Summary of Key Points
- •
Staging of the mediastinum is a key component in the evaluation of patients with lung cancer and includes both preoperative and intraoperative components.
- •
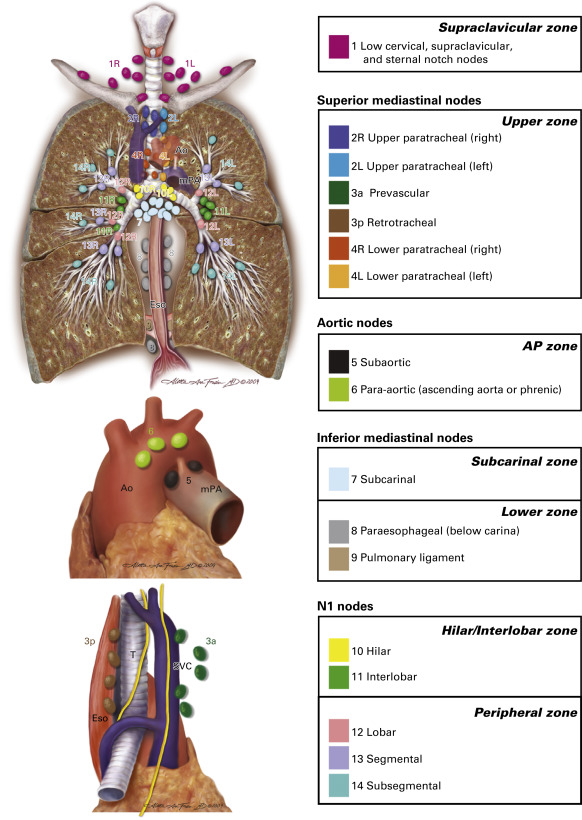
The International Association for the Study of Lung Cancer lymph node map provides standard definitions of each nodal station and allows precise, uniform nomenclature when staging mediastinal and pulmonary lymph nodes.
- •
The importance of lymph node assessment in the staging of nonsmall cell lung cancer is well recognized, but despite this, inadequate lymph node assessment is too common.
- •
Variation in the extent of mediastinal lymph node staging has been a source of confusion in the literature.
- •
Systematic sampling may be performed by endobronchial ultrasound–transbronchial needle aspiration (EBUS–TBNA) or endoscopic ultrasound (EUS) or mediastinoscopy prior to a planned resection or at the time of the planned resection.
- •
Minimally invasive needle techniques including EBUS–TBNA and EUS–fine-needle aspiration are now considered the tests of first choice to confirm mediastinal disease in accessible lymph node stations.
Staging of the mediastinum is a key component in the evaluation of patients with lung cancer and includes both preoperative and intraoperative components. The purpose of mediastinal staging is to distinguish those patients who may benefit from surgery from those who should have other forms of treatment. The preoperative assessment of the mediastinum includes computed tomography (CT) and positron emission tomography (PET) scans and has been addressed in Chapter 21, Chapter 22 . Invasive or operative mediastinal staging includes the techniques of mediastinoscopy, mediastinotomy, thoracoscopic staging, staging at the time of planned resection, and needle biopsy techniques such as endobronchial ultrasound (EBUS) or endoscopic ultrasound (EUS). Invasive mediastinal staging provides histologic or cytologic confirmation of the status of the mediastinal lymph nodes. It is important to differentiate mediastinal node assessment for the purpose of staging versus a possible therapeutic benefit. Whether mediastinal lymph node dissection (MLND) improves survival is controversial.
Patients who have proven metastases in mediastinal lymph nodes have a poor prognosis because of increased risk of systemic disease. Identification of N2 disease prior to resection is preferable to avoid a noncurative resection as surgery alone is inadequate, whereas patients without mediastinal node involvement are candidates for surgery.
Pathologic confirmation of nodal status by the techniques described in this chapter is important because the diagnostic accuracy of imaging tests such as CT and PET is insufficient for clinical decision making. The positive predictive value of CT ranges from 0.18 to 0.88. On average, the likelihood of an enlarged node on CT being positive for metastatic disease is only 60%. Similarly, nodes that are positive on PET are pathologically positive for metastatic disease only 75% to 85% of the time. This means that 15% to 40% of the time patients may be denied curative intent therapy because of imaging tests. Abnormal imaging should be confirmed or refuted pathologically.
This chapter will address the relevant anatomy with respect to the International Association for the Study of Lung Cancer (IASLC) lymph node map ( Fig. 24.1 ), the definitions used in discussing staging of the mediastinum, the indications for invasive staging, the techniques available for invasive staging, and the appropriate use of these techniques.
Lymph Node Anatomy of the Mediastinum and the Iaslc Lymph Node Map
The IASLC lymph node map was published in 2009 (see Fig. 24.1 ). The map illustrates the key lymph node anatomy of the mediastinum and lung. The goal of the IASLC Staging Committee in creating this new map was to reconcile the differences between the Japanese and Mountain–Dresler lymph node maps and to provide specific anatomic definitions of the lymph node stations. The key changes include the description of level 1 nodes as supraclavicular and suprasternal nodes, the division between right- and left-sided nodes defined as the left lateral border of the trachea, definition of the subcarinal nodes as level 7 (not level 7 and 10 as on the Japanese map), and the clear division of lymph node levels based on clearly identified anatomic landmarks ( Table 24.1 ). This map provides standard definitions of each nodal station and allows precise, uniform nomenclature when staging mediastinal and pulmonary lymph nodes. Mediastinal nodes (N2 and N3) are numbered 1–9. Hilar and intrapulmonary (N1) nodes are numbered 10–14.
| Lymph Node Station | Anatomical Limits |
|---|---|
| Supraclavicular Zone | |
| 1: Low cervical, supraclavicular, and sternal notch nodes | Upper border: lower margin of cricoid cartilage. Lower border: clavicles bilaterally and, in the midline, the upper border of the manubrium. 1R designates right-sided nodes, 1L left-sided nodes in this region. For lymph node station 1, the midline of the trachea serves as the border between 1R and 1L. |
| Upper Zone | |
| 2: Upper paratracheal nodes | 2R: Upper border: apex of the right lung and pleural space, and, in the midline, the upper border of the manubrium. Lower border: intersection of the caudal margin of innominate vein with the trachea. As for lymph node station 4R, 2R includes nodes extending to the left lateral border of the trachea. 2L: Upper border: apex of the lung and pleural space, and, in the midline, the upper border of the manubrium. Lower border: superior border of the aortic arch. |
| 3 Prevascular and retrotracheal nodes | 3a: Prevascular. On the right: Upper border: apex of chest. Lower border: level of carina. Anterior border: posterior aspect of sternum. Posterior border: anterior border of superior vena cava. On the left: Upper border: apex of chest. Lower border: level of carina. Anterior border: posterior aspect of sternum. Posterior border: left carotid artery. 3p: Retrotracheal. Upper border: apex of chest. Lower border: carina. |
| 4: Lower paratracheal nodes | 4R: includes right paratracheal nodes, and pretracheal nodes extending to the left lateral border of the trachea. Upper border: intersection of the caudal margin of innominate vein with the trachea. Lower border: lower border of the azygos vein. 4L: includes nodes to the left of the left lateral border of the trachea, medial to the ligamentum arteriosum. Upper border: upper margin of the aortic arch. Lower border: upper rim of the left main pulmonary artery. |
| Aortopulmonary Zone | |
| 5: Subaortic (aortopulmonary window) | Subaortic lymph nodes lateral to the ligamentum arteriosum. Upper border: the lower border of the aortic arch. Lower border: upper rim of the left main pulmonary artery. |
| 6: Para-aortic nodes (ascending aorta or phrenic | Lymph nodes anterior and lateral to the ascending aorta and aortic arch. Upper border: a line tangential to the upper border of the aortic arch. Lower border: the lower border of the aortic arch. |
| Subcarinal Zone | |
| 7: Subcarinal nodes | Upper border: the carina of the trachea. Lower border: the upper border of the lower-lobe bronchus on the left; the lower border of the bronchus intermedius on the right. |
| Lower Zone | |
| 8: Paraesophageal nodes (below carina) | Nodes lying adjacent to the wall of the esophagus and to the right or the left of the midline, excluding subcarinal nodes. Upper border: the upper border of the lower-lobe bronchus on the left; the lower border of the bronchus intermedius on the right. Lower border: the diaphragm. |
| 9: Pulmonary ligament nodes | Nodes lying within the pulmonary ligament. Upper border: the inferior pulmonary vein. Lower border: the diaphragm. |
| Hilar/Interlobar Zone | |
| 10: Hilar nodes | Includes nodes immediately adjacent to the mainstem bronchus and hilar vessels including the proximal portions of the pulmonary veins and the main pulmonary artery. Upper border: the lower rim of the azygos vein in the right; upper rim of the pulmonary artery on the left. Lower border: interlobar region bilaterally. |
| 11: Interlobar nodes | Between the origin of the lobar bronchi. 11s: between the upper-lobe bronchus and bronchus intermedius on the right. a 11i: between the middle and lower bronchi on the right. a |
| Peripheral Zone | |
| 12: Lobar nodes | Adjacent to the lobar bronchi. |
| 13: Segmental nodes | Adjacent to the segmental bronchi. |
| 14: Subsegmental nodes | Adjacent to the subsegmental bronchi. |
Lymph Node Stations and Choice of Staging Technique
Standard mediastinoscopy or videomediastinoscopy can access lymph node stations 1, 2R/L, 4R/L, 7, and 10R. Stations 5 and 6 can be accessed by extended mediastinoscopy, parasternal mediastinotomy (Chamberlain procedure) or anterior mediastinoscopy, and video-assisted thoracic surgery (VATS). Prevascular nodes (3a) may be accessible by parasternal mediastinotomy or anterior mediastinoscopy and VATS. VATS can access ipsilateral nodal stations as well as hilar nodes and even interlobar nodes if the fissure is explored.
Needle techniques include EBUS–transbronchial needle aspiration (EBUS–TBNA) and EUS–needle aspiration (EUS–NA).
EBUS–TBNA can access all the nodes accessed by mediastinoscopy and additionally stations 11 and 12 bilaterally. EUS–NA can access stations 8 and 9 as well as the same stations as mediastinoscopy, but not the hilar nodes.
Techniques such as video-assisted mediastinal lymphadenectomy (VAMLA) and transcervical extended mediastinal lymphadenectomy (TEMLA) have been used for staging, but are more appropriately considered as techniques for mediastinal node dissection. These techniques may have therapeutic as well as staging applications.
Indications for Invasive Mediastinal Staging
The American College of Chest Physicians (ACCP) evidence-based clinical practice guidelines, the European Society of Thoracic Surgeons (ESTS) guidelines, and Cancer Care Ontario (CCO) Program in Evidence-Based Care Practice Guidelines are concordant in their recommendations for indications and techniques for invasive staging ( Table 24.2 ). Invasive staging by needle techniques is recommended if available, but surgical biopsies are recommended if the needle techniques are negative because of the low negative predictive value of needle biopsy techniques.
|
|
|
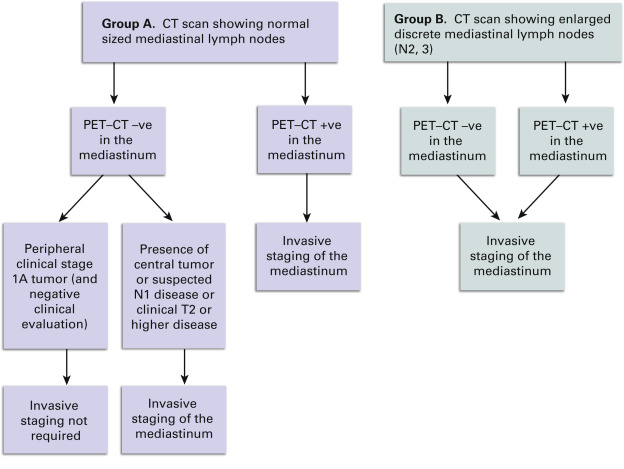
ACCP does not recommend invasive staging for patients with extensive mediastinal infiltration. However, invasive staging techniques may be used for diagnostic purposes. ACCP, ESTS, and CCO guidelines on preoperative mediastinal lymph node staging recommend that invasive mediastinal staging is not required for peripheral stage IA tumors with no suspicion of mediastinal lymph node involvement on CT and PET scans ( Fig. 24.2 ).
Definitions of Mediastinal Lymph Node Staging
Variation in the extent of mediastinal lymph node staging has been a source of confusion in the literature. The standard descriptions listed in Table 24.3 should be used when describing the extent of mediastinal lymph node assessment: sampling or random sampling, systematic sampling (SS), complete MLND, extended MLND, and lobe-specific systematic lymph node dissection (see Table 24.3 ). The term systematic nodal dissection refers to a combination of mediastinal as well as hilar and intrapulmonary node dissection.
| Sampling or random sampling : sampling of lymph nodes guided by preoperative or intraoperative findings; for example, sampling of single enlarged lymph node. |
| Systematic sampling: sampling of predetermined lymph nodes and lymph node stations; for example, sampling of stations 2R, 4R, 7, and 10R for right-sided tumors. |
| Mediastinal lymph node dissection: complete removal of all mediastinal lymph node bearing tissue based on anatomic landmarks. |
| Extended lymph node dissection: removal of bilateral paratracheal and cervical lymph nodes by formal dissection. |
| Lobe-specific systematic node dissection: removal of mediastinal lymph node bearing tissue based on the location of the tumor. |
Random sampling is not considered adequate for staging purposes. Certainly any suspicious or enlarged nodes should be removed as part of a lung cancer operation or when performing a staging procedure, but removal of only an enlarged or suspicious node is not sufficient. SS is the minimum assessment considered acceptable and is defined as the removal or biopsy of lymph nodes from predetermined lymph node stations based on the location of the tumor and known lymphatic drainage patterns. The specified stations represent the minimum number of stations to be assessed. MLND is a formal dissection of the lymph node bearing areas of the mediastinum including the paratracheal zone, the subcarinal space, the inferior mediastinum, and, on the left, the subaortic space and the para-aortic space. It is not simply the removal of individual lymph nodes from those regions but rather removal of all the lymph node bearing tissue within predetermined anatomic boundaries.
Extended mediastinal dissection is the formal removal of bilateral mediastinal and cervical lymph nodes. It is usually performed by VAMLA or TEMLA or by open techniques. This may be used for staging, but is more often a therapeutic technique.
Lobe-specific systematic lymph node dissection refers to the formal dissection of lymph node bearing tissues based on the anatomic location of the cancer. For example, for a left lower lobe tumor, the lymph node dissection would include the subcarinal and inferior mediastinal node dissection. Systematic node dissection refers to the combination of systematic mediastinal lymph node sampling (SS) and systematic hilar and intrapulmonary lymph node dissection.
Required Lymph Node Stations for Invasive Staging
Recommended sampling includes stations 2R/L, 4R/L, and station 7. Any nodes that are enlarged, show increased uptake on PET–CT, or are suspicious in any way should also be sampled. Guidelines from ESTS, National Institute for Health and Care Excellence (NICE), Scottish Intercollegiate Guidelines Network (SIGN), and CCO recommend that appropriate lymph node assessment include SS of at least three mediastinal lymph node stations (preferably 5), one of which should be station 7.
Choice of Staging Technique
SS may be performed by EBUS–TBNA or EUS or mediastinoscopy prior to a planned resection or at the time of the planned resection. If mediastinal nodes are suspicious for metastases, it is preferable to schedule the staging procedure in advance of the planned resection. If performed at the time of planned resection, on-site cytopathology or frozen section analysis is required as the finding of metastases in mediastinal lymph nodes is considered an indication for either nonoperative therapy or perhaps neoadjuvant therapy in selected cases. MLND is usually performed at the time of planned resection and may be performed by open thoracotomy or VATS. MLND may be performed as an alternative to SS based on surgeon preference. If no preresection invasive mediastinal staging has been done, MLND should be performed. MLND also should be performed if any N1 nodes are found to contain metastases or if at the time of resection, SS has identified N2 disease.
There has been considerable controversy over the role of SS compared with MLND. As a staging procedure, SS is adequate in patients with early stage only (clinical T1 or T2, N0 or nonhilar N1 nonsmall cell lung cancer [NSCLC] based on the ACOSOG Z0030 trial). If SS is negative for metastases in such patients, SS is adequate and further dissection by MLND does not confer a survival advantage even though 3.8% of patients in the ACOSOG Z0030 trial were identified by MLND as having occult N2 disease. However, the ACOSOG Z0030 results do not apply to patients with larger tumors (T3/T4) or hilar nodal disease. In such patients, MLND is still recommended.
The importance of lymph node assessment in the staging of NSCLC is well recognized, but despite this, inadequate lymph node assessment is too common. The consequence of inadequate staging will be reduced lung cancer survival if nodal disease goes undetected and untreated.
Invasive/Surgical Staging Techniques
Mediastinoscopy
Definition
Mediastinoscopy is a surgical endoscopic technique that allows the exploration of the superior mediastinum along the tracheobronchial axis, from the sternal notch to the subcarinal space and along both main bronchi.
Technique
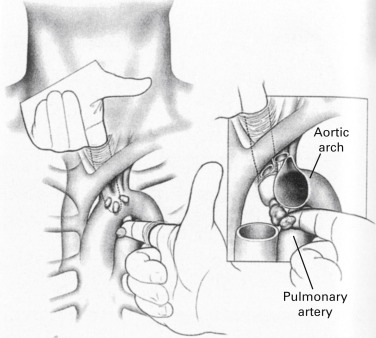
Under general anesthesia and orotracheal intubation, with the patient in the supine position and the neck slightly extended, a 3-cm to 5-cm collar incision over the sternal notch is performed and carried through the subcutaneous tissue and platysma. The pretracheal muscles are separated laterally to expose the trachea. The pretracheal fascia is incised with scissors and the pretracheal plane is developed. The mediastinum is explored by finger dissection as far caudally as possible ( Fig. 24.3 ). Finger palpation of the superior mediastinum allows the surgeon to identify anatomic landmarks, such as the innominate artery and the aortic arch, and to assess the texture of the mediastinal tissues, the consistency of the paratracheal lymph nodes, and the relation of central tumors to the mediastinal structures.
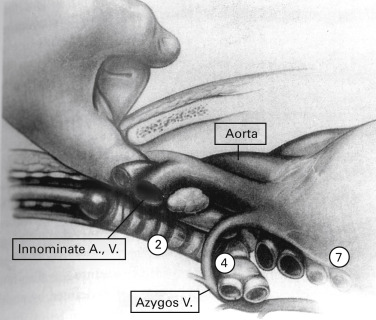
Finger palpation creates a mediastinal space into which the mediastinoscope is inserted. With the mediastinoscope in place, peritracheal dissection is completed by gently sweeping the adjacent tissues away from the airway with the dissection–suction–coagulation device ( Fig. 24.4 ). Before taking any biopsies, the following structures should be identified: the innominate artery lying anterior to the trachea, the aortic arch lying over the trachea on the left, the azygos vein at the right tracheobronchial angle, and the pulmonary artery anterior to the carina ( Fig. 24.5 ).
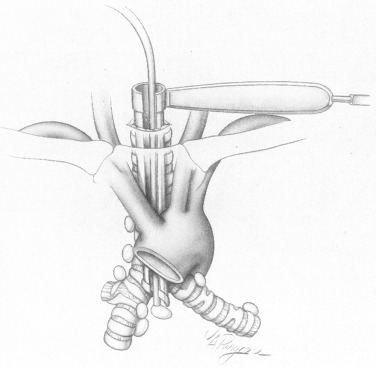
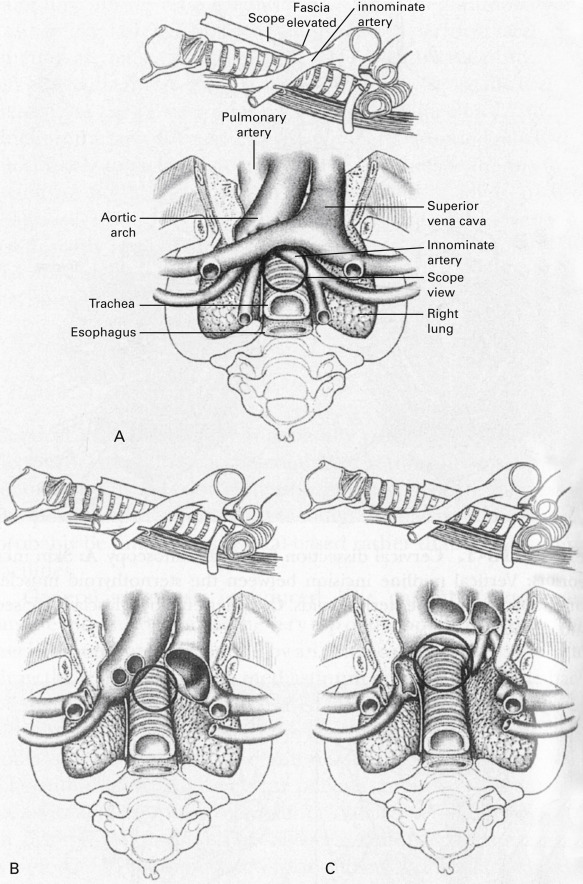
Mediastinoscopy allows biopsies of the pretracheal nodes (station 1), the right and left, superior and inferior, paratracheal nodes (stations 2R/2L and 4R/4L, respectively), the subcarinal nodes (station 7), and the right and left hilar nodes (stations 10R/10L; see Fig. 24.1 , Table 24.1 ). For a clinically acceptable mediastinoscopy in standard clinical practice, the upper and lower paratracheal spaces and the subcarinal space should be explored and any lymph nodes identified should be subjected to biopsy. Any nodes that are enlarged on CT or show metabolic activity on PET scan should be explored and subjected to biopsy.
Nodal exploration should start on the contralateral side of the tumor to rule out N3 disease and then proceed in a systematic way to explore and perform biopsy on all accessible nodal stations. Mediastinoscopy also allows assessment of mediastinal invasion by either the primary tumor (T4) or by mediastinal nodes, which would preclude surgical resection. All biopsy sites should be controlled for bleeding before the incision is closed in two layers.
Results
A review of 26 reports published between 1983 and 2011, including 9267 patients who had undergone conventional mediastinoscopy, reported a median sensitivity of 0.78 and a median negative predictive value of 0.91. An additional series of 995 patients who had undergone video-assisted mediastinoscopy and were reported in seven papers published from 2003 to 2011 showed a median sensitivity of 0.89 and a median negative predictive value of 0.92. By convention, specificity and positive predictive value of mediastinoscopy is 1, although positive results are not confirmed by other tests. Videomediastinoscopy is reported to be more thorough in terms of number of nodes and nodal stations explored but there is no clear evidence of greater safety or improved staging although it has educational advantages.
Complications
Reported complications include pneumothorax, wound infection, mediastinitis, esophageal perforation, tracheobronchial injury, left recurrent laryngeal nerve palsy, chyle leak, hemothorax, and bleeding from any of the vessels of the superior mediastinum, but complications are rare and occur in about 3% of cases. Serious bleeding complications occur in about 0.4% of cases and may be managed by packing, but may require median sternotomy. Mortality related to mediastinoscopy usually is below 0.5%.
Limitations
Mediastinoscopy does not reach the subaortic, para-aortic, prevascular, retrotracheal, and inferior mediastinal lymph nodes.
Technical Variants for Mediastinoscopic Lymphadenectomy
VAMLA and TEMLA are procedures performed from the collar incision used for mediastinoscopy; their objective is not the taking of biopsies of the mediastinal nodes, but their systematic removal. Experience with these techniques is limited to a few centers.
VAMLA is performed with a two-blade spreadable mediastinoscope and has the objective to remove en bloc the subcarinal and the right inferior paratracheal lymph nodes, and to remove individually the left inferior paratracheal lymph nodes. The initial results from the two groups that developed VAMLA were very good, with sensitivity and negative predictive value of 1.
TEMLA is also performed from the cervical incision, but the sternum is elevated with a sternal retractor fixed to a metal frame. The objective of TEMLA is to perform a complete mediastinal lymphadenectomy from the supraclavicular lymph nodes in the midline to the paraesophageal nodes. Most of the operation is performed in the open fashion; the mediastinoscope is used to complete the subcarinal and paraesophageal nodal dissection, and the thoracoscope to facilitate the removal of subaortic and para-aortic lymph nodes. Staging results of TEMLA based on 256 patients are as follows: sensitivity, 0.94; negative predictive value, 0.97; and diagnostic accuracy, 0.98. TEMLA is reported to be superior to mediastinoscopy and to EBUS, EUS, or their combination both for staging and restaging lung cancer after induction therapy.
Parasternal Mediastinotomy
Definition
Parasternal mediastinotomy is a procedure that allows the exploration of the anterior mediastinum through a right or left parasternal incision.
Indicatio\ns
The ACCP and the ESTS guidelines on mediastinal staging recommend the exploration of the subaortic (station 5) and para-aortic (station 6) lymph nodes in patients with cancer of the left upper lobe when all other explored lymph nodes are negative. The natural lymphatic dissemination of left upper lobe tumors is to these two nodal stations, which cannot be reached with mediastinoscopy. Prevascular lymph nodes (station 3a) if abnormal on CT or PET scan can be accessed by right or left parasternal mediastinotomy. Left parasternal mediastinotomy is especially valuable to explore tumors of the aortopulmonary window and to assess whether there is contact or tumor infiltration of the aortic arch.
Technique
A 4-cm to 7-cm transverse incision is performed over the second costal cartilage on the right or on the left down to the pectoralis muscle. The fibers of the pectoralis major muscle are separated in a craniocaudal fashion exposing the costal cartilage. The cartilage is usually excised subperichondrially or, alternatively, the exploration may be carried out through the intercostal space without cartilage resection. The internal mammary vessels may be ligated or retracted. After this, the mediastinal pleura is separated laterally with finger dissection to expose the anterior mediastinum. On the left, the subaortic space and the ascending aorta can be explored, either directly or with the assistance of a mediastinoscope (anterior mediastinoscopy; Fig. 24.6 ). On the right, the prevascular nodes can be reached. Parasternal mediastinotomy is a versatile procedure. Besides the exploration of the anterior mediastinum, it also allows the opening of the mediastinal pleura and the exploration of the hilum and pleural space. The pericardium can also be opened and explored to rule out direct tumor invasion or metastatic dissemination. Lung biopsies can also be performed from this incision. These additional procedures are best performed with unilateral ventilation.