92
Physiologic Changes of Aging
Olivia Le Saux, Sophie Watelet, Marine Haution-Bitker, Florence Murard-Reeman, Clémence Lecardonnel, Mahdi Harchaoui, Marc Bonnefoy, and Claire Falandry
INTRODUCTION
Aging is an inevitable, irreversible, and complex biological process that negatively impacts multiple organ systems and their ability to function (1). Over time, it leads to a decrease in physiologic reserve and an increased risk of many diseases. Ultimately, it will result in death. The development of these changes is not homogeneous and, therefore, will not happen at the same rate in every person (individual variability) nor at the same rate for every organ (intra individual variability). These changes are responsible for the vulnerability of elderly patients in critical situations. For example, the aging of the central nervous system makes the patient more prone to confusion, the aging of the renal system to dehydration or drug-related toxicity. Genetic, epigenetic, and environmental factors affect this process (2). Beyond these biological processes, aging is also associated with a significant shift in social role, which is not discussed in this chapter.
Bouchon conceptualized the loss of function of any organ during aging as being explained by three conditions (3):
1. Physiologic aging leads to a progressive loss of function, without reaching the threshold of insufficiency.
2. The function can be worsened by a chronic disease.
3. The function can be worsened by acute disease leading to insufficiency.
These last two conditions are modifiable, leading to the necessity to adapt care in the elderly.
We will review age-associated physiologic changes according to different systems. The main strategies to prevent organ insufficiency or geriatric deconditioning are summarized in Table 2.1.
TABLE 2.1 Summary of Age-Related Changes, Their Consequences, and Preventive Measures
10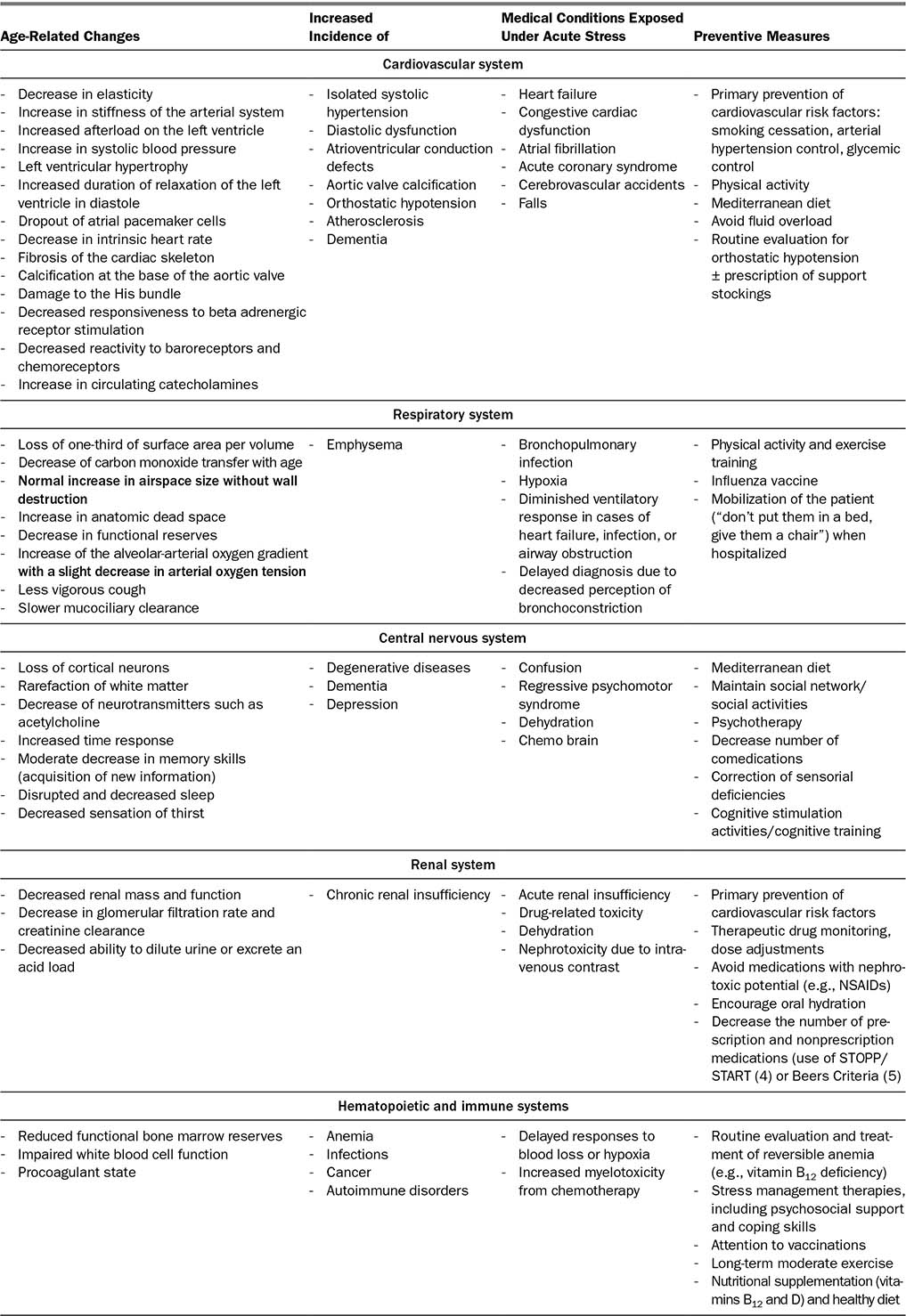
1112131415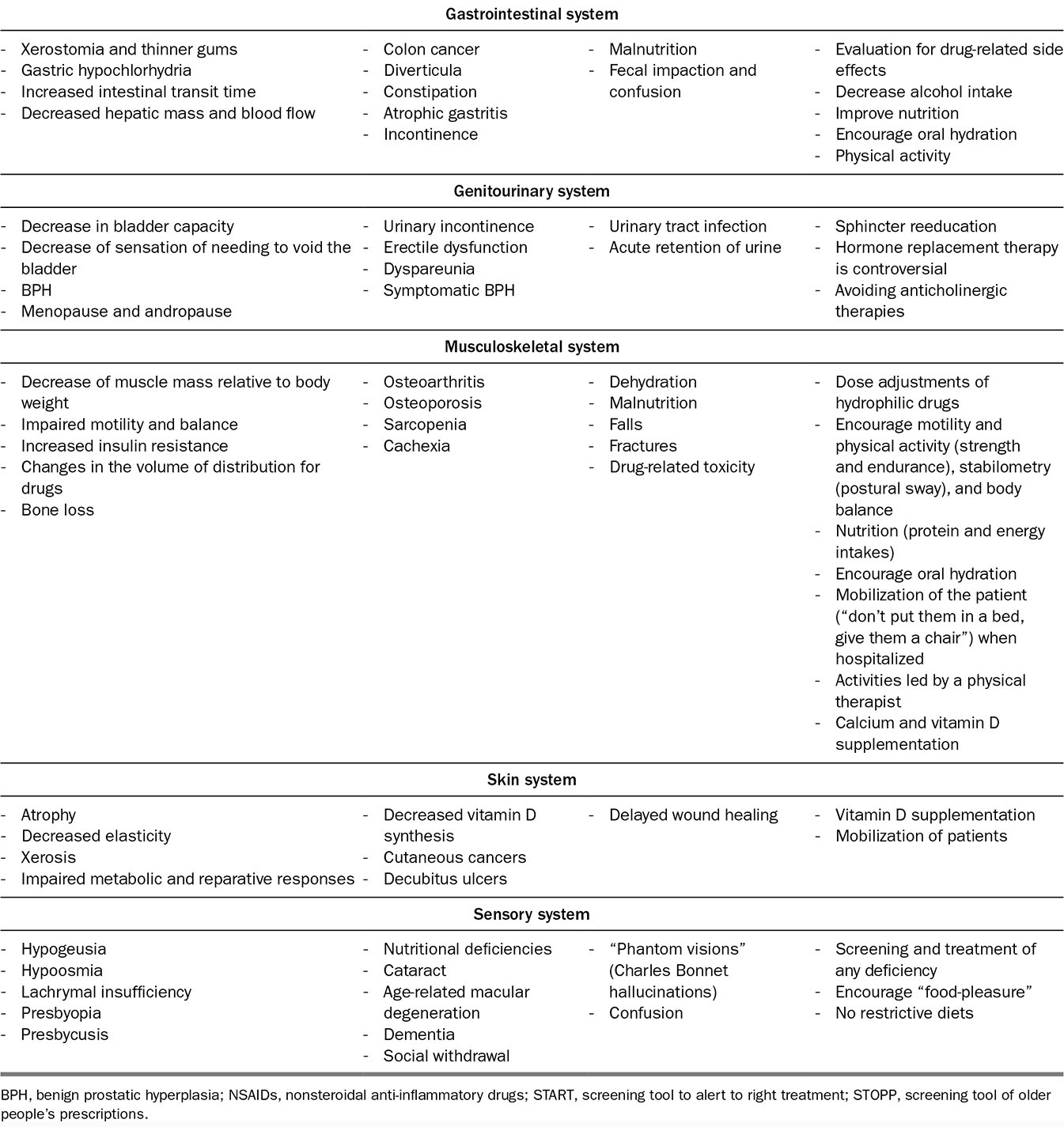
16AGE-ASSOCIATED PHYSIOLOGIC AGING
Cardiovascular System
Changes in the cardiovascular system are very prevalent in the aging process. Age-related changes are listed in Table 2.1. The most important changes and their consequences are (6):
■ Decreased aortic compliance, which results in an increased left ventricular afterload, increased systolic blood pressure, decreased diastolic blood pressure, and an increase in pulse pressure.
■ Modest left ventricular hypertrophy in response to a dropout of myocytes, along with increased left ventricular afterload and prolonged relaxation of the left ventricle during diastole.
■ Dropout of atrial pacemaker cells, resulting in a decrease in intrinsic heart rate.
■ Thickening of the annulus of both the aortic and mitral valves, with development of valvular calcification.
■ Apoptosis of sinoatrial pacemaker cells, fibrosis, and loss of His bundle cells, as well as fibrosis and calcification of the fibrous skeleton of the heart that can lead to various auriculoventricular blocks.
■ Decreased responsiveness to beta adrenergic receptor stimulation, decreased reactivity to baroreceptors and chemoreceptors, and increased circulating catecholamines, resulting in a marked decrease in the maximum heart rate in response to exercise or other stressors.
Respiratory System
There are multiple physiologic changes of the respiratory system associated with aging (7). The most important changes and their consequences are:
Decrease in static elastic recoil of the lung, in respiratory muscle performance, and in compliance of the chest wall, resulting in increased work of breathing compared with younger subjects and a diminished respiratory reserve in cases of acute illness (8).
Decrease in expiratory flow rates (small airway disease).
Less vigorous cough and slower mucociliary clearance, leading to increased frequency of infections.
Central Nervous System
Structural changes associated with aging include decrease of brain volume (predominant in the frontal and temporal lobes and in the white matter), neuronal loss, decrease in neuronal size, and synaptic density (9). There is less available acetylcholine due to decrease in the number of cholinergic neurons, with less synthesis and release of acetylcholine (10). Dopamine and corresponding receptors in the striatum and substantia nigra may also be decreased in normal aging (11).
17Neurocognitive changes are neither uniform nor inevitable. Some functions, such as language ability and vocabulary or visuospatial abilities, are resilient to brain aging. Other abilities, such as visual confrontation naming, verbal fluency, visual construction skills, conceptual reasoning, memory, selective attention, and processing speed, decline gradually over time (12). Episodic memory shows lifelong decline, whereas semantic memory shows late-life decline (13). Nondeclarative memory remains unchanged across the lifespan. Retention of information is preserved in cognitively healthy older adults; however, rate of acquisition and memory retrieval decline with aging (14). Executive function changes include a decline with age in concept formation, abstraction, mental flexibility, response inhibition, and inductive reasoning. In contrast, ability to appreciate similarities, describe the meaning of proverbs, and reason about familiar material remains stable throughout life.
Renal System
Structural and functional changes are summarized in Table 2.2 (15,16). The consequences of these age-related changes include decreased renal function overall, leading to a decrease in glomerular filtration rate and creatinine clearance and a decreased ability to dilute urine or excrete an acid load. It is important to emphasize the role of calculating glomerular filtration rate in all older adults as part of their overall physical evaluation. Due to decrease in overall muscle mass with age, serum creatinine level is not an accurate representation of renal function.
Hematopoietic and Immune Systems
Bone marrow is considered a self-renewing tissue. However, hematopoietic stem cells (HSC) experience phenotypic and functional changes with aging: expansion of the HSC compartment, skewing of differentiation toward myeloid progenitors, and decreased regenerative capacity (17,18). These changes lead to:
a. Immunosenescence: decreased efficiency of adaptive immune responses. Naive T and B cells decline, although the functions of memory cells are relatively preserved.
b. Inflammation (“inflammaging”): dysfunction in innate immunity associated with a pro-inflammatory profile. The increase in functional CD8+ lymphocytes T with aging also contributes to inflammation, due to their production of pro-inflammatory cytokines.
Immunosenescence has clinical consequences such as increased risk of infections, cancer, and autoimmune disorders, and less effective responses upon exposure to new antigens (e.g., through vaccinations) (19). Inflammaging and in particular elevations in levels of tumor necrosis factor (TNF), interleukin-6 (IL-6), IL-1, and C-reactive protein (CRP) are strong independent risk factors for morbidity and mortality in older people (20).
Aging is associated with myeloid-biased blood cell composition and increased prevalence of myeloid malignancies such as myelodysplasia and myeloproliferative neoplasms. There is increased incidence of anemia, and also increased incidence of chemotherapy-induced short-term and long-term toxicities with increased and cumulative risk of chemotherapy-induced neutropenia, secondary myelodysplasias, and acute leukemias (21).
18TABLE 2.2 Summary of Structural and Functional Changes With Aging
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree