Most patients with gynecologic cancer are middle-aged to elderly and have a high incidence of coexisting medical problems at the time of presentation. The medical status of all patients requiring aggressive surgery needs to be optimized preoperatively, to ensure the optimal postoperative outcome. The early identification, evaluation, and management of emerging medical problems are essential. This chapter discusses the preoperative evaluation of the surgical patient, and the postoperative management of some of the most common medical problems encountered by patients with gynecologic cancers.
Preoperative Evaluation
The cornerstone of all perioperative medical management is the anticipation of specific problems. Careful and accurate assessment of preoperative risk is the first step in developing a perioperative plan that maximizes successful outcomes.
Cardiovascular
Surgery can represent a major cardiovascular stress because of depression in myocardial contractility, changes in sympathetic tone induced by general anesthetic agents, and rapid changes in intravascular volume that occur as a result of blood loss and “third spacing” of fluids. The magnitude of cardiovascular stress depends on patient characteristics, the nature and site of the operation, the duration of the operation, and whether it is elective or emergent. Perioperative myocardial ischemia and infarction are the most significant predictors of adverse cardiovascular outcomes in the years following surgery.
Cardiovascular Risk Factors
Multiple studies have been published over the last 30 years assessing clinical risks for cardiac events during surgery (1–5). The risk of perioperative cardiovascular complications depends on the patient’s underlying health and the nature of the planned surgery. A commonly used “simple index” called the Revised Cardiac Risk Index identifies five clinical variables as independent predictors of cardiac complications (Table 18.1). This simple index is among the best validated ways to estimate cardiovascular risk and forms the foundation of the 2007 revision of the American College of Cardiology (ACC) and the American Heart Association (AHA) guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery (5,6).
Table 18.1 Revised Cardiac Risk Index

The 2007 ACC/AHA guidelines (in addition to the 2009 ACCF/AHA focused update on perioperative β-blockade) offer a simplified yet comprehensive approach to the assessment of cardiac risks for patients undergoing noncardiac surgery (6,7). An algorithm based on this approach is shown in Figure 18.1. This approach represents a consensus view derived from a review of the literature to date and is periodically updated on the ACC website (http://www.acc.org). An important overriding theme of these guidelines is that cardiac stress testing and interventions (such as coronary stenting or coronary bypass graft surgery) are rarely necessary simply to lower the risk of surgery. Such interventions are likely unnecessary unless they would have been performed if the patient were not undergoing surgery. No test should be performed unless it is likely to influence patient treatment.
If the patient has an active cardiac condition that indicates major clinical risk (Table 18.2), the surgery should be delayed or cancelled until the condition is stabilized unless the surgery is emergent (6,8). For stable patients, the algorithm can be used as follows: obtain information from the patient and use the Revised Cardiac Risk Index to determine how many of the clinical risk factors (from Table 18.1) the patient has. One point is assigned for each of the six possible clinical risk factors. Based on how many points are assigned, patients can be characterized as high risk (3 or more points), intermediate risk (1 to 2 points), or low risk (0 points).
The first step is to determine the urgency of surgery. If emergent surgery is needed, there is no time for any cardiac assessment beyond what history is available and the patient should proceed immediately to the operating room. For urgent or elective surgery, there is more time to accurately assess a patient’s cardiac risks. If the patient has an active cardiac condition (Table 18.2), all but emergency surgeries should be delayed until the cardiac condition is properly evaluated and treated.
Table 18.2 Major Active Cardiac Conditions


Figure 18.1 Stepwise approach to preoperative cardiac assessment in gynecologic surgery. *For active cardiac conditions, refer Table 18.2; **risk of surgery is shown in Table 18.3 ***MET, metabolic equivalent (see Table 18.4) ****Clinical Risk Factors refers to Table 18.1. (From Fliesher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Circulation. 2007;116:e418–e499.)
Table 18.3 Cardiac Risk Stratification for Noncardiac Surgical Procedures

Often patients need very little evaluation and no additional cardiac testing. Patients who are undergoing low-risk surgery (Table 18.3) do not need further evaluation. Patients with good functional capacity (metabolic equivalents [METs] levels ≥4) (Table 18.4) without symptoms and active conditions can proceed with gynecologic surgery without further evaluation (Fig. 18.1) (9).
Table 18.4 Estimated Energy Requirements for Various Activities

If the patient has poor or unknown functional capacity, the presence of clinical risk factors from the Revised Cardiac Risk Index will help to determine the need for further evaluation. The most challenging patients to evaluate preoperatively are those with at least one cardiac risk factor and with poor or uncertain functional capacity undergoing intermediate risk surgeries (most gynecologic surgeries). Evidence from a randomized trial suggests that preoperative cardiac stress testing is of no value in patients with only one or two cardiac risk factors (10). Preoperative stress testing is best reserved for patients with three or more cardiac risk factors in whom preoperative revascularization is feasible, and would have been considered regardless of whether or not the patient was having surgery. The guidelines emphasize that stress testing should be performed only if it will change management (6,11). Perioperative β-blockade will be discussed in more detail below, but based on the evidence, β-blockade cannot be recommended for patients with intermediate risk and extreme caution should be used if these drugs are newly prescribed preoperatively. Dipyridamole-thallium imaging or dobutamine stress echocardiography can be considered for noninvasive testing in these patients (12,13). If noninvasive testing shows only minor abnormalities (minimal areas of myocardial ischemia), the patient can proceed with surgery with medical management including β-blockade, if appropriate. With optimal medical therapy, these patients have no greater incidence of perioperative cardiac events than those with no evidence of ischemia (14). If stress testing reveals moderate or severe abnormalities, subsequent care should include cardiology consultation. Considerations would include cancellation or delay of surgery, coronary revascularization followed by noncardiac surgery, or intensified care in such patients (6).
Myocardial Infarction
Even with careful and accurate preoperative assessment and preparation, postoperative myocardial infarction (MI) can still occur after surgery under general anesthesia. The risk factors for perioperative MI are related to the underlying risk of ischemic heart disease. Before the advent of more modern management of ischemic heart disease, the risk of a second MI after anesthesia and general surgery was considered too high during the first few months post-MI (15). Current cardiologic practice, including revascularization, angioplasty, or very aggressive medical therapy with lipid-lowering agents and use of β-blockers, makes this rule less useful. It is now commonly believed that with proper management, patients can undergo surgery 6 weeks after MI if necessary.
Traditionally, it has been felt that coronary revascularization with coronary artery bypass surgery lowered the risk in patients with coronary artery disease (CAD) (16,17). Recent evidence, however, suggests that only certain patients with severe CAD benefit from coronary revascularization (significant left main stenosis, three-vessel CAD with a decreased left ventricular ejection fraction [EF < 50%], two-vessel CAD with proximal left anterior descending artery stenosis and either EF < 50% or ischemia on stress-testing, or active acute coronary syndromes such as acute MI) (6). If none of the factors listed above are present, aggressive medical therapy, including β-blockade if appropriate, is as effective as revascularization at reducing surgical risk, including postoperative MI and long-term mortality, even in high-risk ischemic patients (e.g., patients with significantly abnormal preoperative dobutamine stress echocardiograms) (18,19). Studies show that prophylactic percutaneous coronary intervention (PCI) confers no additional benefit when added to rigorous medical therapy in patients with stable CAD (stable angina) (20).
With the increasing use of PCI, elective or nonurgent surgery should be delayed for at least 14 days after balloon angioplasty. When a stent is placed, dual oral antiplatelet therapy with aspirin and clopidogrel is needed to reduce the risk of stent restenosis and stent thrombosis. Therefore, clopidogrel and aspirin therapy should be instituted following placement of a coronary stent and elective or nonurgent surgery should be delayed at least 30 days after the placement of a bare-metal coronary stent, and 365 days after the placement of a drug-eluting stent. When surgery is undertaken, perioperative aspirin should be continued in each of these situations (6,21). In fact, perioperative aspirin therapy should be continued in all patients with previous cardiac stent placement if possible (discussed in detail below).
A postoperative MI can be painless. The risk of MI is throughout the first week, but the incidence is thought to peak on the third postoperative day. Perioperative MI is associated with significant perioperative mortality (30–50%) and reduced long-term survival (22,23), so appropriate surveillance is prudent in high-risk patients.
Electrocardiography (ECG), beginning in the immediate postoperative period and continuing at least through postoperative day 3, is prudent (24,25). Cardiac troponin assays are now part of the universal definition for MI. They should be monitored in high-risk patients and often can detect postoperative MI earlier than other tests (6,26–30). Because the risk of perioperative MI is increased in patients who are subjected to intraoperative hypotension, measures must be taken to maintain high-risk patients in a normotensive state during surgery. If intraoperative hypotension occurs, the patient should be considered at high risk of postoperative MI and monitored appropriately.
Theoretically, β-blockers would be expected to facilitate the development of intraoperative hypotension because of the additive myocardial depressive effect of these medications with general anesthesia. However, abrupt discontinuation of a-blocker medication can be associated with a dangerous rebound syndrome (i.e., acute hypertension and coronary ischemia), with the incidence of the syndrome peaking at 4 to 7 days after discontinuation of the drug (31,32). Patients tolerate general anesthesia in the presence of continued β-blocker treatment. All patients on chronic β-blocker therapy should continue β-blockers in the perioperative period (7). While several studies in the 1990s suggested that perioperative β-blocker use reduced postoperative nonfatal MI and mortality in many patients including those with intermediate risk, subsequent research has cast great doubt on this claim (33–35). The largest placebo-controlled trial of perioperative a-blocker use to date (the POISE trial) showed an increase in mortality and stroke in those receiving β-blockers compared with placebo (35). This study caused the ACC and AHA to publish a focused update in which the only definite recommendation for perioperative β-blockade was that β-blockers be continued in patients who were already receiving chronic β-blocker therapy, because ischemia can be precipitated by abrupt discontinuation (31,32). Although β-blockers may still be considered in high-risk patients with inducible ischemia, CAD, or multiple coronary risk factors (at least 3 points on the Revised Cardiac Risk Index), the ACC/AHA update emphasizes the mixed evidence and potential dangers of aggressive treatment (7).
If β-blockers are prescribed for high-risk patients, the recommended target heart rate for effective a-blockade is below 65, but not lower than 50 beats per minute. The appropriate duration of β-blockade is debated, but should certainly begin before surgery and continue throughout the hospitalization. If possible, there may be additional benefit if β-blockers can be started 1 month before surgery to titrate the heart rate, and be continued after hospitalization for at least 30 days if adequate postoperative medical follow-up can be arranged (34,36).
There is substantial evidence that perioperative statin therapy reduces cardiovascular risk in patients undergoing vascular surgery (37–39). Statins should not be stopped abruptly in patients on chronic therapy, as there appears to be a rebound effect during which the risk of cardiovascular events increases after abrupt cessation (40). Aspirin therapy should be continued in the perioperative period in most cases where there is a risk of cardiovascular complications. Aspirin discontinuation has been shown to result in an increase in adverse cardiac events occurring an average of 10 days after aspirin cessation (41,42). There is a theoretical increase in bleeding risk in patients taking aspirin, but a meta-analysis of studies comparing surgical bleeding in patients taking low-dose aspirin found no difference in the severity of bleeding events (with the exception of intracranial surgery and possibly transurethral prostatectomy) or mortality (42). For most surgeries aspirin can be safely continued in the perioperative period.
Congestive Heart Failure
Heart failure is associated with a poorer outcome after noncardiac surgery (1,2,5). The cause of heart failure should be identified if possible, as this may have implications concerning perioperative risk (6). It may be reasonable to perform an echocardiogram in symptomatic patients, but the utility of this is still questionable (43). Patients with moderate or severe congestive heart failure should be treated before surgery with appropriate medications to optimize their cardiovascular status. Perioperative use of a pulmonary artery (Swan–Ganz) catheter is no longer recommended, as studies have not shown clear benefit to these devices in managing high-risk surgical patients (44).
Arrhythmias
Cardiac arrhythmias that are hemodynamically significant or symptomatic should be treated and stabilized prior to elective or nonurgent surgery. Atrial fibrillation is the most frequently seen arrhythmia, and may require electrical or pharmacologic cardioversion. Alternatively, a rate-control strategy can be attempted with β-blockers, calcium channel blockers, or digoxin. Ventricular arrhythmias, such as simple premature ventricular contractions, complex ventricular ectopy, or nonsustained tachycardia usually require no therapy unless they are associated with hemodynamic compromise or occur in the presence of left ventricular dysfunction or ongoing cardiac ischemia (45,46). Careful evaluation for underlying cardiopulmonary disease, drug toxicity, metabolic disturbances, and infection should be undertaken in patients who have any arrhythmia in the perioperative period (47).
Conduction Disturbances
High-grade Conduction Abnormalities
Patients who do not have permanent pacemakers and who have third-degree heart block at the time of presentation are at substantial risk of cardiopulmonary arrest during surgery. Typically, they are unable to mount an appropriate pulse response to the vasodilatation and decreased myocardial contractility induced by general anesthesia, or to the volume depletion induced by surgical blood loss. Patients with high-grade conduction abnormalities, including complete heart block, may require temporary or permanent transvenous pacing.
Bifascicular Block
In patients with lower degrees of heart block, specifically bifascicular block (right heart block with left axis deviation), the risk of development of a higher degree of ventricular block during surgery is not significantly increased, provided there is no history of previous third-degree heart block or syncope. Such patients rarely require insertion of a temporary pacemaker (48). Patients with bifascicular block who have a history of third-degree heart block should be managed for complete heart block with preoperative cardiologic evaluation and likely pacemaker insertion.
A new bifascicular block developing in the setting of acute MI carries a high risk of progression to complete heart block. Therefore, if this problem occurs after surgery, the patient should be considered at significant risk for the development of complete atrioventricular block. Such patients require a cardiology consultation and insertion of a temporary pacemaker.
Pacemakers
Patients with permanently implanted pacemakers should have a preoperative pacemaker evaluation to allow examination of all pacemaker functions. This precaution ensures that backup demand pacemaker failure will not be uncovered unexpectedly with the vagotonic stimuli associated with general anesthesia in abdominal surgery. Patients with implanted defibrillators typically have their devices turned off shortly before surgery and then turned back on shortly afterward.
Even newer pacemakers and defibrillators can sense the electromagnetic impulses created by electrocautery, especially when the electrocautery plate is close to the pacemaker unit. It is prudent to place the indifferent electrocautery electrode as far as possible from the chest and to use electrocautery sparingly. An added precaution consists of keeping a magnet available in the operating room to convert a pacemaker rapidly from the demand to a fixed pacing mode. Inappropriate discharges from the implanted defibrillator are avoided by having the device turned off during the time of surgery (49). Those with permanent pacemakers should have their device assessed for proper function after surgery (6).
Endocarditis Prophylaxis
Guidelines for the prevention of infective endocarditis (IE) do not recommend administration of antibiotics solely to prevent endocarditis for patients who undergo a genitourinary (GU) or gastrointestinal (GI) tract procedure. As a result of a lack of published data demonstrating a benefit from prophylaxis, current guidelines differ substantially from previous guidelines, and far fewer patients should be recommended for IE prophylaxis than previously thought (50). Very few data exist on the risk or prevention of IE with a GI or GU tract procedure. Enterococci are part of the normal flora of the GI tract and are the primary bacteria from this area likely to cause IE. In patients with the highest risk cardiac conditions (prosthetic cardiac valve, previous IE, or congenital heart disease) who are to receive antibiotic therapy to prevent wound infection, it may be reasonable to include an antibiotic that is active against enterococci, such as penicillin, ampicillin, or vancomycin. However, no published studies demonstrate that such therapy will prevent enterococcal IE (50).
Hypertension
The significance of mild to moderate hypertension (stage 1 or 2 with systolic blood pressure below 180 mm Hg and diastolic blood pressure below 110 mm Hg) in patients undergoing surgery remains controversial. This controversy stems from the difficulty in sorting out the risk of hypertension per se from the risk of hypertension in the setting of hypertensive or atherosclerotic heart disease.
Numerous studies have shown that uncomplicated mild to moderate hypertension (stage 1 or 2), regardless of treatment status, is not an independent risk factor for perioperative complications (1,2,51,52). The presence of hypertension may be of consequence, because it has been reported that patients with preoperative hypertension may demonstrate marked intraoperative blood pressure lability and postoperative hypertensive episodes (53). Certain medications used for the treatment of chronic hypertension, including angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor antagonists (ARBs), seem to make intraoperative hypertension more likely (54,55).
Table 18.5 Causes of Perioperative Hypertension

It is generally agreed that severe hypertension (stage 3 with systolic pressure greater than 180 mm Hg, diastolic greater than 110 mm Hg) should be controlled with effective oral medications in the days to weeks prior to undertaking an elective operation. Another option for severe hypertension is the use of rapidly acting intravenous agents, which usually can bring blood pressure under control in a few hours. One randomized trial was unable to demonstrate a benefit to delaying surgery in a select patient group with severe hypertension (56).
The causes of perioperative hypertension are presented in Table 18.5. Patients with both hypertensive and atherosclerotic heart disease may be at greater risk than those with uncomplicated hypertension alone. As is the case for cardiac complications, the type of surgery is important in understanding the risk of hypertension. Hypotension as a result of any cause remains a concern in patients with CAD.
Hypertensive management begins with identification, followed by development of a plan for control (57). Most antihypertensive medications should be given on the morning of surgery. If tolerated by blood pressure, it is important to continue β-blockers and clonidine to avoid withdrawal and potential heart rate or blood pressure rebound. Because of the possible problem of intraoperative hypotension, several authors have suggested holding ACE inhibitors and ARBs on the morning of surgery (58,59). Most clinicians hold diuretics to avoid volume depletion. Although diuretic use is associated with volume depletion and hypokalemia, the importance of correcting mild degrees of diuretic-induced hypokalemia in the absence of significant heart disease is controversial (60). Potassium repletion should never be rapid, and is safest by the oral route or by adjustment of medication.
In the postoperative period, many patients, especially the elderly, need less antihypertensive medication because of the salutary effects of bed rest and relative sodium restriction. If blood pressure is not elevated, it is wise to plan on reinstating drugs stepwise, beginning with the most active agent at approximately half the usual dose and finally adding the diuretic, if used, sometime later. An exception to this would be the use of β-blockers, which, because of the concern about rebound hypertensive effects and cardiac ischemia if they are stopped abruptly, should be continued in the postoperative setting. Likewise, clonidine should be continued in the perioperative period to avoid rebound hypertension. Patients whose only antihypertensive drugs are thiazide diuretics are best observed in the immediate postoperative period. Patients who need additional antihypertensive therapy in the immediate preoperative period should not be treated with diuretics because of the risk of associated hypovolemia and hypokalemia.
Pulmonary
It is estimated that pulmonary complications are at least as common as cardiac complications after noncardiac surgery (61,62). Atelectasis, postoperative pneumonia, respiratory failure, and exacerbation of an underlying pulmonary condition can all develop after abdominal and pelvic surgery. Pulmonary complications are often associated with the highest costs and the longest hospital stays after surgeries (A). Clinicians should attempt to identify patients at increased risk for pulmonary complications, and reduce these risks whenever possible (Fig. 18.2).
Pulmonary Risk Factors
Pulmonary risk factors are typically grouped as “procedure related” or “patient related.” For “procedure-related” risks, it has been shown that the closer a procedure is to the diaphragm, the greater the perioperative pulmonary risks (63). This is presumably caused by the higher likelihood of diaphragmatic dysfunction, or related postoperative pain and shallow inspiration. For this reason upper abdominal surgeries create more postoperative risk than lower abdominal procedures.

Figure 18.2 Pulmonary evaluation and postoperative care. *Measures to reduce pulmonary complications (see Table 18.6). COPD, chronic obstructive pulmonary disease.
General anesthesia, emergency surgery, and prolonged surgeries (greater than 3 hours in duration) increase the risk of postoperative pulmonary complications (63). There is some evidence that minimizing and changing to shorter-acting neuromuscular blockers during an operation can reduce postoperative pulmonary complications (64,65). Although comparative data are limited, most clinicians believe that epidural anesthesia and analgesia and the use of laparoscopic rather than open surgery should reduce postoperative pulmonary risks.
A review of multiple studies has confirmed several patient-related risk factors for postoperative pulmonary complications: advanced age; ASA (American Society of Anesthesiologists) class ≥2; heart failure; functional dependence; and chronic obstructive pulmonary disease (COPD). A serum albumin level <3.5 mg/dL and overt malnutrition were also associated with increased pulmonary complications, and current smoking seems to increase postoperative pulmonary risks (66).
Although COPD is a known risk factor for postoperative pulmonary complications, there is considerable debate about the routine use of spirometry to screen for this condition before nonthoracic surgery. The American College of Physicians (ACP) recommends spirometry only if the history and physical examination suggest an undefined lung condition (67). A history of prolonged cigarette smoking, dyspnea, chronic cough, sputum production, wheezing, or prolonged expiration and hyperinflation noted on examination would all be suggestive findings for COPD. Patients with these findings should typically receive pulmonary function testing with spirometry (and possibly a blood gas), whether an operation was intended or not.
Although COPD increases the risk of postoperative complications, it does not typically make these risks prohibitive. Even patients with severe COPD can tolerate abdominal surgery when properly prepared (68). When patients with COPD are identified before elective operations, every effort should be made to optimize their lung function and minimize any other perioperative risks.
Studies have not shown asthmatic patients to have significant risks of serious postoperative complications when managed appropriately (69). Obesity considered in isolation is not associated with increased pulmonary risks. However, many of the comorbidities often seen with obesity, such as obstructive sleep apnea, are known to increase perioperative risk (70). Sleep apnea patients are often prescribed positive pressure airway masks to assist their breathing at home during sleep. This equipment should be available in the postoperative period to assist with any apneic breathing episodes. Obese patients (and other patients with unusual upper airway anatomy) may present difficulties for intubation, and fiber optic instruments may be needed in the operating room. Pulmonary hypertension, whether associated with sleep apnea or not, is associated in some studies with increased postoperative risks in noncardiac surgery (71).
Pulmonary Risk Reduction
Physicians should attempt to reduce perioperative risks whenever possible (Table 18.6). Some identified risks (such as location of surgery, type of anesthesia, age, poor general health status, and fixed airways obstruction) cannot be improved. Smoking cessation should be encouraged. Several studies have shown increased reduction in postoperative complications (both pulmonary and nonpulmonary) with each week of smoking cessation prior to surgery (72). Patients with asthma should be optimized to their baseline pulmonary status before surgery. National guidelines recommend bringing asthmatics under “good control” prior to any elective surgery (73). This may involve bronchodilator use, inhaled steroids, and/or oral steroids.
COPD management typically involves inhaled a-agonists and anticholinergics. Noninvasive ventilation has been used successfully in postoperative patients with COPD (74). Both COPD and asthmatic patients should continue their home medications during their postoperative course. Exacerbations of either during the postoperative period can be treated with additional doses of inhaled or nebulized bronchodilators and systemic corticosteroids (oral or intravenous) if needed. For acute COPD exacerbations, antibiotics are usually indicated and have been shown to improve outcomes in patients with a change in sputum volume or purulence, and in patients who require mechanical ventilation. There is strong evidence that noninvasive ventilation helps avoid mechanical ventilation and improves outcomes and mortality in patients with severe dyspnea and clinical signs of respiratory muscle fatigue or respiratory acidosis (75).
Because low lung volumes produced by anesthesia, operative site pain, and bowel distension all contribute to respiratory dysfunction in the postoperative period, clinicians have prescribed deep-breathing exercises, intermittent positive pressure breathing (IPPB), and simple incentive spirometry to attempt lung expansion in the postoperative period. Although the actual value of these techniques was never carefully studied, most clinicians believe that their benefits exceed any small risks (76). Adequate pain control is important to improve deep breathing and lung expansion after abdominal surgery. Systematic reviews emphasized more judicious use of nasogastric tubes in the postoperative period to reduce postoperative pneumonia and atelectasis (77). Guidelines suggest that these tubes be used selectively for nausea and vomiting, inability to tolerate oral intake, or for symptomatic abdominal distension.
Table 18.6 Measures to Reduce Pulmonary Complications

Diabetes Mellitus
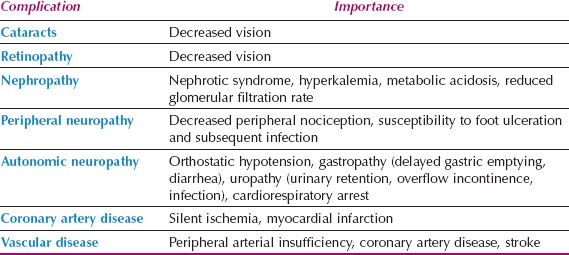
According the Centers for Disease Control and Prevention, in 2010 diabetes mellitus affected approximately 25.6 million or 11.3% of all people 20 years of age and older. Type I diabetes is an autoimmune disease that attacks pancreatic beta cells. People with type I diabetes have a near-total lack of insulin as a result of pancreatic beta cell destruction and become ketoacidotic if insulin is withheld. Although common in juveniles, type I diabetes can occur in adults. People with type II diabetes are not insulin deficient in an absolute sense and are not generally prone to ketoacidosis. The problem in type II diabetes is usually one of relative insulin resistance. Insulin treatment is not limited to type I disease because many patients with type II do require some insulin therapy. Patients with type II diabetes are usually older and overweight. Both groups may experience the complications listed in Table 18.7. Many elderly patients have mild type II diabetes of recent onset related to obesity, are well controlled with diet or oral hypoglycemic drugs, and have few overt complications, but may have occult atherosclerotic vascular disease.
The management of diabetes begins with some understanding of the factors that influence perioperative glucose metabolism. Insulin is the principal glucose-lowering hormone; cortisol, glucagon, growth hormone, and catecholamines are the principal glucose-raising hormones. In the preoperative period, stress and the “dawn” phenomenon may elevate blood glucose. The dawn phenomenon is early morning hyperglycemia resulting from nocturnal surges of growth hormone. During surgery, cortisol, epinephrine, and growth hormone levels rise. In this period, there is hyperglycemia in diabetic and nondiabetic patients alike. This is caused by glycogenolysis, inhibition of glucose uptake, and decreased insulin release. After surgery, in nondiabetic patients, the hyperglycemia is brought under control by increased endogenous insulin release over a period of 4 to 6 hours. Patients with diabetes may need additional exogenous insulin.
Table 18.7 Complications of Diabetes Mellitus
In addition to these hormonal factors, several other factors are important in modulating the blood glucose level in the perioperative period. Inactivity, stress, and intravenous glucose infusions tend to raise blood glucose. Decreased caloric intake and semistarvation tend to lower blood glucose. Because the net effect of these factors is sometimes difficult to anticipate, it is important to monitor blood glucose levels frequently.
Oral Hypoglycemics
There are many more oral agents being used to treat diabetes than in the past (Table 18.8). Sulfonylureas such as glyburide (Diabeta) remain the most popular. Most sulfonylureas are excreted primarily by the kidney. These drugs are typically withheld 24 to 48 hours before surgery, depending on their half-life. They can be restarted when the patient starts eating.
The biguanide metformin (Glucophage) is used frequently. Metformin should be used with extreme caution (and is frequently held) in the perioperative period and probably should be avoided altogether in systemically ill gynecologic oncology patients. There is a serious risk of lactic acidosis if renal function declines as a result of chemotherapy, dehydration, congestive heart failure, sepsis, radiologic contrast agents, or third spacing. It should not be used in patients with liver disease.
Acarbose (Precose) is a complex oligosaccharide glucosidase inhibitor that delays the digestion of ingested carbohydrates. There is little use for this drug in the perioperative period. Repaglinide (Prandin) is a meglitinide that stimulates release of insulin from the pancreas. It is used to cover mealtime blood sugar and may have a limited role in blood sugar management during the perioperative period when patients are not eating. Its safe use depends on stable renal and hepatic function.
The thiazolidinedione rosiglitazone (Avandia) improves peripheral use of glucose by improving insulin sensitivity. This class of medication has a risk of fluid retention. It is not recommended for patients with NYHA class III or IV status. The use of thiazolidinediones around the perioperative period should be exercised with caution, especially in patients with cardiac disease or in those who have received more intravenous fluids during the perioperative period.
Exenatide (Byetta) is a glucagon-like peptide-1 receptor agonist that acts like an incretin mimetic agent. It enhances insulin secretion from pancreatic beta cells, suppresses glucagon secretion, and slows gastric emptying. This medication can be continued perioperatively when a patient is starting to take oral nutrition. Liraglutide (Victoza) is formulated as weekly injectable medication, which may not be ideal for inpatient blood sugar management.
The latest oral diabetic medication is dipeptidyl peptidase-4 (DPP-4) enzyme inhibitor, Sitagliptin (Januvia). DDP-4 breaks down incretin hormones. Sitagliptin, therefore, increases the level of incretin hormones (glucose-dependent insulinotropic polypeptide [GIP] and glucagon-like peptide-1 [GLP-1]) by inhibiting their breakdown. Incretins enhance glucose-dependent insulin secretion, glucose-dependent suppression of inappropriately high glucagon secretion, slowing of gastric emptying, reduction of food intake, and promotion of beta-cell activity. This medication is not useful during the perioperative period if patients are nil per os (NPO). Dose adjustment is needed for renal insufficiency patients.
Table 18.8 Characteristics of Oral Hypoglycemics

Insulin
Insulin is the mainstay of in-hospital management of diabetes because it is easily titrated during management. In spite of many oral and other subcutaneous diabetic therapies, insulin remains an important tool in the inpatient management of diabetes. There are various types of insulin available for treatment of diabetes (Table 18.9). The use of different insulin types depends on the goals of treatment. Long-acting insulins like glargine and detemir are used to cover basal blood sugar needs. Short-acting insulins like ultrashort-acting insulin or regular insulin are used to cover mealtime blood sugar or any elevated blood sugar not covered by the basal insulin.
Management
Hyperglycemia is known to impair neutrophil function, wound healing, and to increase the risk of wound infection (78–83). In addition, it impairs cardiac ischemic preconditioning (a protective mechanism for ischemic insult), enhances neuronal damage following ischemia, decreases nitric oxide, increases platelet activation, increases inflammatory markers, and increases reactive oxygen species, all of which have a significant impact on a patient’s morbidity and mortality (84). Several studies have shown that hyperglycemic patients have a higher mortality, increased risk of infection, poorer functional recovery, more postoperative complications, and longer length of stay (85–92). Previous studies have shown that tight glycemic control improved mortality, decreased risk of infection, and decreased length of ICU stay (93–101). One study of critically ill surgical ICU patients showed that tight glycemic control with blood glucose at or around 110 mg/dL reduced blood stream infections, acute renal failure (ARF) requiring dialysis, blood transfusion, length of mechanical ventilation and critical care, and in-hospital mortality (94). Mortality at 12 months was reduced in the intensive insulin therapy patient (94). Subsequent studies of tight glycemic control in critically ill patients have failed to show improved mortality. Some of the studies showed increased mortality in the tighter glycemic control group and increased risk of severe hypoglycemia (95,96). In a meta-analysis of 26 trials, the pooled relative risk of death was 0.93 (95% CI 0.83 to 1.04) comparing intensive therapy to conventional therapy, and half reported hypoglycemia with a relative risk of 6 (95% CI 4.5 to 8). Surgical ICU patients appear to benefit from intensive insulin therapy with relative risk of 0.63 (9% CI 0.44 to 0.91) (97).
Table 18.9 Characteristics of Insulin

In light of these recent studies, the American Association of Clinical Endocrinologists and American Diabetic Association recommend controlling preprandial blood sugar to <140 mg/dL and random blood sugar <180 mg/dL in critical care patients. Some surgical ICU patients can have tighter blood sugar control, with preprandial blood sugar between 80 and 110 mg/dL. For noncritical care patients, the goal of preprandial blood sugar should be <140 mg/dL and random blood sugar should be <180 mg/dL (Table 18.10).
Details of the management of the diabetic patient who is taking an oral hypoglycemic agent are presented in Table 18.11. A patient with well-controlled diabetes who takes sulfonylureas is at risk of hypoglycemia if sulfonylureas are given while the caloric intake is reduced. Sulfonylureas should be held on the morning of surgery or longer if the medication has a long duration of action. Dextrose infusion should be given to prevent any hypoglycemia. Glucose monitoring should be performed at regular intervals to ensure that the blood sugar falls within an acceptable range. Any hyperglycemia can be treated with supplemental insulin. When oral nutrition is reinstated, sulfonylureas can be resumed. Thiazolidinediones can be continued during the perioperative period, but should not be used if the patient received excessive amounts of fluids or developed NYHA Class III or IV heart failure. Glucosidase inhibitors, meglitinides, glucagon-like peptide-1, and DDP-4 inhibitors can be resumed only when the patient is on oral nutrition. Metformin should not be used during the perioperative period for the reasons mentioned above.
Table 18.10 Target Goals of Inpatient Blood Glucose Control

Alternatively, patients can be started on a basal-bolus insulin regimen perioperatively instead of resuming oral hypoglycemic medications. A patient’s oral nutritional intake is often unpredictable because of postoperative nausea and vomiting. Postoperative hyperglycemia is common due to stress hormone release. Total insulin need is between 0.3 and 0.6 unit/kg/d depending on the patient’s insulin-resistance status (102,103). Half of that insulin dose should be given as basal insulin with glargine or detemir. The other half should be divided into four doses given every 6 hours or before each meal and at bedtime, using ultra short-acting or short-acting insulin. Any additional hyperglycemia should be covered with supplemental insulin with a corrective scale. Basal insulin dosage can be increased daily by 10–20% if blood sugar is not controlled, or it can be decreased daily by 10–20% if the patient has episodes of hypoglycemia.
Patients on insulin treatment should not be on sliding scale insulin only, as that strategy produces fluctuating high and low blood sugar levels, and the blood sugar may be still inadequately controlled (102,104–106). In a randomized controlled trial comparing sliding-scale insulin to a basal-bolus insulin regimen, the latter resulted in significantly improved glycemic control, without significant risk of hypoglycemia (102).
The management of the diabetic patient who routinely takes insulin at home is presented in Table 18.12. Because caloric intake is reduced on the day of surgery, total daily insulin dose should be reduced. Usually, half the dose of basal insulin is given the night before or the morning of surgery to cover endogenous glucose production. Patients should be started on a dextrose infusion to prevent hypoglycemia. Glucose monitoring should be performed to make sure that the patient’s blood sugar is within an acceptable range. Postoperatively, patients are likely to have increased stress hormone levels and are on a dextrose infusion; thus, hyperglycemia is often seen despite the patient being on nil orally. Frequent blood sugar monitoring is needed for management of these hyperglycemic episodes with corrective dose insulin. After patients recover from the surgery and start to take oral nutrition, they can be resumed on their home insulin regimen. Caution should be exercised with any changes in the patient’s nutritional status.
Table 18.11 Details of Perioperative Diabetes Management for Well-Controlled Patients Taking Oral Hypoglycemics

Table 18.12 Details of Perioperative Insulin Management for Well-Controlled Patients Taking Insulin

In critically ill patients or those with uncontrolled diabetes, continuous insulin infusion is a better strategy for glycemic control (93–101). Continuous insulin infusion with glucose infusion maintains normal insulin sensitivity during the perioperative period and decreases blood cortisol, glucagons, fat oxidation, and free fatty acids when compared with controls (107). An insulin infusion can be started at 1 unit/hr and the rate titrated by 0.5 unit/hr increments to keep blood glucose levels below 140 mg/dL. Five percent dextrose infusion with or without potassium at a rate of 50 to 100 mL/hr should be given to avoid any hypoglycemia.
Despite studies that have not shown improved mortality with tighter blood sugar control in critically ill patients, keeping the blood glucose levels below 180 mg/dL is believed to decrease infection risk and improve morbidity. Postoperative diabetic management can be difficult and many hospitals have endocrinology consultants available to assist surgical teams in managing postoperative diabetic patients with hyperglycemia.
Thyroid Disorders
Hypothyroidism
Hypothyroidism is common and may go undetected in patients being prepared for surgery (108). Symptoms include cold intolerance, recent or progressive constipation, hoarseness, fatigability, and changes in cognition. Signs include associated goiter, skin dryness, and a delayed relaxation phase of peripheral reflexes (best demonstrated in the Achilles tendon). Studies have suggested that unrecognized mild to moderate hypothyroidism is clinically important, but fears of hyponatremia, prolonged respirator dependency, hypothermia, delayed recovery from anesthesia, or death are probably unwarranted (109,110). One retrospective study suggested that such patients have more intraoperative hypotension, postoperative ileus and confusion, and that infection is less often accompanied by fever (109).
For patients who are suspected before surgery of being hypothyroid, thyroid hormone levels should be measured. Hypothyroid patients should be treated with replacement hormone and rendered euthyroid before surgery. In urgent situations, patients who are not myxedematous should be given 1 or 2 days of oral replacement before surgery, with careful postoperative follow-up (111–113).
Hyperthyroidism
Hyperthyroidism can be a dramatic illness, with tachycardia, fever, and exophthalmos associated with goiter. Other common symptoms and signs include weight loss, fatigue, diarrhea, heat intolerance, tremor, hyperreflexia, and muscle weakness. Hyperthyroidism may be occult in older patients. Unexplained tachycardia, weight loss, arrhythmias, or fever may be the only clinical indicators and should always raise suspicions of unrecognized hyperthyroidism in surgical patients. With proper preparation, hyperthyroid patients undergoing thyroid surgery do well (114). There are scant data concerning the problems of the hyperthyroid patient undergoing nonthyroidal surgery, such as radical hysterectomy. Exacerbation of the illness into a “thyroid storm” is the usual concern. Because of this, when any patient is suspected before surgery of being hyperthyroid, thyroid hormone levels should be measured. If the diagnosis is confirmed, elective surgery should be delayed until treatment has produced a euthyroid state (113,115). In the postoperative period, thyroid hormone levels should be measured when any patient has persistent unexplained tachycardia, fever, or tachyarrhythmias.
Corticosteroids
Patients taking corticosteroids or those who took them in the recent past should be evaluated for the need of supplemental corticosteroid coverage. Patients taking less than the equivalent of 5-mg prednisone daily should not have adrenal suppression (116–118). There is variability between patients in their response to suppression of the hypothalamic–pituitary–adrenal (HPA) axis by exogenous steroid. In a prospective cohort study, 75 patients were given short-term, high-dose glucocorticoid treatment of at least 25 mg prednisone daily for 5 to 30 days (119). Forty-five percent of the patients experienced HPA suppression. Of those patients, the majority recovered within 14 days. However, a couple of patients remained suppressed at 3 and 6 months.
In a retrospective study, 279 patients were taking prednisone or its equivalent steroid at doses of 5 to 30 mg/d for between 1 week and 15 years (120). Human corticotropin-releasing hormone (CRH) was used to assess HPA suppression. There was a trend toward an inverse correlation between dosage and duration of therapy and the plasma cortisol response to CRH. There were numerous patients taking high-dose steroids for more than 100 weeks who still had an intact HPA axis. Despite this variability, suppression of the HPA axis should be anticipated in patients taking more than 25 mg of hydrocortisone, 5 mg of prednisone, or 0.75 mg dexamethasone per day for more than 3 weeks (121).
To further clarify whether patients on chronic steroids have suppressed HPA axis, a cortrosyn (ACTH) stimulation test can be performed. Baseline cortisol and ACTH levels should be obtained, then 250 μg of ACTH is given intramuscularly or intravenously. A cortisol level is obtained 30 minutes after ACTH is given. If the stimulated cortisol level does not rise above 18 μg/mL, the patient is suspected of having a suppressed HPA axis and stress dose steroid should be given perioperatively (119,122–124). In addition to an abnormal ACTH stimulation test, patients who have findings of Cushing syndrome or findings of adrenocortical insufficiency such as hypotension, hyponatremia, or hyperkalemia should be considered for stress dose steroids perioperatively.
Corticosteroid supplementation for patients suspected of adrenal suppression will depend on the type of surgery performed. Patients having minor surgeries like hernia repair or colonoscopy should be able to take their usual dose of oral steroid on the day of surgery without additional supplementation. Patients undergoing moderate surgical stress, such as hysterectomy, should take their usual steroid dose on the morning of the surgery and be supplemented with 50 mg intravenous hydrocortisone on call to surgery, followed by 25 mg intravenously every 8 hours for three doses. They should resume their usual oral steroids the following morning. Patients undergoing major surgery, such as primary cytoreduction for advanced ovarian cancer, should take their usual steroid dose on the morning of surgery and be supplemented with 100 mg intravenous hydrocortisone on call to surgery, followed by 50 mg intravenously every 8 hours, tapering the dose by half each day over the next 24 to 48 hours. The patient can resume oral steroids in the morning after tapered off intravenous stress dose hydrocortisone (125,126). For those patients who continue to not be able to take oral medications, equivalent dose of intravenous hydrocortisone should be given in the mornings.
Thromboembolic Disease Prevention
Almost all hospitalized patients are at risk for venous thromboembolism (VTE) and should receive some type of prophylaxis to reduce these adverse events. Surgical patients, in particular, are at increased risk for deep venous thrombosis (DVT) and associated pulmonary embolism (PE) related to immobility and the operative stimulation of the coagulation cascade. One older analysis of historical data suggested that nearly a third of hospitalized surgical patients might develop VTE, and perhaps 1% may develop fatal PE if no prophylaxis was given (127). More recent data collected by the American Heart Association suggest that up to two million Americans will develop a DVT each year, with almost one-third of them also developing a PE, resulting in 60,000 deaths annually (128).
Although young, ambulatory patients without additional risks who undergo short (<30 minutes) surgeries may not need specific interventions other than early mobilization, almost all other postoperative patients should receive some type of thromboprophylaxis. Gynecologic surgical patients known to be at particularly high risk include those with malignant disease; those undergoing open abdominal (vs. vaginal or laparoscopic) surgery; elderly patients; and those who have had previous venous thrombotic events. A more complete list of risk factors for venous thromboembolic disease in hospitalized patients is shown in Table 18.13.
Preventive therapy for venous thrombosis in the setting of gynecologic surgery includes graded compression stockings and mechanical compression devices placed on the lower extremities, and various subcutaneous anticoagulation regimens with unfractionated heparin, low molecular weight heparins, and newer agents such as fondaparinux. Higher doses of the latter agents may have associated risks for postoperative bleeding.
There are conflicting reports on the relative protective benefits of each of these treatments. At least one randomized trial showed that proper use of compression stockings may be as effective as subcutaneous heparin in major surgeries for gynecologic malignancies (129). Other trials have suggested that higher doses of subcutaneous unfractionated heparin (three times a day) or low molecular weight heparin may be more protective than lower doses of unfractionated heparin, especially in cases of malignancy (130). Some surgeons have advocated the use of pneumatic compression devices and subcutaneous anticoagulants in their highest risk patients, although there are no clear data that these treatments are additive in effectiveness in gynecology patients.
Table 18.13 Factors Related to Increased Risk of Thromboembolic Disease

Thromboprophylaxis in hospitalized patients is typically continued at least until hospital discharge. One study of patients with gynecologic malignancies undergoing open abdominal surgery has suggested that 4 weeks of postoperative prophylaxis is cost-effective, and improves patient outcomes (131). Additional studies are needed to determine optimal length of VTE prophylaxis in high-risk populations.
Table 18.14 Recommendations for Venous Thromboembolic Prophylaxis in Gynecologic Surgery: Ninth (2012) American College of Chest Physicians Guidelines for Antithrombotic Therapy for Prevention and Treatment of Thrombosis
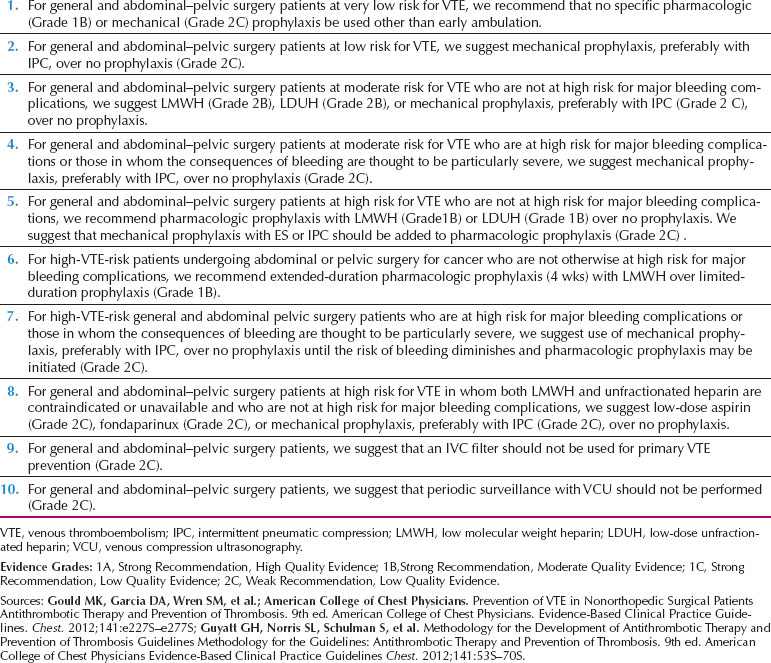
A recent consensus statement summarizes recommendations for prevention of VTE in postoperative gynecologic patients (Table 18.14).
Preoperative Testing
The question of how much preoperative laboratory testing is warranted is the subject of considerable interest and debate (132,133). Two randomized trials on patients undergoing cataract surgery, and one randomized trial on ambulatory surgical patients that included orthopedic, general, plastic, ophthalmologic, urologic, spinal, and neurosurgical patients showed no difference in adverse events between patients with and without preoperative laboratory testing (134–136). A recent review of the effectiveness of preoperative testing in noncardiac elective surgical patients has revealed a scarcity of evidence supporting preoperative testing, but has suggested that testing based on pathologic findings in a patient’s medical history and physical examination would be prudent (137). Many previous studies have demonstrated that unless clinical indicators are present, preoperative test results will usually be normal, falsely positive, or truly positive with no significant clinical outcome on perioperative complications (136,138–142).
Table 18.15 Surgical Grade

The National Institute for Clinical Excellence of the United Kingdom developed guidelines in 2003 in an attempt to give some directions for clinicians on preoperative testing for elective surgery. The guidelines incorporated as much evidence as possible, but the evidence base is often lacking, so recommendations are frequently based on experts’ opinions and consensus (143). These guidelines categorize patients by age; surgical grade (minor, intermediate, major, major+); anesthetic grade as per American Society of Anesthesiologists (ASA); and comorbidity (cardiac, respiratory, and renal) (Tables 18.15 and 18.16). Preoperative test recommendations cover chest x-ray, complete blood count, electrocardiogram (ECG), coagulation studies, blood chemistry, renal function tests, blood glucose, urine analysis, blood gases, and lung function tests. The complete guidelines are available online (143).
Patients who are under 40 years, healthy, and undergoing minor surgery generally do not need any preoperative testing. Gynecologic oncology patients are at least Surgical Grade 3—major surgery. Such patients often have clinical indicators that support additional testing, particularly when they are 60 years or older, have higher ASA Class, and/or multiple comorbidities. A preoperative ECG should be obtained on all women over 60 years, or younger if the patient has cardiac disease. A complete blood count and chemistries are recommended. Glucose level is not recommended by the guidelines in many patients, but is usually warranted to exclude diabetes because of the increased morbidity and mortality associated with inpatient hyperglycemia. Chest x-ray is generally not recommended, but can be considered if the patient has respiratory symptoms or an abnormal chest examination. Urinalysis should be considered in all gynecologic patients. Coagulation studies are not recommended in the majority of the patients, except for those patients with hepatic comorbidity or cardiac comorbidity in ASA Class 3 status. For those patients with metastatic liver cancer or who are significantly malnourished, coagulation studies prior to surgery are reasonable. Blood gases can be considered in patients with multiple comorbidities. Pulmonary function tests are not recommended for gynecologic patients even if they have respiratory comorbidity (Tables 18.17–18.19).
The guidelines on preoperative testing are a general roadmap for the clinician. Combining the patient’s medical conditions and symptoms with the guidelines will provide a more focused approach to preoperative testing.
Table 18.16 American Society of Anesthesiologists (ASA) Class

Table 18.17 Grade 3 Surgery (Major) with Cardiovascular Disease

Screening for Hemostatic Defects
A good history and physical examination are most helpful in screening patients for hemostatic defects before operations. Some of the most important information involves the outcome of prior hemostatic stress and the family history. Minor surgical procedures should not have required transfusion, and a history of postoperative bleeding 2 or 3 days after surgery is also suspicious. Many patients have had tooth extractions. Bleeding should not last more than 24 hours and should not start again after stopping. A familial history of bleeding or suspected bleeding should be investigated. Patients should be questioned about nosebleeds, intestinal bleeding, and heavy menstrual bleeding. Large ecchymosis and mucosal bleeding on examination can be a cause for concern.
Studies suggest that screening for hemostatic defects has no benefit in patients without a history of oral anticoagulation use or significant liver disease that impairs the liver’s ability to synthesize coagulation factors, even in neurological patients (144–146). Despite the lack of evidence, coagulation screening tests are still frequently ordered preoperatively. A platelet count, INR (international normalized ratio), and PTT (partial thromboplastin time) are the most commonly ordered tests for this purpose. A low platelet count can be caused by decreased production, sequestration into the spleen, or increased destruction. For platelet counts less than 100,000/cc, platelet transfusions may be necessary before the operation, depending on additional risks. Certain commonly prescribed drugs (such as aspirin and nonsteroidal anti-inflammatory drugs [NSAIDs]) can inhibit platelet function and should be held for a week before the operation if possible. As stated previously, low-dose aspirin therapy should be continued in many patients with cardiovascular disease, and communication between the primary care physician and surgeon is essential in weighing the risks of adverse cardiac events and bleeding. Renal dysfunction is another common cause of acquired platelet dysfunction.
Table 18.18 Grade 3 Surgery (Major) with Respiratory Disease

Elevated INR and PTT values reflect blood coagulation protein deficiencies or inhibitors. Patients with elevated values may require plasma factor replacement before surgery to minimize their bleeding risks, and further hematologic testing is warranted to identify the specific deficiency or inhibitor.
There are some patients with normal screening laboratory tests who have suggestive histories or examinations for hemostatic defects. One possible culprit might be Von Willebrand disease—an inheritable coagulation defect in platelet function and the most common hereditary bleeding disorder. Identification and perioperative management of this and other more uncommon bleeding disorders may require further laboratory testing and the expertise of a hematologist (147).
Perioperative Antibiotics for Wound Infection Prophylaxis
A dose of intravenous antibiotics such as vancomycin, cefazolin, or cefotetan should be given within 1 hour of incision to decrease the risk of surgical site infections (148). Additional doses can be given at intervals of one or two times the half-life of the drug if there is extended surgery. If bowel resection is anticipated, mechanical bowel preparation on the day before surgery has been the traditional practice. More recent studies have shown no difference in infection or postoperative complication rates among patients with or without a mechanical bowel preparation (149,150), and a recent clinical practice guideline has recommended omission of mechanical bowel preparation for patients undergoing elective colorectal surgery (151).
Table 18.19 Grade 3 Surgery (Major) with Renal Disease

Postoperative Management
Cardiovascular
Hypertension
High blood pressure, both labile and persistent, is a common problem for acutely ill patients. Perioperative hypertensive episodes occur commonly in hypertensive patients and occasionally in normotensive patients because of pain, anxiety, stress, medications, and other factors (Table 18.5). Perioperative hypertension is most common during laryngoscopy and induction (primarily because of sympathetic stimulation) and immediately after surgery, often in the recovery room.
Patients with pre-existing hypertension usually require some continuation of their daily antihypertensive medication when they are brought into the hospital or are critically ill, but the dosages may need titration and diuretic use for blood pressure control is infrequent. Many agents can be converted to an intravenous form or administered with minimal fluid down a gastric tube if the patient is not eating or drinking. The use of β-blockers as antihypertensives in the acute care setting may have additional benefits by decreasing the risks of atrial fibrillation and myocardial ischemia in vulnerable patients, and these agents are often selected as first-line agents for this reason. Some studies suggested that routine use of these agents might decrease cardiovascular mortality after noncardiac surgery in patients at risk (152). At the minimum, care should be taken that β-blockers are not discontinued suddenly, as this can cause rebound hypertension and associated problems. Sublingual, short-acting calcium channel blockers (e.g., nifedipine) should be avoided because their use can lead to reflex tachycardia and myocardial ischemia.
Corticosteroid medications can cause hypertension in susceptible patients. Mild antihypertensive medication may be necessary until the steroid dose is lowered or discontinued.
Many hypertensive episodes resolve spontaneously. Patients with pain and anxiety are best treated with appropriate analgesics and anxiolytics. When evaluating the hypertensive postoperative patient, adequacy of ventilation and stable cardiac status should be verified by examination, arterial blood gases, and ECG. Bladder distension may cause elevated blood pressure and should be relieved. A patient may require a continuous intravenous infusion to control severe hypertension. Intravenous drugs with short half-lives should be chosen to allow safe titration (the vasodilator nitroprusside or short-acting β-blockers are two popular choices) and the patient should be changed to longer-acting agents as their condition stabilizes (153).
Myocardial Injury and Ischemia
Patients undergoing oncologic treatment may have underlying coronary artery disease (CAD). The variable stresses in the postoperative period, such as inflammation, increased hypercoagulable state, and hypoxemia, can lead to myocardial injury or ischemia. One study of unselected patients aged over 50 years undergoing noncardiac surgery showed that the risk of postoperative cardiac events was nearly 1.5% (5). Most postoperative MIs occur during the first 3 days, at a time when patients may be receiving narcotics that may mask the symptoms of ischemia.
Patients experiencing postoperative MI have an increased risk of hospital mortality. These patients need to be managed promptly by a surgeon and a consulting cardiologist, and observed closely in a monitored bed for any complicating features such as arrhythmia, pulmonary edema, and shock. In addition to correcting anemia, hypoxia, and starting β-blockers in those that can tolerate them, the treatment of such acute coronary syndromes involves the use of anticoagulants such as aspirin and heparin. The use of these agents must be balanced against the risk of postoperative bleeding. MIs are typically divided into ST segment or non-ST segment elevation injuries, depending on the ECG appearance. MI patients with ST elevation or with hemodynamic instability have improved outcomes if they can receive rapid angiography and angioplasty, while those in a stable condition with non-ST segment elevations can often be managed medically (at least initially). Unless contraindicated, all patients with postoperative MI should be on aspirin, β-blockers, HMG co A reductase inhibitors, and ACE inhibitors by the time of discharge (154).
Arrhythmia
Every physician working in an acute care hospital should be familiar with the use of a defibrillator and the algorithms developed by the American Heart Association for Advanced Life Support (155). Fluid shifts, electrolyte changes, and myocardial ischemia can put the patient receiving treatment for gynecologic malignancy at increased risk for heart rhythm abnormalities.
The tachyarrhythmias, both ventricular and supraventricular, can be quite dangerous and should be electrically cardioverted immediately if the blood pressure is low, or the patient is unstable. In less urgent situations, a variety of antiarrhythmic medications are available for chemical conversion and stabilization. Many of these medications are proarrhythmic as well, and a search for an underlying cause of the rhythm disturbance is indicated to reduce the propensity for recurrence. Often, when the electrolyte imbalance or other precipitant has been corrected, the heart rhythm normalizes, and these agents can be discontinued. Persistent or unstable ventricular arrhythmias are often managed in the acute setting with intravenous amiodarone. Supraventricular tachycardias may respond to vagal maneuvers or a rapid bolus of adenosine.
Atrial fibrillation is the most common postoperative tachyarrhythmia and deserves special mention. After the blood pressure is stabilized in a patient with atrial fibrillation, attempts should be made to control the heart rate. Popular drugs for rate control include β-blockers (if left ventricular function is preserved), diltiazem, or digoxin. If the rhythm persists after other precipitants are corrected (e.g., hypokalemia, hypoxemia, fluid overload), many clinicians would attempt chemical or electrical conversion if the atrial fibrillation has not been present more than 48 hours. Restoration of normal sinus rhythm often improves cardiac output (CO), and mitigates the risk of stroke from left atrial thrombus formation in the fibrillating chamber. Patients with atrial fibrillation lasting longer than a few days and who have no contraindication should be considered for anticoagulation, to decrease their risk of a stroke (156).
Bradyarrhythmias often arise from excessive vagal stimulation. Nausea, bladder distension, pain, and endotracheal tube manipulation can all stimulate excessive vagal tone. As with tachyarrhythmias, attention to blood pressure is paramount. Those patients who develop hypotension should receive atropine and catecholamines. Patients not responding to these agents may need urgent transvenous pacemaker placement. Transcutaneous pacing can be attempted if available at the bedside.
Shock
Shock is defined as a clinical syndrome in which the patient shows signs of decreased perfusion of vital organs. Typical findings include alterations in mental status, cold and clammy skin, oliguria, and metabolic acidosis. Patients with shock have a substantial decrease in blood pressure, but no absolute value is used to define shock.
The therapeutic approach to these patients is facilitated by a functional classification of shock states. Each class of shock has its own pathophysiologic process, and requires a different management strategy. Traditionally, four varieties of shock have been described:
1. Hypovolemic shock—secondary to fluid losses and decreased cardiac filling pressures (e.g., postoperative bleeding or intravascular fluid redistribution).
2. Distributive shock—secondary to inappropriate “vasodilation” and venous pooling (e.g., sepsis syndrome, anaphylaxis, decreased vasomotor tone from spinal anesthesia, and adrenal insufficiency from steroid withdrawal).
3. Cardiogenic shock—secondary to decreased myocardial contractility and function (e.g., acute MI or ischemia, or congestive cardiac failure).
4. Obstructive shock—secondary to mechanical obstructions in the cardiovascular circuit (e.g., PE, cardiac tamponade).
Common causes of shock in the perioperative management of gynecologic malignancy include the following:
1. Hemorrhage (hypovolemic)
2. Sepsis (distributive)
3. Postoperative MI (cardiogenic)
4. Pulmonary embolus (obstructive)
A careful physical examination, review of laboratory and test results, and consideration of the clinical situation often suggests the etiology of a particular shock syndrome. Therapy should begin promptly, as information is being gathered. In the past, invasive hemodynamic monitoring with pulmonary artery catheters was often used to diagnose and manage these conditions. This has declined significantly as studies have shown no clear benefit to their use (157).
Regardless of whether measured directly with a pulmonary catheter, or deduced from less invasive data, each of the shock states has an expected hemodynamic profile. Therapy is targeted to the underlying defect in cardiovascular performance:
1. Hypovolemic shock is treated with crystalloid or colloid infusion to increase cardiac filling and CO and perfusion to vital organs. There is no clear evidence that colloids have any benefits over crystalloids for this purpose (158). Hypovolemic shock is the most common form of shock in the surgical patient, and treatment of the hypotensive, oliguric patient typically begins with a “fluid trial.”
2. Distributive shock often requires vasopressor management with catecholamines active at the alpha receptors on the vasculature. This helps restore adequate resistance to create a perfusing blood pressure. Equally important is to commence treatment of the presumed cause of the vasodilation: Prompt antibiotics and source control for cases of suspected sepsis, steroids if secondary adrenal insufficiency is a possibility, or withdrawal of the offending agent and anti-inflammatory treatment if an allergic reaction is suspected. There are published guidelines for the treatment of septic shock (159).
3. Cardiogenic shock is characterized by inadequate CO, such that inotropic management with catecholamine and dopaminergic compounds is often necessary to maintain adequate contractility. Vasodilator therapy can be helpful in cardiogenic shock because it unburdens the failing heart’s afterload. This allows contractility to improve without excessive cardiac work, which might exacerbate myocardial ischemia.
4. Obstructive shock can be difficult to manage and might require a combination of measures to maintain adequate filling pressures and contractility. Like distributive shock, it is important to attempt reversal of the precipitant quickly (e.g., anticoagulation for PE, pericardiocentesis for tamponade) because the patient’s ultimate outcome depends on this.

Figure 18.3 Algorithm for the management of hypotension. UOP, urine output; PE, pulmonary embolism.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree