POSTTRANSLATION
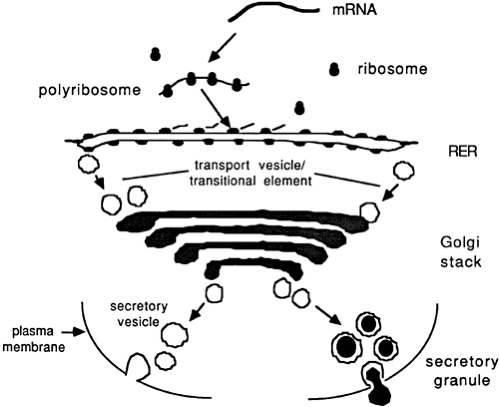
Up to the translational and cotranslational steps in the ER, all secretory, membrane, lysosome, endogenous ER, and Golgi proteins have traversed the same biosynthetic path. After this point, the major task of sorting and transferring the proteins to the correct intracellular destinations must be completed. This complex process occurs in the Golgi stack and requires sorting signals among the proteins and sorting mechanisms in this organelle. A polypeptide hormone destined for regulated secretion must exit the ER, traverse the Golgi stack, and arrive properly in the secretory granule (Fig. 3-12).
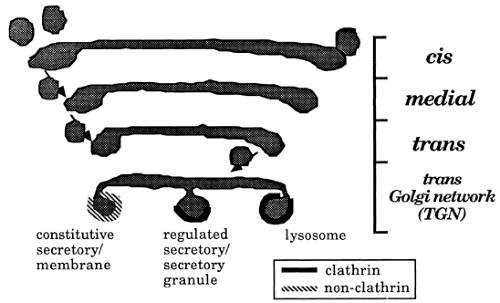
The Golgi stack comprises a series of flattened, saccular membranous compartments that encompass four histologically and functionally distinct regions: the cis, medial, and trans regions of the Golgi complex and the trans-Golgi network (TGN)43,44 (Fig. 3-13). The cis-Golgi region is most proximal to the transitional elements of the RER, and the TGN is most distal. The maintenance of distinct Golgi-specific antigens, unique enzyme markers, and different lectin-binding characteristics suggest that the compartments are not contiguous.
A vesicle transfer model has been proposed to account for transport of materials from the RER to the TGN. In this model, membrane vesicles form from the upstream compartment by budding at the rims of the Golgi plates and rejoin the adjacent downstream compartment by vesicle fusion and the interaction of microfilaments. The reiterative process of budding and fusion of secretory or transport vesicles causes vectorial transfer of proteins from the RER to the TGN in a unidirectional and energy-dependent process.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree