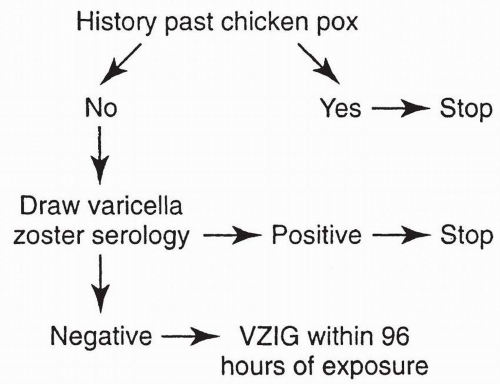
a few weeks. This test may be helpful in some diagnostic situations but is available in few laboratories. Although a positive rubella IgM titer indicates recent primary rubella infection, its absence does not necessarily exclude it, as in some patients, IgM may disappear in less than 4 to 5 weeks. When a patient is first seen with an elevated titer, it is not always possible to determine whether there has been acute primary rubella infection, even though these other tests are properly used. According to the CDC, persons generally can be presumed to be immune to rubella if they have documented vaccination with at least one dose of measles, mumps, and rubella (MMR) vaccine or other live rubella-containing vaccines, when administered on or after the first birthday, or if they have laboratory evidence of rubella immunity, or if they were born before 1957 (except women who could become pregnant). Birth before 1957 is not acceptable evidence for rubella immunity for women who could become pregnant because it provides only presumptive evidence of rubella immunity and does not guarantee that a person is immune. If a person has an “equivocal” serologic test result, that individual should be considered susceptible to rubella unless they have evidence of adequate vaccination or a subsequent serologic test result indicating rubella immunity. Postinfection immunity to rubella appears to be long lasting and is probably lifelong.
TABLE 15.1 ▪ RISK OF CONGENITAL RUBELLA AFTER MATERNAL INFECTION BY GESTATIONAL AGE AT INFECTION | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
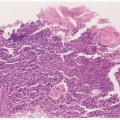
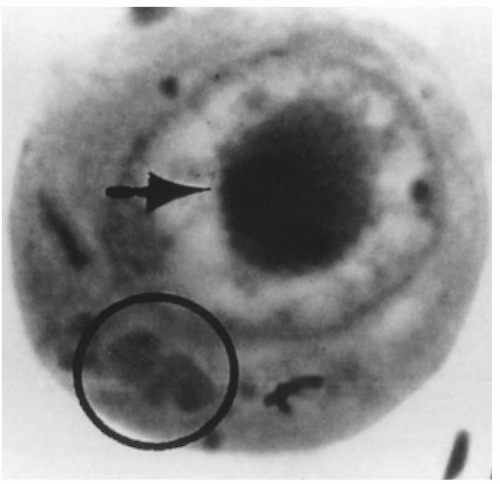 FIGURE 15.1 Intranuclear (arrow) and cytoplasmic inclusion (circle) in a pathognomonic “owl’s eye” cell of cytomegalic inclusion disease. |
Most importantly, only children born to mothers with primary CMV infection developed bilateral hearing loss (8%).
TABLE 15.2 ▪ SEQUELAE IN CHILDREN WITH CONGENITAL CYTOMEGALOVIRUS INFECTION ACCORDING TO TYPE OF MATERNAL INFECTIONa | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||
TABLE 15.3 ▪ PRIMARY CYTOMEGALOVIRUS INFECTION: EFFECT OF GESTATIONAL AGE ON TRANSMISSION IN UTERO AND DISEASE IN OFFSPRING | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||
greater risk for fetal infection than does infection occurring in the third trimester. More recently, another group demonstrated a significant correlation between higher CMV titers in the amniotic fluid and symptomatic disease in the newborn. Symptomatic infants had a mean titer of 1.0 × 107 GE/mL while asymptomatic infants had a mean titer of 5.1 × 105, but no clinically useful breakpoint was clearly identified.
months, IgM-specific antibody can be detected in the serum. Moreover, IgM may remain positive for up to 18 months and in 10% of women with recurrent CMV, IgM can be detected.
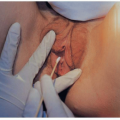
 FIGURE 15.3 An infant with congenital cytomegalovirus infection. Infant had jaundice, hepatosplenomegaly, and petechiae. |
tests resulted in antenatal diagnosis of CMV in 13 of 16 infected fetuses (sensitivity, 81%) in one study. Amniocentesis diagnosed 12 of 13 antenatally identified cases of CMV infection. Of these 12 cases, four had a negative result on the first amniocentesis before 20 weeks; 4 to 8 weeks later, the results of a second amniocentesis were positive. Diagnostic sensitivity of amniocentesis increases with the time interval after primary screening. Thus, when the interval is <8 weeks, the sensitivity is 50%, but increases to 91% after 13 weeks. Thus, with a strong suspicion (i.e., documented maternal primary CMV) if the initial assessment is negative, the testing should be repeated later. Detection of CMV IgM antibody in fetal blood had a sensitivity of 69% (9 of 13 cases). More recently, another group assessed amniocentesis, fetal blood sampling and serial ultrasounds in the prenatal diagnosis of CMV. Nearly 23% (26 of 114) of fetuses were infected with prenatal diagnosis identifying 20 of these cases, resulting in a sensitivity of 77% and specificity of 100%. In this study, amniocentesis best diagnosed fetal infection while ultrasound examination and fetal blood sampling identified fetuses at risk for severe sequelae.
TABLE 15.4 ▪ DIAGNOSIS OF FETAL CMV INFECTION | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||
diagnosis is uncertain, the V-Z virus can be indirectly identified by PCR or rapid antigen tests based on immunofluorescence techniques. Culture is available for direct identification but requires 3 to 5 days and is rarely used. Skin scrapings are the preferred specimen. Rapid antigen tests are the best test for detection of acute V-Z infection.
TABLE 15.5 ▪ COMPLICATIONS OF ACUTE VARICELLA INFECTION (CHICKENPOX) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
improved since the introduction of high dose acyclovir therapy (1985), mortality still remains a substantial concern.
TABLE 15.6 ▪ MATERNAL MORTALITY ASSOCIATED WITH VARICELLA DURING PREGNANCY | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Chest symptoms
Neurological symptoms (other than headache)
Hemorrhagic rash or bleeding
Severe varicella disease
> 100 lesions
Numerous mucosal lesions
Significant immunosuppression
In pregnant women
Near term
Poor obstetric history
History of smoking
Chronic lung disease
developing the congenital varicella syndrome after exposure in the first or second trimester is reviewed in Table 15.7. Overall, only 1% of infants exposed to V-Z virus during the first or second trimester developed stigmata of congenital varicella, with a range of 0% to 9%. In a large prospective study in Germany and the United Kingdom of 1,373 women who had varicella prior to 36 weeks’ gestation, nine cases of congenital varicella syndrome were identified, for a risk rate of 1%. Interestingly, the highest risk (2.0%) was noted between 13 and 20 weeks’ gestation (7 of 351 pregnancies; 95% CI, 0.8-4.1%). Before 13 weeks, only 2 of 472 pregnancies (0.4%) (95% CI, 0.05-1.5%) were identified.
TABLE 15.7 ▪ RISK OF CONGENITAL VARICELLA-ZOSTER SYNDROME WITH ACUTE MATERNAL VARICELLA-ZOSTER INFECTION DURING THE FIRST TRIMESTER OF PREGNANCY | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
TABLE 15.8 ▪ CLINICAL DATA IN INFANTS WITH DEVELOPMENTAL DEFECTS BORN TO 37 WOMEN WITH VARICELLA-ZOSTER INFECTIONS DURING EARLY PREGNANCY | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
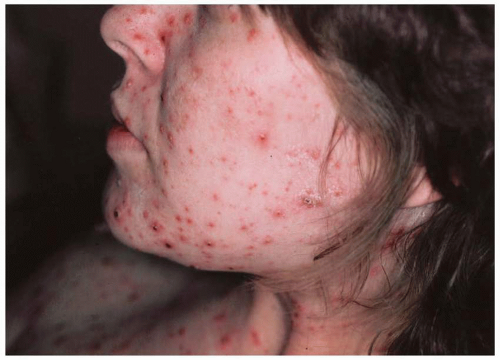
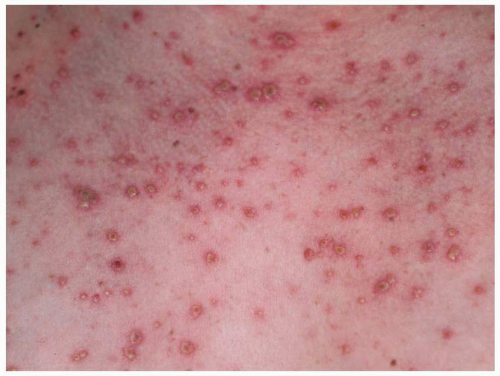
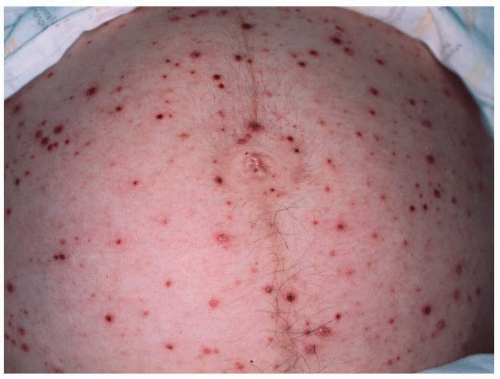
fetus is at high risk for life-threatening, neonatal varicella infection. Infants at risk are those whose mothers contract varicella within 2 days of birth or within the first 5 days after delivery. Congenital varicella infection has been reported in 10% to 20% of term infants born to mothers with varicella within 4 to 5 days of delivery, and the case fatality was 20% to 30%. Infants born 5 or more days after maternal illness onset developed either mild varicella or no infection at all.
Cutaneous scars
Musculoskeletal abnormalities
Limbs contractures, hypoplastic
Cerebral anomalies
Ventriculomegaly
Hydrocephalus
Microcephaly with polymicrogyria
Porencephaly
Ocular anomalies
Congenital cataracts, microophthalmia
Hepatic and intestinal echogenic foci
Hydrops
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree