Plasma Cell Disorders
INTRODUCTION
Plasma cell disorders are a group of related diseases arising from a common progenitor belonging to the B-cell lineage. They are characterized by the expansion of plasma cells in the bone marrow (BM) and nearly always accompanied by the presence of a monoclonal immunoglobulin (Ig) or Ig fragment in the serum and/or urine of patients (1). Monoclonal gammopathy of undetermined significance (MGUS), multiple myeloma (MM), Waldenstrom’s macroglobulinemia (WM), primary amyloidosis, and heavy chain diseases (HCD) all belong to this group of disorders. Dysproteinemias or plasma cell dyscrasias are some of the other terms used to refer to this unique group of disorders.
Normal B-cell differentiation is characterized by a process of Ig VDJ rearrangement, somatic mutation, and Ig class switching resulting in maturation of antibody-producing plasma cells. Ig variable (VH) gene sequence analysis indicates that myeloma tumor cells are postfollicular, and originate from a memory cell undergoing isotype switch events. Translocations involving switch regions indicate that the final oncogenic molecular event in myeloma occurs late in B-cell ontogeny. Five heavy-chain isotypes (M, G, A, D, E) and two light-chain isotypes (κ and λ) are present and are typically identified by serum or urine protein electrophoresis as a sharp spike in the gamma region. The isotype is further identified and quantitated by immunofixation and is referred to as the monoclonal component, that is, arising from the neoplastic clone. This is a useful biomarker and helps determine responses in patients with plasma cell disorders. More recently an ELISA-based assay is being used to detect light chains in the serum. This is particularly useful in patients with amyloidosis, light chain MM, and nonsecretory MM.
MONOCLONAL GAMMOPATHY OF UNDETERMINED SIGNIFICANCE
Monoclonal gammopathy of undetermined significance (MGUS) is seen in 1% of patients over age 50 and 3% of people over age 70 and is associated with the presence of a monoclonal Ig which is less than 3.5 g/l (2). It is differentiated from MM by fewer than 5% monoclonal BM plasma cells and lack of end organ damage like bone lesions, anemia, hypercalcemia, or renal dysfunction. In a large series of 1384 patients followed longitudinally at the Mayo Clinic, 115 patients progressed to MM (relative risk [RR] 25), IgM lymphoma (RR 2.4), primary amyloidosis (RR 8.4), macroglobulinemia (RR 46), chronic lymphocytic leukemia (RR 0.9), or plasmacytoma (RR 8.5). The risk of progression of MGUS to MM or related disorders is about 1% per year (2); risk factors for progression include high serum monoclonal protein (≥1.5 g/dl), non-IgG MGUS, and abnormal serum free light chain ratio (3). At this time, treatment for MGUS and smoldering MM consists of risk stratification and close observation (2). Clinical trials are however being conducted to target this population.
MULTIPLE MYELOMA
 EPIDEMIOLOGY
EPIDEMIOLOGY
MM is a plasma cell dyscrasia characterized by a clonal proliferation of lymphoid B cells and infiltration of the BM by plasma cells (1, 4). It is the second most common hematologic malignancy, and is responsible for at least 2% of cancer-related deaths. It is estimated that 21,700 new cases of MM and 10,710 deaths from MM will occur in the United States in 2012 (5). African-Americans and Pacific Islanders have a reported high incidence of MM followed by Europeans and North American Caucasians. Low incidence rates have been reported for Asians living in Asia and the United States (6). In addition to MGUS, potential risk factors for the development of MM include exposure to irradiation or petroleum products. Familial cases have also been reported, suggesting a possible genetic predisposition. Myeloma has also been found to occur with somewhat greater frequency in farmers, paper producers, furniture manufacturers, and wood workers.
 BIOLOGY
BIOLOGY
Cytokines like interleukin-6 (IL-6) and insulin-like growth factor-1 (IGF-1) confer a growth and survival advantage to MM cells. Regulation of the mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3’-kinase/Akt kinase (p13 kinase/AKT) pathways by these and other cytokines and activation of the survival Janus Kinase/signal transducer and activator of transcription (JAK/STAT) pathway may be involved in the pathogenesis. The role of adhesion molecules and the BM microenvironment is also critical to tumor cell growth and survival. Adhesion molecules mediate both homotypic and heterotypic adhesion of tumor cells to either extracellular matrix (ECM) proteins or bone marrow stromal cells (BMSCs). After class switching in the LN, adhesion molecules, including CD44, VLA-4, VLA-5, LFA-1, CD56, Syndecan-1, and MPC-1, mediate homing of myeloma cells to the BM, and adhesion to BMSCs and ECM. Such binding not only localizes tumor cells to the BM microenvironment, but also stimulates IL-6 transcription and secretion from BMSCs with related paracrine growth of myeloma cells. In addition to homing to the BM, loss of some of these adhesion molecules results in migration at the time of disease progression. The binding of MM cells to BMSCs also regulates cell cycle progression and plays a role in the development of adhesion-mediated drug resistance (4). A variety of chromosomal abnormalities are found in MM. Based on conventional cytogenetic analysis, chromosome 13 deletions were predominant and associated with a poor prognosis. Techniques like spectral karyotyping have identified chromosomal abnormalities in the majority of MM patients and are broadly classified into hyperdiploid (HRD) or nonhyperdiploid (NHRD) tumors. Five recurrent IgH translocations have been seen in MM including MMSET and FGFR3 (15%), cyclin D3 (3%), cyclin D1 (15%), c-maf (5%) genes, and MAFB (2%) accounting for a prevalence of 40%. All MM tumor cells have high levels of cyclin D1, D2, and D3 including MGUS suggesting a unifying early oncogenic event (7, 8). Dysregulation of cyclin genes renders MM cells more susceptible to proliferative stimuli resulting in selective expansion in response to BMSCs and/or cytokines like IL-6 and IGF-1. On the basis of gene expression profiles, a new molecular classification has been proposed that categorizes patients based on the five major translocations and cyclin D overexpression in MM. Whole genome and whole exome sequencing of 38 MM patients identified mutations in genes involved in protein translation (e.g., mutations in DIS3 [also known as RRP44] and FAM46C), histone methylation, blood coagulation, and members of the NF-κB signaling (9). Four percent of patients had activating mutations in the BRAF kinase, suggesting a potential role for BRAF inhibitor-directed therapy.
 DIAGNOSTIC CRITERIA
DIAGNOSTIC CRITERIA
The diagnosis of MM is based on the presence of well-defined major and minor criteria (Table 30-1) (10). These criteria help distinguish MM from other plasma cell dyscrasias and B-cell malignancies associated with a paraprotein. They also help differentiate MM from solitary plasmacytoma. Solitary plasmacytomas are collections of monoclonal plasma cells originating in either bone (solitary osseous plasmacytoma, SOP) or soft tissue (extramedullary plasmacytoma, EMP) and are very responsive to local radiation treatment. The mean age of diagnosis of solitary plasmacytomas is usually younger than MM and approximately 70% of EMPs can be cured with local treatment alone. EMPs typically involve the lymphoid tissues of the upper aerodigestive tract. SOPs have a higher rate of progression to MM and therefore patients with plasmacytomas require prolonged follow-up (11).
TABLE 30-1 DURIE–SALMON CRITERIA FOR DIAGNOSIS OF MYELOMA
Adapted from Durie BG. Staging and kinetics of multiple myeloma. Semin Oncol. 1986; 13: 300–309.
 CLINICAL AND LABORATORY FEATURES
CLINICAL AND LABORATORY FEATURES
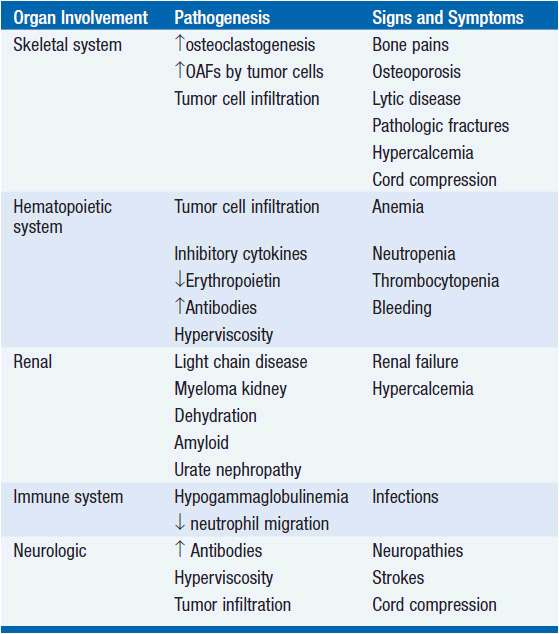
The mean age at diagnosis for MM is 62 years for men and 61 years for women. A slight male preponderance has been described. BM infiltration with plasma cells is commonly seen (Figure 30-1). Most of the symptoms associated with MM are a result of the end-organ damage caused by either MM tumor cell infiltration and/or the associated paraprotein. Symptoms of bone pain and anemia remain the most common presenting features affecting 80% of the patients. Other features include renal insufficiency, hypercalcemia, and symptoms associated with infection and hyperviscosity (12) (Table 30-2).
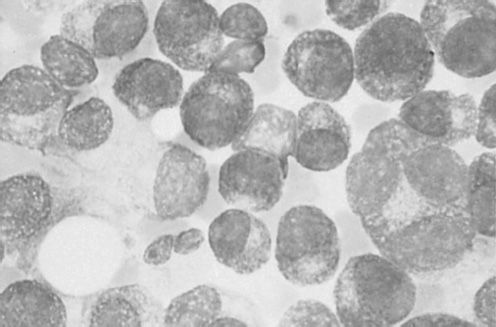
FIGURE 30-1 Photomicrograph of clusters of plasma cells in a bone marrow specimen.
Bone disease and hypercalcemia MM typically presents with osteolytic bone lesions (Figure 30-2). These may be associated with pathologic and compression fractures of the vertebral bodies. Bone scans and serum alkaline phosphatase are usually normal because of the absence of osteoblastic activity. Increased tumor burden results in production of osteoclast-activating factors like lymphotoxin (LT), TNF-α, hepatocyte growth factor (HGF), IL-6, IL-1, metalloproteinases, RANKL, and insulin-like growth factor-binding protein 4 (IGFIV) resulting in increased osteoclastogenesis and increased bone resorption. Bone resorption leads to increased calcium in extracellular fluid.
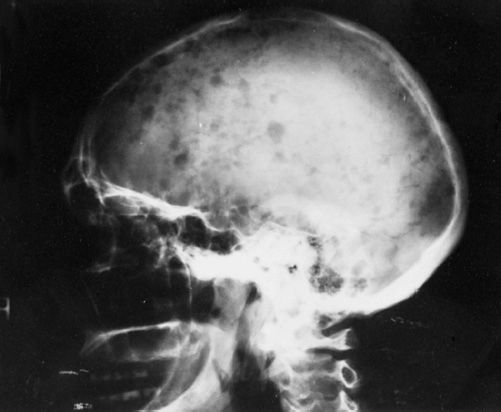
FIGURE 30-2 Radiograph of the head showing lytic lesions in the skull. The lesions have no osteoblastic activity and do not appear on nuclear medicine bone scans. (Photo provided by Dr. Geraldine Schechter.)
A skeletal survey is routinely performed on patients with MM to evaluate for bone disease. MRI is more sensitive and is being increasingly used in patients. In addition to defining bone disease, it can highlight BM infiltration.
Anemia Anemia in MM can be due to a number of factors, including tumor infiltration of the BM, renal impairment, the myelosuppressive effects of tumor products and chemotherapy, and a deficient production of erythropoietin (EPO) relative to the degree of anemia.
Renal failure Renal failure in MM predicts for an adverse outcome. The causes of renal failure in MM are often multifactorial and include hypercalcemia, MM kidney, hyperuricemia, toxicity from intravenous urography, dehydration, plasma cell infiltration, pyelonephritis, medications like nonsteroidal anti-inflammatory drugs, and amyloidosis.
Hyperviscosity Hyperviscosity is characterized clinically by spontaneous bleeding with headache and neurologic and visual disorders. Hyperviscosity is commonly seen with IgM paraproteins, although the IgG3 subclass has also been associated with this syndrome.
Recurrent infections Patients with MM are at an increased risk of infections because of the underlying hypogammaglobulinemia. Streptococcus pneumoniae and hemophilus infections usually occur early and typically during response to chemotherapy. Gram-negative infections occur in refractory, advancing disease; in the setting of previous antibiotic therapy; instrumentation; immobilization; colonization with hospital flora; and azotemia. Fatal infections may be hospital acquired, emphasizing the need to minimize indwelling foreign bodies such as catheters in patients with MM.
Cardiac failure The median age of patients with MM is more than 60 years; such patients are at an increased risk of cardiovascular disease. Patients are uniquely susceptible to cardiac ischemia and/or congestive heart failure (CHF) due to myocardial infiltration with amyloid, causing dilated or restricted cardiomyopathy, hyperviscosity syndrome, and/or anemia. MM patients are also susceptible to high-output CHF of unclear etiology.
Neuropathies In MM, a symmetric, distal sensory or sensorimotor neuropathy is most common and is associated with axonal degeneration, with or without amyloid deposition. In some cases, neuropathy is associated with monoclonal antibodies directed against peripheral nerve myelin. Laboratory evaluation identifies a monoclonal Ig in serum and/or urine in the majority of cases. In most series, 50%–60% of patients with myeloma have both serum and urinary monoclonal protein; 20%–30% of patients have serum without urinary protein; 15%–20% patients have monoclonal protein in urine only, and only 1%–2% patients do not secrete monoclonal protein in blood and/or urine but have evidence of BM infiltration with plasma cells. IgG or IgA monoclonal proteins are most common, and IgD or IgE proteins are rare. The natural history of myeloma is a progressive increase in tumor growth. The M protein doubling time, which is reflective of the myeloma growth rate, shortens with each relapse. Eventually marrow failure develops, with sideroblastic anemia, leukopenia, and thrombocytopenia. The median interval from marrow failure to death is 3 (range 1–9) months. Infection (52%) and renal failure (21%) account for the majority of deaths in patients with myeloma.
 PROGNOSTIC FACTORS
PROGNOSTIC FACTORS
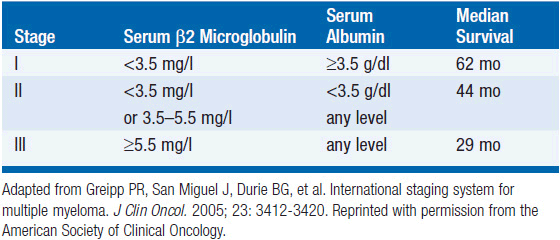
Multiple attempts have been made to define clinical and laboratory parameters which have prognostic significance. The Durie–Salmon staging system has been most commonly utilized (13). Staging in this system correlated with tumor cell mass and was further subdivided into A and B based on renal function. Based on this system, survival duration is 61.2 months for patients with Stage IA disease, 54.5 for Stages IB + IIA + IIB, 30.1 months for Stage IIIA, and 14.7 months for Stage IIIB disease. A newer staging system has been has been validated in over 10,000 MM patients based on presenting serum albumin and β2-microglobulin levels (14). This system classifies patients into three stages with median survivals of 62 months for Stage I, 44 months for Stage II, and 29 months for stage III disease (Table 30-3).
FISH and conventional karyotype may be used to risk-stratify patients (15–17). Gene expression profiling is also used to identify patients with high risk disease, although this does not as yet guide clinical decision making (18).
A quarter of newly-diagnosed patients will have intermediate- to high-risk disease, including patients with deletion 17p (leading to loss of tumor suppressor p53), t(4:14), or t(14;16) by FISH. The majority of patients will have standard risk disease, including patients with hyperdiploid karyotype. Patients with standard risk disease have an expected median survival of over 6–7 years, whereas patients with high-risk disease have a median survival of roughly 3 years (19).
 TREATMENT
TREATMENT
Between 5% and 10% of MM patients have an indolent course (see Table 30-1) and do not require immediate therapy. For the majority of patients, MM treatment is indicated at the time of diagnosis. Treatment is subdivided into specific antitumor therapy and supportive care measures.
SUPPORTIVE CARE MEASURES
Bone disease and hypercalcemia The use of bisphosphonates for the treatment of MM-related bone disease has greatly improved the quality of life of MM patients. Pamidronate has demonstrated efficacy in a prospective randomized trial by reducing skeletal-related events, including pathologic fractures, radiation therapy to bone, and spinal cord compression in patients with Durie–Salmon stage III MM and ≥ one lytic bone lesion (20). More potent bisphosphonates, such as zoledronate, have undergone clinical evaluation and offer the advantage of shorter infusion times compared to pamidronate.
In addition to playing an important supportive role, bisphosphonates may have a direct antitumor effect. The MRC Myeloma IX trial compared zoledronic acid to oral clodronic acid and found that zoledronic acid reduced mortality by 16% and increased median overall survival from 44.5 months to 50 months (21). The benefit of zoledronic acid on skeletal morbidity was also seen in patients without bone lesions at baseline (22). A key concern with bisphosphonates, especially zoledronic acid, is the risk of osteonecrosis of the jaw (ONJ) (23). In the MRC Myeloma IX trial, the rate of ONJ was 4% (21). Attention to dental hygiene and minimizing invasive procedures may reduce the risk of ONJ (24).
Denosumab is a monoclonal antibody to RANK ligand that also inhibits osteoclasts and showed promising activity in MM in a phase II trial (25). While denosumab was superior to zoledronic acid in patients with solid tumors and bone metastases, denosumab was inferior in a subset analysis of MM patients in a phase III trial (26). However, interpretation is limited based on the small numbers in the trial. A larger phase III study (NCT01345019) focusing on patients with MM is ongoing. Vertebroplasty (injection of methyl methacrylate or bone cement) and kyphoplasty (use of an inflatable balloon followed by instillation of bone cement) are percutaneous procedures for treating compression fractures, and have also been used in the setting of MM (27, 28).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree