Platelets are derived from the fragmentation of the cytoplasm of giant bone marrow cells, the megakaryocytes (MKs). MKs are the largest cells of the marrow, up to 50
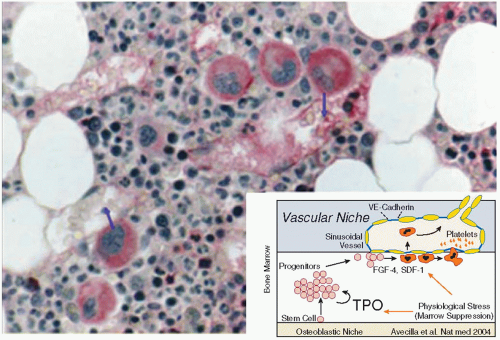
µm in diameter, and are characterized by a single polylobulated nucleus. They constitute <0.05% of marrow cells. MKs arise and mature in the bone marrow, along with the precursors of other blood cells. Immature MKs are located near the osteoblastic niche and migrate to the subenthothelium of venous sinusoids during maturation (
FIGURE 23.1). Given a normal platelet count (150 to 400 × 10
9/L) and platelet life time (8 to 10 days in humans), an average of 10
11 platelets are produced every day to maintain the blood platelet level.
MKs are unique cells that display a single polyploid nucleus and grow by undergoing a unique phenomenon called endomitosis during which the nucleus increases its deoxyribonucleic acid (DNA) content without dividing. Following polyploidization, the cytoplasmic mass of MK increases roughly proportionally to the ploidy status. After achieving full maturation, the MK cytosol is filled with a network of membranes formed by invagination of the plasma membrane, is loaded with secretory granules, and is ready to deliver a myriad of newly formed platelets. Platelets detach from cytoplasmic MK protrusions called proplatelets that extend into the lumen of bone marrow sinusoids and are shed into the peripheral blood from the tips of the extensions. Study of MKs is difficult because of their low numbers in the bone marrow, but scientific advances and techniques such as intravital imaging have aided our understanding of MK maturation and platelet formation. The development of in vitro MK culture systems, allowing the preparation of pure MK populations and the identification of specific and early markers permitting the immunologic detection of MK and their precursors, have made the study of MK feasible. Finally, the identification, purification, and cloning of thrombopoietin (TPO), the major regulator of platelet production, and the availability of recombinant TPO for experimental purposes have facilitated the preparation of large enriched MK populations.
This chapter focuses on the cellular and molecular aspects of MK differentiation and its regulation in humans. Descriptions of relevant animal models are presented as well. TPO and its receptor MPL-R are discussed in detail in
Chapter 25 and details of platelet maturation are provided in
Chapter 24.
ULTRASTRUCTURE OF MK DIFFERENTIATION
MK differentiation is characterized by structural changes that are easily observed under the electron microscope. Immunoelectron microscopy, in particular immunogold labeling, is a sensitive tool that has greatly contributed to the description of this process.
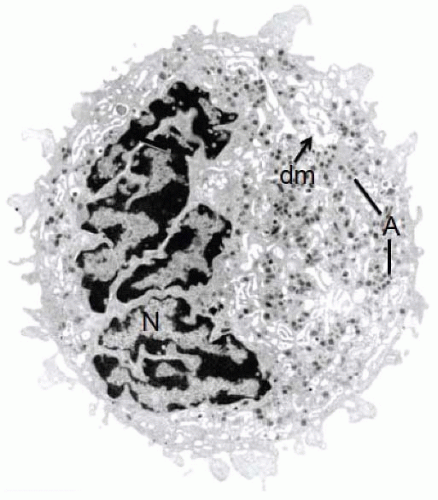
Demarcation membrane system. During the maturation process, the demarcation membrane system (DMS) develops as a network of smooth membrane channels located initially near the plasma membrane. These channels derive from multiple invaginations of the MK plasma membrane
13 (
FIGURE 23.3). The demarcation membranes are always in contact with the extracellular medium, as demonstrated by the penetration of electrondense extracellular tracers such as horseradish peroxidase. The DMS grows rapidly and becomes widespread within the whole cytoplasmic volume, expanding by more than 700% within 72 hours. Although some differences between the protein composition of the DMS and plasma membrane have been detected by freeze fracture studies,
14 the main platelet surface (glycoprotein [GP]) receptors are expressed equally on both.
15
The DMS is the source of proplatelet membrane
16 and is the precursor of the membranes of future platelets (plasma membrane and surface connected canalicular system); the mechanism of platelet shedding is discussed in detail below.
Because mature MKs accumulate near marrow sinusoids and represent a potential physical barrier for other marrow cells migrating into the circulation, migrating cells are able to penetrate the open channels of the DMS and transmigrate across the MK volume. This natural phenomenon, termed “emperipolesis,” is occasionally observed in normal bone marrow and is distinct from phagocytosis since the emigrating cell maintains its integrity.
17Golgi complexes and centrioles. Several Golgi complexes within a single MK follow the migration of several centrioles. The localization of centrioles within the cell is random, as are the Golgi complexes. The latter are found more frequently near the cell center in the perinuclear region. Their number appears to be roughly proportional to ploidy. Endogenously synthesized platelet granule proteins can be initially detected immunologically in the trans-Golgi network (TGN) where they undergo glycosylation, concentration, and precipitation. Granule proteins endocytosed from the extracellular medium are not detected in the TGN.
18 Membrane GPs also transit through the Golgi complex. It is noteworthy that immunolabeling of the GP Ib/IX/V complex is weak, whereas GPIIb/IIIa and P-selectin, major components of the
α-granule membrane, are seen more prominently in the Golgi cisterns and arising vesicles.
19
Endoplasmic reticulum. Rough ER and free ribosomes are abundant in immature MK. The smooth ER that derives from the rough ER after loss of ribosomes remains abundant until the end of the maturation process and is closely associated with the DMS. It contains a number of enzymes, including NADH-cytochrome
c reductase, cyclooxygenase, thromboxane synthase, and Ca
2+ Mg
2+ adenosine triphosphatase. In humans, a peroxidase activity, the platelet peroxidase, is located in the ER and reflects the presence of cyclooxygenase, an early marker of the cell line. The ER gives rise to the platelet-dense tubular system containing the same cytochemical activity.
10 In rodents, acetylcholinesterase (AchE), a marker absent from mature human MK, is also specifically located in the ER.
Formation and packaging of cytosolic granules. Four different types of granules are formed during MK maturation:
α-granules, dense granules, lysosomes, and peroxisomes. Their contents are detailed in
Table 23.1; the following sections focus on their mechanism of formation.
α-Granules. α-Granules appear early during MK maturation, concurrent with the development of the DMS.
20 They are the more numerous granule population in the mature MK (
FIGURE 23.3).
α-Granules arise from the TGN, where a dark nucleoid is rapidly
visible within budding vesicles.
α-Granules are unique organelles in that they acquire their protein contents by two distinct mechanisms: (a) MK biosynthesis
21 and (b) plasma protein endocytosis, either receptor mediated or through a fluid phase.
22,23 Endocytosis may continue in platelets.
24,25 The cytoplasmic markers are initially detected in a diffuse staining pattern when located in the cisterns of synthesis and later in a granular pattern when packaged in granules. Two types of information have been provided by immunolabeling
α-granules proteins. First, endocytosed proteins (e.g., fibrinogen and immunoglobulins) appear later in the MK than endogenously synthesized proteins (e.g., von Willebrand factor [vWF]),
18 and their distribution patterns within the maturing MK cytoplasm are distinct. Endocytosed proteins are centrifugal and maximal at the cell periphery, whereas synthesized proteins are centripetal and predominant in the juxtanuclear area.
26 vWF multimers are apparent in the form of 20-nm tubular structures, first appearing in the TGN and then in the
α-granules.
27 Stored proteins may not be uniformly distributed among
α-granules. For example, angiogenic proteins seem to be selectively located in distinct granule populations according to their function, pro- and antiangiogenic, leading to differential release from platelets.
28 The
α-granule membrane contains numerous receptors that appear during MK maturation: P-selectin (CD62P), GPIIb/IIIa, CD36, CD9, and PECAM1.
19,29 Some are detectable in the Golgi apparatus and appear to be transported directly to
α-granules, but others appear to transit
via the plasma membrane and to be internalized. Evidence based on intracellular trafficking suggests that the GPIIb/IIIa present in the
α-granule membrane originates directly from an internalized plasma membrane pool.
30 Finally, fully formed platelet
α-granules appear as highly elaborated and compartmentalized structures, storing hemostatic and adhesive proteins and numerous growth factors (
Table 23.1). Multivesicular bodies, present in MK and only occasionally in platelets, represent a sorting compartment, intermediately between the TGN and
α-and dense granules.
31,32 In contrast to secretory cells such as endothelial cells, virtually no constitutive granule secretion occurs in MK; secretion follows the regulated pathway and secreted proteins are packaged within secretory granules.
33
Dense granules. Dense granules are the final marker of the maturation of MK cytoplasm at the ultrastructural level.
34 Indeed, these granules acquire their dense appearance quite late, but their limiting membrane and intrinsic receptors are probably formed earlier.
35 They arise from TGN and their constituents are sorted from the
α-granule components in multivesicular bodies.
32 They are lysosome-related organelles containing high concentrations of several low molecular weight molecules necessary for normal blood hemostasis. These include calcium, serotonin, adenine nucleotides, pyrophosphate, and polyphosphate. Their absence results in the hemorrhagic
δ-storage pool deficiency disorders. In the Hermansky-Pudlak syndrome,
δ-storage pool deficiency is associated with tyrosinase-positive albinism, revealing a relationship between platelet-dense granules and the melanosomes of melanocytes. There are similarities in the biogenesis and secretory code of both types of granules that are linked to the small G-protein rab27 deficient in the ashen strain of mice.
36 Murine models have also shown that a specific gene (
Slc35d3 gene) is involved in dense granule formation independent of lysosomes and melanosomes.
37
Lysosomes and peroxisomes. These granules can be detected cytochemically within maturing MK because of their specific enzymatic content-acid hydrolases
38 and catalase, respectively.
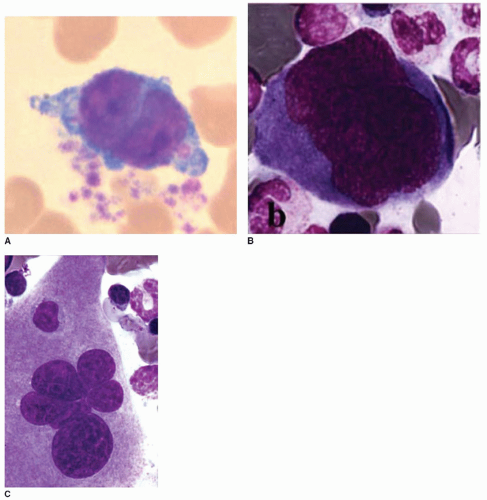
The MK nucleus. The nucleus of MK displays several sparse rounded lobes with abundant euchromatin and conspicuous nucleoli (type I) (
FIGURE 23.2A). Its maturation is characterized by a constant size increase and segmentation and by chromatin clumping (type II) (
FIGURE 23.2B). Through the unique process endomitosis, nuclear lobulation and ploidy increase without division, with the DNA duplicating to levels of 4N to 64N (rarely 128N). A correlation between ploidy and the number of nuclear lobes has been found on squash preparations. Close to the final maturation stage of MK, the nucleus changes texture and aspect (type III) (
FIGURE 23.2C). Electron microscopy shows that the nuclear lobes tend to elongate and gather, the chromatin becomes coarser, and long clefts of cytoplasm extend between each lobe. These structural changes herald the start of proplatelet formation and platelet shedding.
Megakaryocyte activation. MKs are able to react to agonists in a manner similar to platelets.
39 Thrombin induces dramatic morphologic changes as early as the megakaryoblastic stage, indicating the early expression of protease-activated receptors. In the presence of agonists, the MK plasma membrane becomes bristled and sends out numerous thin pseudopods, the nucleus becomes eccentric, cytosolic organelles centralize, and secretory granules fuse with DMS after which they discharge their contents. This phenomenon, which leads to inappropriate secretion of granule contents, including growth factors, may be implicated in pathologic states such as fibroblast activation and myelofibrosis.
40
ENDOMITOSIS
At the end of the proliferative phase, 2N MK precursors become polyploid. MK polyploidization is unique among mammalian cells in that a multiple of 2N chromosomes is enclosed in a single nucleus surrounded by a single nuclear membrane. To achieve polyploidization, MKs undergo several rounds of DNA duplication and the nucleus segments but without nuclear and cytoplasmic division.
MKs are the only cells that become polyploid during their normal differentiation, whereas other cells may become polyploid after a stress. Polyploidy is a way to increase platelet production because the MK cytoplasm volume increases in parallel with ploidy. An increase in MK ploidy is one of the first events to occur after the induction of acute severe thrombocytopenia in mice: within 24 hours, the modal MK ploidy increases from 16N to 32N. This increase is maximal at 48 hours and ploidy returns to basal values within 120 hours.
As a first maturation step, MKs increase their ploidy and stop DNA duplication at any point between 2N and 64N, rarely going up to 128N. In humans, as in most mammals, the modal ploidy is 16N. However, in some mouse species such as C3H mice, the modal MK ploidy is 32N.
61 In the fetus and embryo, MKs have a lower ploidy; most fetal MKs are only 2N or 4N in culture.
62 Nonetheless, low ploidy MKs, either on a physiologic basis or due to malignancy, are able to shed platelets
in vitro.
MK endomitosis is a rare event that remains difficult to observe. Originally, the term endomitosis was given to the mechanism of polyploidization because it was suggested that the mitotic process was occurring without rupture of the nuclear envelope. However, ultrastructural studies have allowed a description of the endomitotic process. The first steps are identical to those of mitosis. They begin with chromosome condensation and disappearance of the nuclear membrane. Several mitotic spindles are subsequently formed with two centrosomes for a tetraploid nucleus. Each centrosome is active and participates in elongation of the mitotic spindle. At metaphase, chromosomes are aligned at the equator and have sizes similar to those of mitotic cells.
MK culture in the presence of TPO, in conjunction with immunolabeling of various components of the mitotic apparatus, has made it easier to precisely describe the endomitotic process. It was initially thought that endomitosis represented mitosis that had skipped anaphase B and cytokinesis.
63,64 In fact, the initiation of endomitosis is identical to a normal mitosis with duplication of the centrosomes, development of a mitotic spindle, chromosome condensation (prophase), rupture of the nuclear envelope, chromosomal alignment at the equatorial plate (metaphase), and separation of the sister chromatids (anaphase).
65 Initially, the late phases of endomitosis were not observed because of their rarity. However, with the use of timelapse videomicroscopy, it has been observed that endomitotic
MKs go through telophase and begin a cytokinesis that fails.
66,67 At the 2N and 4N transition, the two daughter cells that are nearly fully separated make a backward movement and fuse together. The structure of the central spindle has been a matter of debate, but it appears normal at the 2N and 4N transition. The main abnormality involves the cleavage furrow and its ingression which is incomplete. At higher ploidy, the abnormality is more pronounced with only incomplete cleavage furrow ingression.
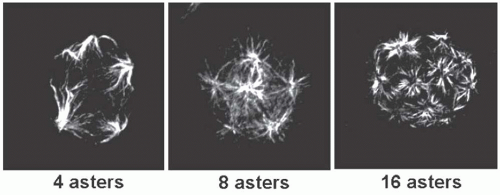
66,67,68,69 When the MK is polyploid, its spindle is multipolar with the number of poles corresponding to the ploidy level; however, the number of chromosomes at each pole does not correspond to 2N because segregation of chromatids is asymmetrical during endomitosis
65 (
FIGURE 23.4). It is surprising that a defect in cytokinesis leads to mononucleated cells because it usually induces multinucleated cells. This suggests that anomalies in chromatid separation may be also present during endomitosis. Preliminary evidence suggests that a large fraction of 4N MKs have two nuclei as a consequence of a pure defect in late cytokinesis. Because the abnormality is more pronounced in MK with higher ploidy, the chromatids are less separated, leading to a defect in karyokinesis and to a single nucleus. However, it has recently been shown that polyploid MKs are able to undergo a mitotic cycle with the generation of 2N cells.
70The cell cycle of a MK undergoing polyploidization is very similar to a mitotic process. There is clearly a succession of G1/S/G2/M phases, but the M phase is incomplete. After the M phase, the cell reenters G1 to undergo a subsequent cell cycle of DNA duplication. However, the molecular mechanisms responsible for the switch from a mitotic to an endomitotic cycle, as well as the mechanisms involved in stopping the process, are still incompletely understood.
REGULATION OF ENDOMITOSIS
The usual regulators of mitosis are active during the endomitotic process. Cyclin D3, cyclin E, and cyclin A are normally expressed during G, S, and G2/M phases, respectively.
71 The presence of cyclin B1 during endomitosis has been a subject of debate. There is now a large body of evidence indicating that cyclin B1 is expressed during the endomitotic cycle and is degraded in anaphase.
64,65 The presence of cyclin B1 is absolutely required for an entry in mitosis because the cdc2 (CDK1) kinase activity is indispensable for rupture of the nuclear envelope and chromatid condensation. Cyclin E may also play an important role in ploidization. Cyclin E
−/− mice have a marked defect in MK ploidization,
72 but a proliferation defect was not observed in the other mitotic cells. In addition, cyclin E overexpression increases MK polyploidy.
73The anaphase-promoting complex is functional in MKs. This proteasome complex regulates the end of mitosis by degrading successive mitotic regulators such as the p55cdc20 subunit and cdh1. During the endomitotic process, cyclin B1, p55cdc20, and Aurora B are normally degraded.
65,74 On the other hand, CDK inhibitors such as p21 and p27 are expressed at high levels in polyploid MKs, but are not directly involved in endomitosis. These inhibitors do not seem to contribute to mitotic arrest as MK ploidy is normal in p21
−/−, p27
−/− and double KO mice.
75 However, p19
Ink4d appears to play a role in endomitotic arrest because p19
Ink4d KO mice have MK with a 64N modal ploidy.
76 It is noteworthy that p19
Ink4d is transcriptionally regulated by RUNX1 as well as several genes coding for platelet function. Thus, RUNX1 may be a link between the regulation of the end of endomitosis and terminal cytoplasmic differentiation.
There are also differences between endomitosis and mitosis. Levels of cyclin D3 and cyclin D1 are extremely high in MK,
71,77 and an increase in cyclin D increases MK ploidy. Cyclin D1 expression is regulated by GATA1 in MK.
77 In addition, it has been shown that STAT1, which is downstream of GATA1, also increases polyploidy and MK maturation.
78 Interferon
γ, which induces STAT1 phosphorylation, induces MK polyploidization as well.
Whatever the molecular mechanisms involved, there are two main abnormalities in the cell cycle of the endomitotic MKs. One abnormality occurs at the end of mitosis and is responsible for the failure in cytokinesis. It has been suggested that this is related to a defect in Aurora B and survivin expression in polyploid MK,
79,80 but no major defect in the expression of these proteins and in their
localization in the central spindle has been found at the 2N to 4N transition.
74,81 Such abnormalities may be present in higher ploidy MKs.
80 Perhaps more germaine to the process, there is accumulation of myosin II at the contractile ring.
74 Myosin II normally plays a central role in cytokinesis by promoting the contractile ring forces required for cytokinesis. It is presently unknown if the defect in myosin II accumulation is primary or is secondary to low Rho/Rock pathway activation. The second cell-cycle abnormality occurs at the G1/S transition. A normal cell cannot become polyploid because an as yet unknown “sensor” detects the level of ploidy and blocks subsequent reentry into the S phase. This checkpoint is mainly regulated by p53.
82 However, a role for p53 in polyploidization remains controversial.
83Whether polyploidization is advantageous for platelet formation has been unclear. Theoretically, an endomitotic cycle could be shorter than a mitotic cycle because the late phases of the cell cycle are abrogated. However, the time for DNA duplication seems identical in both processes. During terminal differentiation, MK must synthesize large amounts of membrane to produce platelets. The endomitotic process could produce considerable economy of nuclear and plasma membrane synthesis which may aid in the efficiency of platelet production. Polyploidization may also be a way to markedly increase protein synthesis. In favor of this hypothesis, it has been shown that all 2N alleles of a gene remain functional during polyploidization; therefore, polyploidization represents true gene amplification.
84 Whether platelets arising from MK of different ploidy have similar or different sizes and function remains a matter of debate. In addition, polyploidy may induce resistance to mutations leading to haploinsufficiency as multiple gene copies are present. Whether polyploidization modifies protein synthesis and gene expression also remains to be determined. It has been reported that there is a linear correlation between mRNA or protein expression and ploidy. However, at high ploidy, expression may reach a plateau. Gene profiling during polyploidization has shown that the processes of polyploidization and terminal differentiation are partially intermingled because expression of genes involved in platelet function increases with ploidy.
85 Perhaps this is because terminal differentiation and polyploidization are regulated by the same transcription factors, for example, GATA1, FLI-1, or RUNX1.
76,77