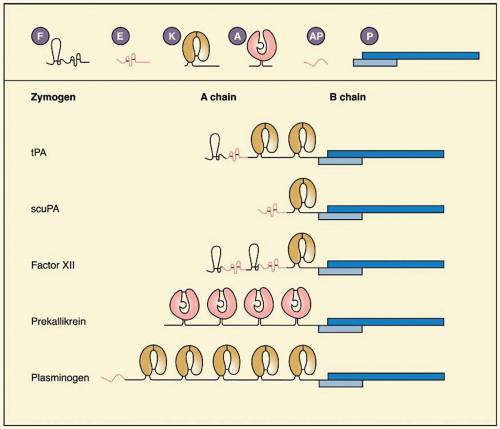
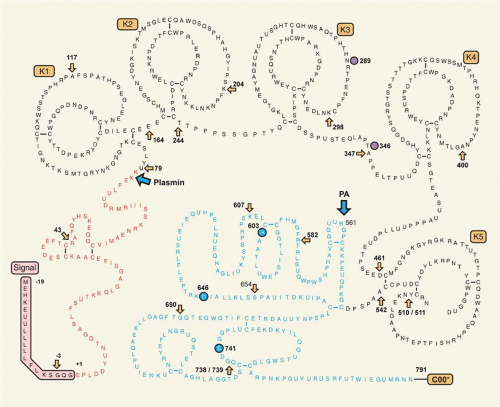
tPA, a serine protease of 68 kDa (EC 3.4.21.68), previously known as vascular PA or extrinsic activator, is a glycoprotein of 527 residues (
FIGURE 20.1). It exerts its effect primarily in the vascular system, because it is produced and secreted by endothelial cells
93; many other cells in culture also synthesize tPA. In normal plasma, the antigen concentration of tPA is approximately 5
µg/L, which corresponds to approximately 70 pM (
Table 20.1),
94 but most is in complex with its primary inhibitor, plasminogen activator inhibitor 1 (PAI-1).
95,96
Expression and Structure
The gene for tPA is located on bands p12-p11 on chromosome 8
97; it contains four exons
98 and comprises 32.7 kb. The mature protein exists in two forms, the
N-terminus being Gly or Ser in Bowes melanoma cell tPA,
99 but predominantly Ser in recombinant tPA,
100 and the numbering in this chapter is based on Ser at position one. tPA expression in cultured cells can be stimulated through multiple intracellular signaling pathways. Vasoactive substances, such as thrombin and histamine, increase tPA synthesis in human umbilical vein endothelial cell (HUVEC), acting through their G-coupled receptors and the protein kinase C pathway.
101,102,103 Steroid hormones
104,105 and retinoids can increase synthesis of tPA.
106,107 The expression of tPA has been reviewed
108 and will not be covered in detail here. It is important to note that not all endothelial cells synthesize tPA
in vivo and that expression is restricted to small vessels.
109,110The structure of tPA (
FIGURE 20.1) shows a finger and EGF domain, and two kringle domains in the A chain, whereas the protease domain resides in the B chain. The finger domain extends from residues 6 to 43. It contains a binding site for fibrin, as mutants lacking kringle domains are still capable of binding fibrin.
111 Consistent with this, a degraded form of tPA that had lost the
N-terminal 12 kDa bound less well to fibrin than wild-type tPA.
112 The binding of the finger domain is independent of LBS, in that it cannot be blocked by
εACA.
113 Structurally, the finger domain is very similar to the seventh type 1 repeat of human fibronectin.
114The EGF domain (residues 44 to 92) is structurally similar to other EGF structures, as shown by nuclear magnetic resonance (NMR).
115 There is some evidence from deletion mutagenesis that the EGF domain binds to the mannose receptor and is involved in tPA clearance.
116 Kringle 2 has affinity for lysine,
ω-amino acids, and fibrin, whereas no function has yet been ascribed to kringle 1.
111 The affinity for the binding of
εACA (a model for
C-terminal lysine residues) and of
N-acetyllysine methyl ester (a model for intrachain lysine residues) is approximately equal, suggesting that unlike PLG, tPA does not have a preference for
C-terminal lysine residues binding.
113 Intact tPA and a variant consisting only of kringle 2 and the protease domains were found to bind to the cyanogen bromide (CNBr) fibrinogen fragment FCB-2, which also binds PLG and acts as a stimulator of tPA-catalyzed PLG activation.
111 In both cases, binding was completely inhibited by
εACA, pointing to the involvement of LBS in this interaction.
111The structure-function relations among kringle 2 and
ω-amino acids and lysine have been studied in detail, using NMR,
117 microcalorimetry,
118 crystallography,
119 and site-directed mutagenesis.
118,120,121 The crystal structure of kringle 2 resembles that of PLG kringle 4; there are, however, differences in the lysine-binding pocket. The core of kringle 2 is formed by a hydrophobic cluster of three tryptophan residues in positions 25, 63, and 74, surrounded by aromatic and hydrophobic side chains that form, at the surface of the kringle, a hydrophobic grove. Ligand binding appears to rely mostly on the integrity of Trp63 and Trp74, and an aromatic residue at position 76, which is normally Tyr.
122 Mutation of the critical amino acids Lys33, Asp55, Asp57, or Trp72 markedly diminished binding to lysine-Sepharose,
εACA, or both.
123The protease domain of tPA has the typical catalytic triad His322, Asp371, and Ser478. The 2.3-Å crystal structure of the protease domain revealed strong structural similarity with other trypsin-like serine proteases, thrombin in particular.
124 The active site cleft is shaped and narrowed by four surface loops. The loop around Arg299 exhibits five additional residues, Arg298-Arg-Ser-Pro-Gly302, compared with chymotrypsin. It projects out of the molecular surface as a
β-hairpin and is of fundamental importance for the interaction with PAI-1.
3 The 60-loop around Arg327 is similar to but shorter than the corresponding loop in thrombin. Further loops are found around Ser381 and Gly465. The fully solvent-exposed hydrophobic region, comprising amino acids 420 to 423 of tPA, which forms a surface loop near one edge of the active site of tPA, is an important secondary site for the interaction of tPA with PLG in the absence of fibrin.
tPA is secreted as a single-chain molecule (sctPA) that is converted to the two-chain form (tctPA) by plasmin by cleavage at Arg275-Ile276. Unlike most serine proteases the single-chain form is not a zymogen but a protease that, in the presence of fibrin, is nearly as active as the two-chain form.
125 Mutagenesis studies have revealed that tPA lacks a specific zymogen triad Asp194, His40, and Ser32 (chymotrypsin numbering).
3,126 The constructed double mutant Phe305His and Ala292Ser was more zymogenic.
126
Release
The major source of tPA in the circulation under basal conditions is thought to be the endothelial cell, from which it is constitutively released.
127 A secondary pool of tPA is present in specialized storage vesicles and is released in response to specific extracellular stimuli.
128,129,130,131 High concentrations of tPA are contained within these storage vesicles that occur in various cells, such as endothelial, neuroendocrine, and adrenal chromaffin cells.
129,131 In rats, the tissue stores of tPA were calculated to be sufficient to maintain a steady-state plasma level of tPA for 2 days in the absence of protein synthesis.
132 Compounds that triggered release within a few minutes include bradykinin, histamine, eledoisin, acetylcholine,
β-adrenergic agents, platelet-activating factor, endothelin, calcium ionophore A-23187, and acidosis.
133,134 These compounds induce calcium influx into the endothelial cell and activate G-protein-coupled receptors.
135 Some interventions that cause elevated tPA act through decreased clearance, including exercise and
α-adrenergic agents.
136 In all situations in which epinephrine levels are increased, such as stress, anxiety, and exercise, tPA antigen levels increase.
96,137Acute release of tPA is established from early studies on venous occlusion.
138,139,140 Young patients with a history of recurrent venous thromboembolism exhibited an abnormal response to venous occlusion and this could be attributed either to subnormal tPA release or, more often, to elevated PAI-1.
95,139,140 In isolated cases with severe forms of von Willebrand disease, a complete lack of response to venous stasis and the infusion of 1-deamino-8-D-arginine vasopressin (DDAVP) was found.
141 These patients may have a functional or structural defect in their endothelial cells. It should be noted, however, that release of von Willebrand factor (vWF) and tPA occur by different mechanisms.
142 Lower PA activity is found in leg veins than in proximal veins and may be one etiologic factor for development of deep venous thrombosis. DDAVP was widely used in the 1980s to study the release potential for tPA in patients with idiopathic or recurrent thrombosis.
143,144,145 Comparison of the effects of DDAVP and sodium nitroprusside showed that nitroprusside produced a greater increase of forearm blood flow but no increase of tPA; however DDAVP stimulated tPA release from the vascular bed.
146 The increase of circulating tPA levels after intra-arterial infusion of acetylcholine and methacholine was shown to be mediated by muscarinic receptors.
146,147,148 Bradykinin and substance P both induced tPA release after being infused into the forearm of volunteers,
149,150 and the releasable pool of tPA, rather than the availability of PAI-1 to inhibit it, was the key factor.
151
Enzymatic Properties of Tissue-Type Plasminogen Activator
tPA is unusually specific among the serine proteases. Its major substrate is PLG, in which it cleaves the Arg561-Val562 bond but is a relatively inefficient activator of PLG in the absence of its cofactor, fibrin.
152 Binding of sctPA and tPA to fibrin is roughly comparable.
153 The
Kd for binding of tPA to fibrin clots in the absence of PLG ranges from 140 to 400 nM.
125,153,154 In the presence of PLG, the affinity of tPA to fibrin increases approximately 20-fold (
Kd 20 nM).
155 These observations are explained by formation of a ternary complex between tPA, PLG, and fibrin or binding of tPA to PLG, which subsequently, upon binding to fibrin, takes on an open conformation. The isolated A chain of tPA has been shown to bind to Glu- and mini-PLG with a
Kd of 100 nM.
156There is a wide range of kinetic parameters reported for the activation of PLG by tPA due to many potential experimental variables, such as different protein preparations, substrate
concentrations, and nature of the fibrin stimulator. In the absence of fibrin,
KM values for the activation of Glu-PLG by tPA range from 9
µM to slightly more than 100
µM.
125,157,158 In general,
KM is three to four times lower with tPA than with sctPA for the activation of Glu-PLG in the absence of fibrin. In the presence of fibrin, this difference is less apparent with
KM values typically two orders of magnitude smaller, with only moderate increases of
kcat. Values for
KM in the presence of fibrin range from 0.16 to 1.1
µM PLG, and
kcat values from 0.1 to 1.1 per second.
125,158 Several authors found nonlinear enzyme kinetics,
156,157,159 and there appear to be two phases in the activation of Glu-PLG by tPA in the presence of fibrin, with an initial
KM of 1.05
µM and
kcat of 0.15 per second. Later in the process, as new high-affinity binding sites for PLG and tPA are exposed in partially digested fibrin,
160,161,162,163,164 KM decreases to 0.07
µM, whereas the
kcat is unchanged.
159 The key message is that PLG is not readily activated by tPA except in the presence of fibrin, because it is only in this situation that the
KM for the reaction is consistent with the circulating PLG concentration of 2
µM.
Distinct sites in the fibrin molecule have been identified as accelerating PLG activation; notably, they are not available for binding in fibrinogen but become exposed in fibrin.
165 The D-region of fibrin binds both PLG and tPA in a region that encompasses the residues A
α148-160. Both these associations are of low affinity and in the circulation the A
α148-160 site would bind only PLG, which is present in much higher concentrations than tPA. Another binding in the D-region encompasses
γ311-336 and
γ337-379, which are linked by a disulfide bond.
166 Antibodies have revealed that there is a tPA binding site that includes
γ312-324.
167 Higher affinity sites for binding tPA and PLG are in the
C-terminus of the
α chain, within the region A
α392-610.
168 The conformational changes that occur on fibrinogen cleavage and fibrin assembly
169 reveal new sites for binding of tPA and PLG, and for enhancement of PLG activation.
Mutations have been engineered in tPA to change its characteristics, with the aim of making it an even more effective therapeutic agent. The most striking of these is the series of mutations to make tPA resistant to PAI-1. Madison et al.
170 identified the importance of interactions between the positively charged tPA sequence 298 to 302 and the negatively charged PAI-1 sequence 350 to 355. Mutagenesis of Arg298, 299, and 304 resulted in a mutant that was inhibited 120,000 times less rapidly by PAI-1 than wild-type tPA.
171 These observations led, in part, to the development of the new tPA mutant tenecteplase (TNK-tPA), in which the sequence 296 to 299 (Lys-His-Arg-Arg) is replaced by four alanines.
172 This variant also exhibits slower clearance, by virtue of its changed glycosylation, and its enhancement of PLG activation is more selective for fibrin over fibrinogen and the fibrin degradation product DD/E.
173