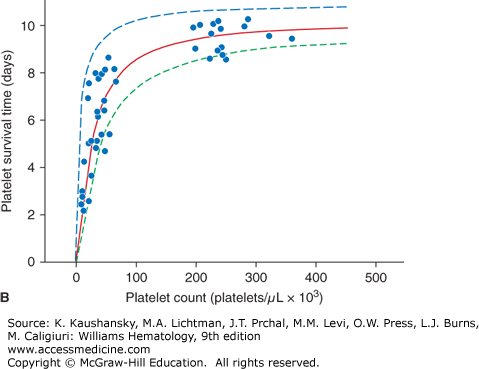
The expected response to a prophylactic platelet transfusion in nonrefractory thrombocytopenic patients is assessed by two parameters: (1) the number of platelets that circulate immediately after transfusion, as measured by platelet recovery; and (2) the survival time of the transfused platelet as measured by days-to-next-transfusion. Platelets circulate for a shorter time in thrombocytopenic patients (≤5 days) compared with normal subjects (8 to 10 days).2 This can be explained by the two mechanisms by which platelets are lost from circulation: (1) senescence, whereby platelets are removed by the mononuclear phagocyte system, and (2) random, whereby platelets are consumed during hemostasis to provide endothelial support.3 The random loss has been estimated to be 7.1 × 109 platelets/L per day. Thus, the more thrombocytopenic a patient is, the higher the percentage of their circulating platelets that will be removed randomly versus lost by senescence (Fig. 139–1A). At a platelet count of 300 × 109/L, approximately 15 percent of the platelets will be randomly removed—a fraction too small to influence the overall platelet survival. However, in a patient with a platelet count of 50 × 109/L, approximately 60 percent of the platelets will be used for endothelial support, and this will have a significant impact on reducing platelet survival time. This random platelet loss at low platelet counts results in a direct relationship between platelet count and platelet survival at platelet counts of less than 100 × 109/L (Fig. 139–1B).
Figure 139–1.
A. Relationship between platelet count and random platelet destruction. Studies were performed on healthy volunteers receiving autologous radiolabeled platelets as well as on patients receiving allogeneic platelet transfusions. Data points were obtained by dividing the fixed number of platelets lost per day by the number of circulating platelets lost from turnover.3 B. Relationship between platelet count and radiolabeled platelet survival measurements in healthy and thrombocytopenic patients. The curve (solid line) was obtained from an equation predicting the dependence of platelet life span on platelet count, assuming a fixed platelet requirement. The data show a high correlation of a finite rate of random platelet destruction per day with a fixed life span of the platelets.3
The number of platelets needed daily to support the random loss of platelets in maintaining endothelial integrity can be calculated. For example, a man weighing 70 kg with an estimated blood volume of 5 L would need 7.1 × 109 platelets/L per day, thus 3.6 × 1010 platelets per day. To account for the platelets pooled in the spleen, an additional 30 percent should be added giving a daily requirement of 4.8 × 1010 platelets for endothelial support. As one random donor platelet concentrate contains on average 8.3 × 1010 platelets, the daily requirement for endothelial support should be easily maintained with the transfusion of only one platelet concentrate per day. A somewhat higher number of platelet concentrates may be needed for patients who are also consuming transfused platelets based on clinical conditions such as sepsis, extensive tumor burden, and others.
Prior to the availability of platelets for transfusion, observational studies found the incidence of spontaneous bleeding increases at platelets counts of 100 × 109/L or less in children with acute leukemia,4 but minor and major bleeding began to increase (>1 percent chance of observable bleeding per patient-day) when the platelet count fell below 50 × 109/L. Major bleeding was only observed below 20 × 109/L, and on only 3 percent of days. Major bleeding was much more common below a platelet count of 5 × 109/L, as high as 33 percent as the count fell toward zero. Notably, many of the patients observed in this study were treated with aspirin for pain and fever resulting in some degree of platelet dysfunction that likely increased their bleeding risk. More recent observations suggest that the amount of bleeding is not dependent on the platelet count as long as it is above 5 × 109/L.2,5 Life-threatening bleeding rarely occurs above platelet counts of 5 × 109/L to 10 × 109/L without disruption of the vessel wall. In the Platelet Dose (PLADO) study, bleeding occurred on 17 percent of the study days at platelet counts between 6 × 109/L and 85 × 109/L and increased to 25 percent when counts fell below 6 × 109/L.6 These data are remarkably consistent with the increase in bleeding time when platelet counts fall below 100 × 109/L,7 although the bleeding time is not an accurate enough test to be relied upon clinically. There was also a marked increase in bleeding at platelet counts below 5 × 109/L as determined by stool blood loss measurements.2
Most recent large platelet transfusion trials have used the World Health Organization (WHO) bleeding scale or some modification of this scale to standardize the incidence and severity of bleeding in thrombocytopenic patients (Table 139–1).8 Grade 1 bleeding is noticeable but without clinical significance. Grade 2 bleeding, which requires some minor intervention to control bleeding, is an observable and reliable measurement for monitoring bleeding risk in platelet transfusion trials. It occurs frequently enough to be a useful end point for comparison of bleeding incidence and severity with different platelet transfusion strategies. In two large platelet transfusion trials, the incidence of Grade 2 bleeding in patients being treated for hematologic malignancies with chemotherapy was between 38 and 73 percent.6,9 In patients undergoing autologous hematopoietic stem cell transplantation (HSCT), bleeding rates were 45 percent and 57 percent; however, it was much higher (79 percent) in patients undergoing allogeneic HSCT. The 79 percent bleeding rate in allogeneic HSCT patients is likely because of their intensive conditioning therapies and the occurrence of graft-versus-host disease (GVHD).
| Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|
| Minor bleeding | Bleeding requires intervention or treatment, e.g., nasal packing, bladder irrigation, platelet transfusion or medications, to treat bleeding Grade 2a: Grade 2 bleeding excluding skin manifestations | Bleeding requires red cell transfusion related to treatment of bleeding or Significant intervention to treat bleeding, e.g., endoscopy or surgery | Bleeding that is fatal or life-threatening |
In a study of 1244 hematology-oncology patients, the 198 pediatric patients had a significantly higher overall risk of Grade 2 or higher bleeding than did adults (86 percent, 88 percent, and 77 percent for patients ages 0 to 5 years, 6 to 12 years, and 13 to 18 years, respectively, vs. 67 percent for adults).10 Children also experienced more days with Grade 2 or higher bleeding (median: 3 days in each pediatric group vs. 1 day in adults; p <0.001). The pediatric patients were at higher risk of bleeding over a wide range of platelet counts, indicating that their excess bleeding risk may be a result of factors other than just platelets.
Hematologic malignancies accounted for approximately 9 percent of all new cancers reported in the United States in 2012.11 More aggressive therapies have led to increased 5-year survival rates, but also resulted in substantial increases in the demand for platelet transfusions to support extended periods of marrow failure. The disturbance of endothelial integrity that frequently occurs with these therapies12 and the associated inflammation can induce hemorrhage in periods of thrombocytopenia.13 Mucositis, GVHD, infection, and organ dysfunction can all increase daily platelet consumption and negatively affect posttransfusion platelet increments and life span. Multiple strategies have been evaluated for maximizing the hemostatic effect of platelets while minimizing platelet use. Prospective randomized controlled trials have evaluated the relative safety of different platelet count thresholds for transfusion, whether platelets should be transfused prophylactically or can be administered therapeutically at the first sign of bleeding, and optimal platelet dose for platelet transfusion.
Prophylactic platelet transfusions were shown to decrease the incidence of bleeding into vital organs noted at autopsy of leukemia patients as early as 1966,14 and have become an integral part of treatment regimens for hematologic malignancy. However, maintaining platelet counts may be difficult owing to very short platelet survivals in severely thrombocytopenic patients.2,6,15 Several prospective randomized platelet transfusion trials have shown no differences in spontaneous bleeding events when patients are transfused at platelet counts below 10 × 109/L versus 20 × 109/L16,17,18 or even versus 30 × 109/L,19 and a threshold for transfusion as low as 5 × 109/L may be safe.20 In one study, 85 patients with acute leukemia were randomized to receive prophylactic platelet transfusions when their platelet count fell to 20 × 109/L, 10 × 109/L, or 5 × 109/L.20 An aliquot of each patient’s red cells were labeled with radioactive51 chromium and daily stool collections were performed to quantify the amount of gastrointestinal mucosal bleeding. There were no differences in stool blood loss, red blood cell transfusion rates, or incidence of bleeding events among the three study arms. However, there was a significant decrease in both the frequency and number of platelet transfusions required among patients randomized to the lower transfusion threshold compared to the higher transfusion thresholds. A platelet transfusion threshold of less than 10 × 109/L in stable patients has been recommended by both the American Association of Blood Banks (AABB)21 and Sanquin Blood Supply in 2014.22 Patients with active infection, fever, or who are bleeding may require higher transfusion thresholds.23
WHO Grade 4 bleeding that causes life threatening hemorrhage or death is a rare occurence.6,9 Several prospective trials have evaluated the potential bleeding risk of withholding platelet transfusions until WHO Grade 2 bleeding occurs with the assumption that Grade 4 bleeding would very rarely take place before Grade 2 bleeding was observed. A trial comparing a therapeutic-only transfusion strategy versus prophylactic platelet transfusion given routinely for a morning platelet count of less than 10 × 109/L in 396 patients who were undergoing intensive chemotherapy for acute myeloid leukemia (AML) (n = 190) or autologous HSCT patients (n = 201) monitored patients for bleeding according to the WHO bleeding scale.24 Despite randomization to the therapeutic-only transfusion arm, 30 percent of patients were transfused with platelets prophylactically and 22 percent for extended petechiae or bruising. The primary end point (number of platelet transfusions) was 33.5 percent higher in the prophylactic transfusion group. However, Grade 4 bleeding occurred in 14 patients with AML in the therapeutic-only transfusion arm, two of which were fatal cerebral hemorrhages. No Grade 4 bleeding occurred in patients undergoing autologous HSCT. In the Therapeutic or Prophylactic Platelet Study (TOPPS), 600 patients with acute leukemia, lymphoma, or myeloma undergoing chemotherapy alone (n = 98), autologous HSCT (n = 411) or reduced-intensity allogeneic HSCT (n = 74) were randomized to a therapeutic-only or prophylactic transfusion strategy.9 Patients in the therapeutic-only transfusion arm had a significantly shorter time to their first Grade 2 or greater bleeding event, and they also experienced more bleeding overall. Although both of these studies showed a decrease in the number of platelet transfusions administered in the therapeutic-only arms compared to the prophylactic transfusion arms, and there were no differences in the number of red cell transfusions, this strategy cannot be considered safe in the majority of patients undergoing HSCT or induction chemotherapy for acute leukemia.
It has been estimated that 4.8 × 1010 platelets are used daily to maintain endothelial integrity in an individual weighing 70 kg with an estimated blood volume of 5 L.3 As long as this minimal number of platelets is provided, higher platelet doses do not appear to decrease the incidence of bleeding in patients with hematologic malignancy. The PLADO trial studied more than 1200 patients with hematologic malignancies who were receiving prophylactic platelet transfusions at a threshold of less than 10 × 109/L during chemotherapy or HSCT.6 Patients were randomized to one of three platelet doses; the accepted current standard dose of 2.2 × 1011 platelets/m2 (expected to be equivalent to four to six pooled platelet concentrates or one apheresis platelet [AP] collection in most adults), a low dose of 1.1 × 1011/m2 (half of standard), or a high dose of 4.4 × 1011/m2 (twice standard). WHO Grade 2 bleeding was common in all patients and similar at all doses. Seventy percent of patients had at least one episode of Grade 2 or greater bleeding with no significant differences among the dose groups (71 percent, 69 percent, and 70 percent, respectively). Grade 3 bleeding occurred in 8 percent of patients and Grade 4 in only 2 percent with no differences among the groups. Only one hemorrhagic death occurred, in a patient in the high-dose group. By treatment category, 79 percent of patients undergoing allogeneic HSCT had at least one episode of Grade 2 or greater bleeding compared to 73 percent of patients undergoing chemotherapy for hematologic malignancy, and 57 percent of autologous or syngeneic HSCT patients. There were no differences in bleeding risk based on transfused platelet dose among any of these patient categories. Lower platelet doses required fewer number of platelets overall, but more frequent transfusions to maintain a platelet threshold of 10 × 109/L. However, as the cost of platelet therapy is predominantly related to the number of transfused platelets rather than the frequency of administration, low-dose therapy may be the most cost-effective strategy, at least during hospitalization.25 Platelet transfusion intervals were significantly longer in the higher-dose groups, resulting in fewer transfusion events, which may make higher-dose transfusions the preferred strategy for outpatients.
Only mild shortening of the period of thrombocytopenia in patients undergoing chemotherapy for hematologic malignancy has been observed with the use of thrombopoietin mimetic agents. Treatment with romiplostim, a thrombopoietin (TPO) receptor agonist, in patients with low-risk/intermediate-1–risk myelodysplastic syndrome increased platelet counts and decreased the number of bleeding events and platelet transfusions. Although the study drug was discontinued because of an initial concern of risk of progression to AML, survival and AML rates were similar in patients receiving romiplostim and a placebo.26 At this time, TPO receptor agonists cannot be routinely recommended as an adjunct to or replacement for platelet transfusions in patients with hypoproliferative thrombocytopenia, but clinical trials are ongoing.
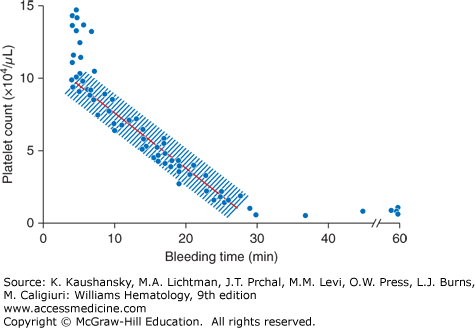
Little data exist to guide platelet transfusions in patients who are undergoing invasive procedures.27 Most recommendations are based on reports of “no harm” observed in groups of patients undergoing planned procedures. Some implications can be drawn from bleeding time measurements performed at various platelet counts (Fig. 139–2).7 At platelet counts of 100 × 109/L or greater, the bleeding time averages approximately 5 minutes. At platelet counts of less than 100 × 109/L, there is an inverse relationship between platelet count and bleeding time; that is, as the platelet count decreases, the bleeding time increases.7 Figure 139–2 provides a graphical comparison of bleeding times at different platelet counts but does not predict the risk of bleeding at surgery. Neither platelet counts nor bleeding times are exact measurements, and small differences in platelet counts are unlikely to make a significant difference in the time it takes for bleeding to cease. The need for a prophylactic platelet transfusion for an invasive procedure must consider platelet count, platelet function, and endothelial integrity, as well as the consequences of prolonged bleeding. Procedures that would result in significant harm with even small amounts of bleeding (e.g., neurosurgical procedures) should probably be performed at higher platelet counts (≥100 × 109/L), although most procedures can safely be performed at lower platelet levels. Abnormal coagulation parameters, drugs or diseases (e.g., uremia) that inhibit platelet function should be corrected as much as possible before any procedure. Although international guidelines exist for platelet counts at which a procedure can be safely performed, evidence for these recommendations are generally weak and of low quality.22,27
Figure 139–2
The relationship between platelet count and bleeding time. With normal platelet function, bleeding time above a platelet count of greater than 100 × 109/L is maintained at approximately 5 minutes. Bleeding time has an inverse relationship with platelet count below a platelet count of 100 × 109/L, prolonging as the platelet count decreases. (Repoduced with permission from Harker LA, Slichter SJ: The bleeding time as a screening test for evaluation of platelet function. N Engl J Med 27;287(4):155–159. 1972.)
Central Venous Catheter Placement Observational studies of central venous catheter placement have reported low bleeding rates of 0 to 9 percent. The largest study included the placement of 604 nontunneled catheters in 193 consecutive patients and found only patients with preprocedure platelet counts below 20 × 109/L were at increased risk of bleeding compared to those with counts above 100 × 109/L.28 Ninety-six percent of bleeding events were Grade 1, and the remaining were Grade 2, requiring only local compression for bleeding cessation. A study of 3170 tunneled catheter placements under ultrasound guidance reported no bleeding complications in 344 patients with platelet counts less than 50 × 109/L, nor in the 42 patients with even lower platelet counts of 25 × 109/L or less.29
Lumbar Puncture Lumbar puncture (LP) can often be safely performed at platelet counts below 20 × 109/L. A study of 5223 LPs performed in 956 pediatric leukemic patients reported that 941 procedures were done at or below 50 × 109/L, with 199 done below 21 × 109/L, with no bleeding complications.30 Even in those patients who had a traumatic LP (>500 red blood cells/high power field), which occurred in 10.5 percent of the patients, there were no adverse clinical outcomes. In a study in 66 adults with acute leukemia undergoing 195 LPs, there were no bleeding complications in the 40 LPs performed at platelet counts of 31 to 50 × 109/L nor in the 35 LPs performed at platelet counts of 20 to 30 × 109/L.31
High-level evidence is again lacking for determining a safe platelet count for major invasive procedures. At this time there are no data to support increased perioperative bleeding risk in patients with platelet counts below 50 × 109/L who are undergoing major surgery.32 However, the presence of other hemostatic abnormalities, especially platelet dysfunction, should be considered. Although there is no evidence to support platelet transfusion in the nonbleeding cardiac surgery patient, a bleeding patient post–cardiopulmonary bypass may benefit from platelet transfusion, even in the setting of a normal platelet count as a result of the detrimental effect of bypass on platelet function.33 The patient undergoing major neuraxial surgery may require higher platelet counts perioperatively to minimize increased accumulation of blood in an enclosed space. Although no specific evidence supports the practice other than normalization of the bleeding time, a platelet count of 100 × 109/L is generally accepted as appropriate for major neurosurgical procedures.21,22
The Thrombocytopenic Patient on Anticoagulation Therapy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree