Practical Approach to Evaluation of Monoclonal Gammopathies
Francis K. Buadi
Joseph R. Mikhael
William G. Morice II
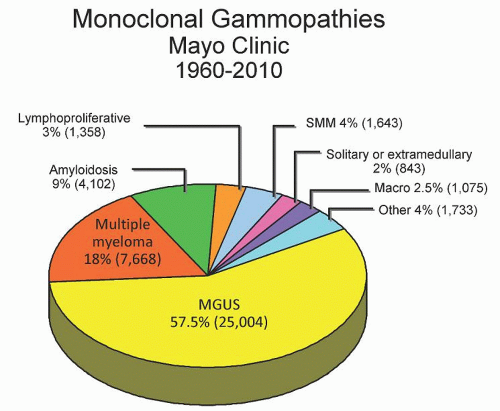
The real incidence of monoclonal gammopathy is unknown; however, it increases with age, with most cases being identified in the 7th or 8th decade of life.1, 2, 3 The presence of a monoclonal protein is indicative of an underlying clonal plasma cell or B cell disorder. These disorders encompass a spectrum of disease entities ranging from clinically benign monoclonal gammopathy of undetermined significance (MGUS) to clinically significant diseases such as multiple myeloma, and also including Waldenström macroglobulinemia, chronic lymphocytic leukemia, and various neurologic and cutaneous diseases.4, 5, 6, 7, 8, 9, 10, 11, 12 Among 43,000 monoclonal gammopathies evaluated at Mayo Clinic from 1960 to 2010 most had MGUS (57.5%), multiple myeloma (18%), or primary (AL) amyloidosis (9%) (Fig. 95.1). However, 4% of these cases were associated with other conditions, such as POEMS syndrome, cryoglobulinemia, Castleman’s disease, and light chain deposition disease, which do require therapy and should not be missed during evaluation. A systematic approach to the patient with a monoclonal immunoglobulin (monoclonal protein) disorder is required, on the one hand to prevent unnecessary testing in the majority who will not need treatment for the underlying condition, and on the other hand to ensure that those with a clinically significant condition will be adequately diagnosed.
This chapter addresses the initial approach to an individual with a monoclonal protein or suspected immunoglobulin disorder. We review the various conditions to consider in a patient with a monoclonal protein, so as to help guide the evaluation. The basic principles on the use of the various tests used, their interpretation, and limitations are also reviewed. Subsequent chapters in this book will deal with the detailed evaluation of specific diseases associated with monoclonal gammopathy.
CLASSIFICATION OF MONOCLONAL IMMUNOGLOBULIN DISORDERS
A prior understanding of the various conditions associated with the production of monoclonal immunoglobulins (monoclonal protein) is essential in the evaluation of an individual with a monoclonal gammopathy. The clinical presentation may be due to the magnitude of the underlying tumor burden or the direct toxic effect of the monoclonal protein. However, a simple way of approaching the evaluation of these cases is to start with the type of monoclonal protein (Table 95.1). Although the various diseases can be broadly classified based on the type of monoclonal protein, there is overlap in the underlying conditions. For example, an IgM monoclonal protein is usually associated with lymphoproliferative diseases such as Waldenström macroglobulinemia and some lymphomas, but may also be associated with cases of IgM-associated multiple myeloma.13
Initial Evaluation
The usual test that will detect a monoclonal protein is not part of the typical healthy adult physical evaluation.14 It is therefore usually detected during the evaluation of a clinical symptom or during further evaluation of an abnormal routine blood test. Abnormalities in routine clinical tests that suggest the possibility of a monoclonal protein disorder include rouleau formation in a peripheral blood smear, elevated total serum protein, proteinuria, anemia, renal dysfunction, or hypercalcemia. Clinical situations that may require evaluation for
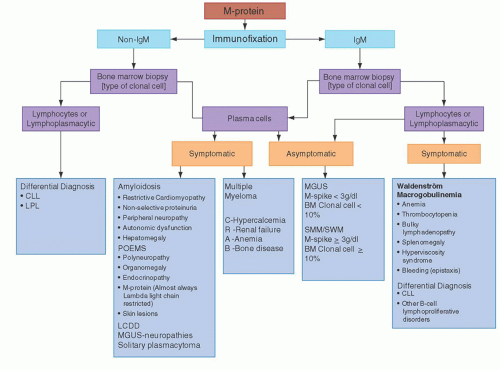
a monoclonal protein include back pain, osteoporosis disproportionate to age, pathologic fracture, osteolytic or sclerotic bone lesions, recurrent sinopulmonary infections, progressive peripheral neuropathy, infiltrative or restrictive cardiomyopathy, and Raynaud’s phenomenon. It must, however, be noted that in these conditions a monoclonal protein may not always be detected by standard testing, and further evaluation may be needed to confirm or exclude an underlying plasma cell or lymphoproliferative disorder, particularly if the index of suspicion is high. Most patients with monoclonal protein are asymptomatic, especially those in whom a routine blood abnormality leads to further testing, resulting in the identification of a monoclonal protein. A simple algorithm taking into consideration the various tests available and clinical presentation is shown in Figure 95.2. Although there may be some overlap, following this algorithm should lead to the correct diagnosis in most cases.
a monoclonal protein include back pain, osteoporosis disproportionate to age, pathologic fracture, osteolytic or sclerotic bone lesions, recurrent sinopulmonary infections, progressive peripheral neuropathy, infiltrative or restrictive cardiomyopathy, and Raynaud’s phenomenon. It must, however, be noted that in these conditions a monoclonal protein may not always be detected by standard testing, and further evaluation may be needed to confirm or exclude an underlying plasma cell or lymphoproliferative disorder, particularly if the index of suspicion is high. Most patients with monoclonal protein are asymptomatic, especially those in whom a routine blood abnormality leads to further testing, resulting in the identification of a monoclonal protein. A simple algorithm taking into consideration the various tests available and clinical presentation is shown in Figure 95.2. Although there may be some overlap, following this algorithm should lead to the correct diagnosis in most cases.
TABLE 95.1 CLASSIFICATION OF MONOCLONAL PROTEINS | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||
Laboratory Evaluation
The following are important tests that will help in the evaluation of a patient with or suspected to have a monoclonal protein.
PROTEIN ELECTROPHORESIS
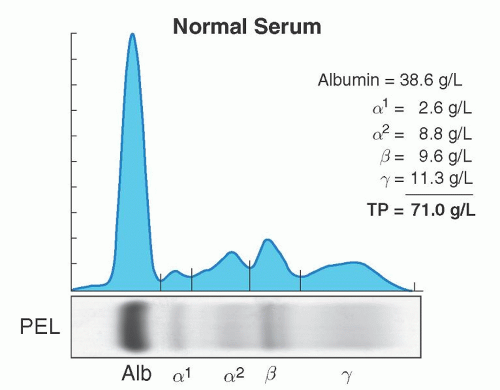
Serum and urine should both be evaluated for the presence of a monoclonal protein. High-resolution agarose gel electrophoresis or capillary zone electrophoresis is the preferred method for screening for a monoclonal protein.15, 16, 17, 18, 19 These tests will separate serum or urine proteins into their various components in an electric field based primarily on their physical properties such as size and charge; the proteins are detected either by staining a solid matrix or by their electrical impedance as they exit the column. There are usually five components seen, albumin, α-1, and α-2, β-, and γ-globulin (Fig. 95.3).
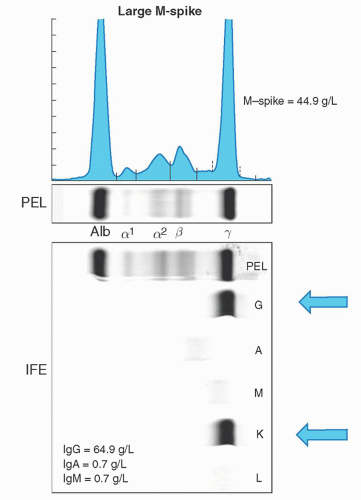
Monoclonal proteins will usually migrate into the gamma regions; however, occasionally they may be seen in the β or α-2 region. Samples with a monoclonal protein usually will result in a spike in the gamma region (Fig. 95.4). This is referred to as the M-protein or M-spike with the “M” referring to monoclonal, not IgM. Further testing is then required to determine the type and quantity. All spikes should be evaluated, since in certain cases there may be two (biclonal gammopathy), or more, different monoclonal proteins in a single M-spike that should not be missed.20, 21, 22, 23 It must also be stressed that the gamma region contains all immunoglobulin isotypes (IgM, IgA, IgD, and IgE) and not only IgG. This same test can be used to determine the amount of monoclonal protein present using the densitometer tracing or peak size by capillary zone electrophoresis (Fig. 95.4). The measured M-protein by densitometry tracing is usually lower than the total involved immunoglobulin measured by nephelometry.16 Protein electrophoresis occasionally
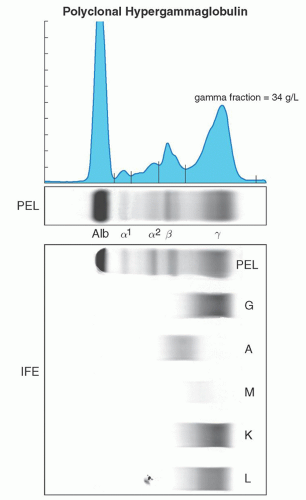
may fail to identify the presence of a monoclonal protein, especially in cases where there is only minimal production of the monoclonal protein, or minimal amounts of immunoglobulin free light chains.24 Currently in such cases performing immunofixation despite a negative protein electrophoresis or using the serum free light chain analysis may be the only way to confirm the presence of an underlying clonal disorder, although tandem mass spectroscopy methods are currently being explored.21, 25, 26 A monoclonal protein spike should not be confused with a polyclonal increase in immunoglobulin, which usually will be seen as a broad-based band in the gamma region and is not associated with a clonal cell disorder27 (Fig. 95.5).
may fail to identify the presence of a monoclonal protein, especially in cases where there is only minimal production of the monoclonal protein, or minimal amounts of immunoglobulin free light chains.24 Currently in such cases performing immunofixation despite a negative protein electrophoresis or using the serum free light chain analysis may be the only way to confirm the presence of an underlying clonal disorder, although tandem mass spectroscopy methods are currently being explored.21, 25, 26 A monoclonal protein spike should not be confused with a polyclonal increase in immunoglobulin, which usually will be seen as a broad-based band in the gamma region and is not associated with a clonal cell disorder27 (Fig. 95.5).
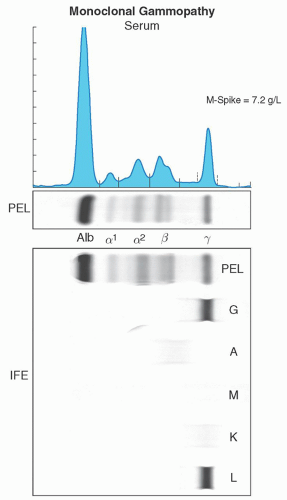 FIGURE 95.4. Images of a serum protein electrophoresis and immunofixation depicting a monoclonal protein. |
IDENTIFICATION OF THE TYPE AND QUANTITATION OF THE MONOCLONAL PROTEIN
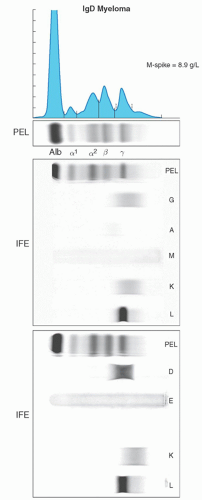
The heavy and light chain isotypes of a monoclonal protein is usually determined by immunoelectrophoresis or immunofixation.15, 16, 21 Immunoelectrophoresis, although less expensive, is infrequently used, as it is relatively insensitive. Immunofixation involves the application of anti-heavy and antilight chain antibodies to the electrophoretic gel and has higher detection sensitivity. Immunofixation will identify both the heavy chain isotype (IgG, IgM, IgA, IgD, or IgE) and the light chain type (κ or λ) and should always be performed on all cases of protein electrophoresis with an M-spike. A case with an IgG heavy chain isotype and a κ-light chain will be reported as an IgG-κmonoclonal protein (Fig. 95.6). Most laboratories will initially only perform immunofixation for IgG, IgA, and IgM. Immunofixation for IgD and IgE must be performed if an M-spike is present and either initial immunofixation studies are negative or detect only a monoclonal light chain (Fig. 95.7). The isotype and quantity of the monoclonal protein is important for classification and prognosis. For example in MGUS, the risk of progression is lower in patients with IgG isotype and an M-spike less than 0.5 g/dl. The type of protein and quantity is also important for monitoring patients during therapy. Immunofixation should still be performed in cases where there is a strong suspicion of the presence of a clonal plasma cell or
lymphoproliferative disorder, even if the protein electrophoresis is negative for an M-spike. Capillary zone electrophoresis with immunosubtraction is an automated system that is slightly more sensitive than high-resolution agarose gel electrophoresis and can be used in the identification, subtyping, and measurement of the M-protein.17, 19 This method is laborious and time consuming, however, making it less frequently used for typing of the monoclonal protein.
lymphoproliferative disorder, even if the protein electrophoresis is negative for an M-spike. Capillary zone electrophoresis with immunosubtraction is an automated system that is slightly more sensitive than high-resolution agarose gel electrophoresis and can be used in the identification, subtyping, and measurement of the M-protein.17, 19 This method is laborious and time consuming, however, making it less frequently used for typing of the monoclonal protein.
QUANTITATION OF IMMUNOGLOBULINS
Immunoglobulin (IgG, IgA, IgM) levels should always be measured at diagnosis and monitored regularly during therapy, especially the involved immunoglobulin. If immunofixation showed an IgD or IgE M-protein, then their levels should also be determined. In most clonal plasma cell disorders the levels of the uninvolved immunoglobulins are suppressed or reduced. Rate nephelometry is a reliable and rapid method for immunoglobulin quantification, although this method does not differentiate between monoclonal and polyclonal immunoglobulin.28, 29 Rate nephelometry also does not provide information on how much of the measure immunoglobulin is abnormal; therefore, in patients with normal total immunoglobulin it is not clinically helpful. This test does have value in clinical care however, as knowing the level in severely hypogammaglobulinemic states will help determine who should receive immunoglobulin replacement, especially those with recurrent sinopulmonary infections.
FREE LIGHT CHAIN ASSAY
A significant population of patients with clonal plasma disorders produces excess amounts of light chain but not a full immunoglobulin molecule: Bence Jones proteinemia.30 Most of these patients will have a negative protein electrophoresis and immunofixation. In these patients the free light chain assay test may be the only way to detect the presence of a clonal plasma cell disorder.24, 25, 26 Immunoglobulin free kappa (κ) and lambda (λ) light chains concentration in the serum is usually dependent on the rate of production from plasma cells and renal clearance. This results in a defined serum concentration and ratio. In clonal plasma cell disorders there is an excess production of only one of the light chain types, resulting in higher levels, with suppression of the uninvolved light chain, leading to an abnormal κ/λ ratio. The levels and ratio, however, may be affected by renal failure, since the light chains are cleared by the kidneys. This test has become very important in the evaluation and monitoring of patients with amyloidosis, since this is the major protein involved in AL-type amyloid deposits.31
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree