Fig. 6.1
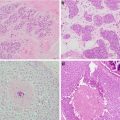
Breast ultrasound prior to placement of cryoablation probe (Courtesy of Dr. Rosa Hwang)
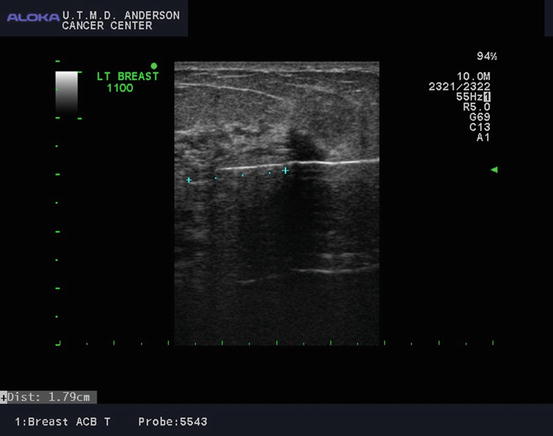
Fig. 6.2
Cryoablation probe inserted with ultrasound guidance through the center of the tumor with the tip extending past the tumor (Courtesy of Dr. Rosa Hwang)
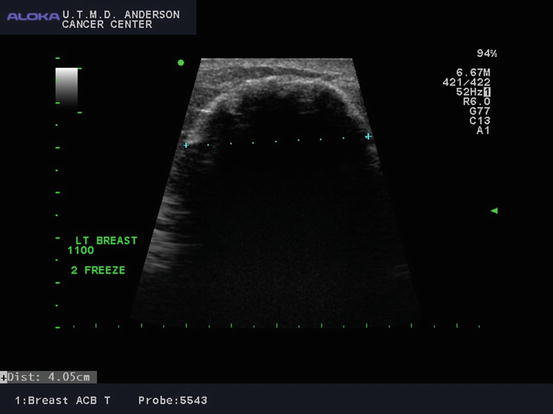
Fig. 6.3
Ultrasound image of ice ball formed after second freeze cycle with tumor cryoablation (Courtesy of Dr. Rosa Hwang)
Cryoablation and the Fibroadenoma Experience
Successful experience of cryoablating fibroadenomas provided a foundation for evaluating cryoablation to treat breast cancers. Cryoablation is Food and Drug Administration (FDA) approved for the treatment of fibroadenomas. In a study with 2.6-year follow-up, Kaufman et al. reported on 37 patients with ultrasound showing a 99% volume reduction in treated fibroadenomas with most (84%) remaining nonpalpable. Long-term results demonstrated excellent patient and physician satisfaction [11].
The larger multi-institutional FibroAdenoma Cryoablation Treatment (FACT) registry of 444 patients reported that the treatment area was palpable at 12 months in 60% of cases with high patient satisfaction (88%). Residual palpable areas were more common in lesions greater than 2 cm [10]. The fibroadenoma experience demonstrated cryoablation’s safety and provided feasibility for applying this technique for breast cancer treatment.
Cryoablation for the Treatment of Invasive Breast Cancer
The concept of cryoablation was developed in the 1800s for palliative treatment of locally advanced uterine and breast cancers using iced saline solution [7, 12]. In recent decades, it has been utilized to treat select primary and metastatic cancers in the liver [13, 14]. For breast cancer, several parameters have been identified for successful cryoablation: unifocal ductal cancer measuring less than 1.5 cm, tumor location away from the skin, discrete tumor margins on imaging, and a visible posterior wall on ultrasound for evaluating the distal active freezing zone.
Small single-institution studies of ultrasound-guided cryoablation followed by surgical excision report variable success rates. With including a larger mean tumor size of 21 mm, Pfleiderer [15] reported limited success in treating 16 invasive cancers with cryoablation followed by surgical excision 5 days later. Five tumors measuring less than 16 mm did not have a remaining invasive component, but two specimens had residual ductal carcinoma in situ (DCIS). Eleven cancers measuring at least 23 mm had incomplete ablation demonstrated on surgical pathology.
A recent study incorporating pretreatment and posttreatment breast MRI demonstrated improved ablation outcomes, likely due to improved patient selection (Figs. 6.4 and 6.5). In 20 patients with invasive ductal cancer (IDC) measuring ≤15 mm, Poplack et al. [16] reported 85% complete ablation. Posttreatment MRI had a 0% sensitivity and 88% specificity with a negative predictive value (NPV) of 83%.
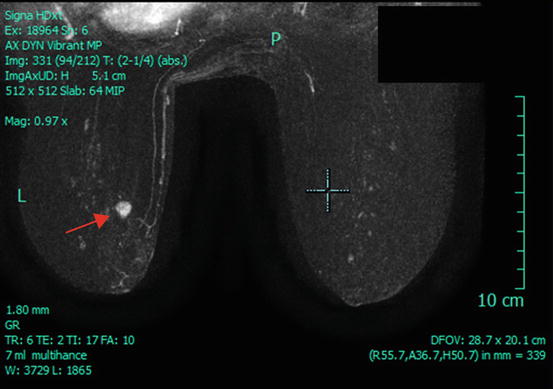
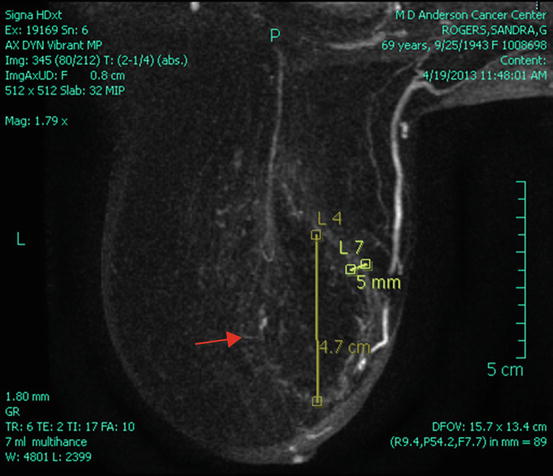

Fig. 6.4
Pre-cryoablation breast MRI demonstrating a unifocal tumor in the left breast (arrow) (Courtesy of Dr. Rosa Hwang)

Fig. 6.5
Post-cryoablation breast MRI. Red arrow marks cryoablation halo cavity; no residual enhancing mass is seen (Courtesy of Dr. Rosa Hwang)
A retrospective study preferred cryoablation when comparing it with radiofrequency ablation (RFA) for the treatment of IDC ≤2 cm in elderly patients [17]. Forty patients underwent cryoablation compared to 40 patients undergoing radiofrequency ablation with a mean age of 75 years. Post-ablation MRI was performed followed by surgical excision approximately 5 weeks later with 18-month mean follow-up. There was complete ablation in 75 patients (94%) on surgical excision, and this was accurately determined on MRI. Two patients undergoing cryoablation (95% success) and three patients undergoing RFA (93% success) had residual disease on surgical excision which was visualized on 4-week post-ablation MRI. The failures were thought to be due to incorrect positioning of the devices and inadequate necrosis area. There were no long-term cosmetic differences between the two groups, but there were two cases of skin necrosis in the RFA group. The authors preferred cryoablation given the analgesic affect from its cooling.
Studies of cryoablation without surgical excision of breast cancer are limited. However, this may become a treatment option for elderly patients with a short life expectancy. In a study of 23 patients with a median age of 85 years having invasive cancers ranging from 5 to 28 mm, there were five recurrences during the 14.6 month-median follow-up [18]. At 24-month follow-up, “complete tumor control” as evaluated on MRI was 9%. There were four hematomas and one skin burn.
Multi-institutional Cooperative Cryoablation Trial: ACOSOG Z1072
The American College of Surgeons Oncology Group (ACOSOG) Z1072 , a multi-institutional phase II trial, recently reported the effectiveness of cryoablation for treating invasive breast cancer [19]. The trial included patients with unifocal IDC ≤2 cm with a limited intraductal component (25%) and preoperative tumor enhancement on MRI. After undergoing cryoablation, patients underwent repeat breast MRI and surgical excision. Physicians were required to have a minimum of 20 ultrasound-guided procedures and 5 ultrasound-guided cryoablation procedures. Cryoablation successfully treated 66 of 87 (76%) of cancers with 16% other specimens having residual invasive cancer and 17% having residual DCIS. If multifocal disease was not defined as an ablation failure, 92% of tumors were successfully ablated. Postsurgical MRI had a negative predictive value of 81% with a 100% NPV for cancers <1.0 cm.
Cryoablation is a promising treatment for small unifocal invasive ductal cancers; however, improvements in imaging modalities for accurate identification of multifocal disease are needed to identify appropriate patients. Complications are low and include temporary site tenderness, hematoma, and skin necrosis [9, 19, 20]. A unique property of cryoablation compared to other ablation techniques is its production of antitumor immune responses. In animal models [21], it provides antigen presentation caused by pro-inflammatory cytokines. In theory, it is possible that the immune response induced by cryoablation may protect against local recurrences and possibly distant metastasis [7, 19]. Cryoablation has not been adequately evaluated as a treatment for invasive lobular carcinoma or extensive in situ disease [7, 22].
Radiofrequency Ablation
Radiofrequency ablation (RFA ) is one of the most common percutaneous techniques in clinical practice for treating hepatocellular carcinoma , renal tumors, and metastasis usually to the liver [23–26]. The feasibility of using RFA for breast cancer was first reported by Jeffrey et al. in 1999 [27] in five patients with locally advanced invasive breast cancer. Surgical resection followed RFA. Analysis by immunohistochemistry demonstrated complete cell death in 80% of the breast tumors.
RFA must be performed in the operating room and under general anesthesia. The technique uses thermal energy through high-frequency alternating currents emitted from the non-insulated tip of a needle electrode. This transmits into the adjacent breast tissue, causing ionic vibrations as the ions attempt to follow the rapidly changing direction of the alternating current. Ionic agitation results in frictional heating to temperatures exceeding 100°C. This in turn causes cell death and coagulation necrosis of the tumor. Higher temperatures cause more destruction to tumor cells and the less exposure needed. Central placement of the electrode into the cancer is also important with this technique [28–30].
In a two-center pilot study of 26 patients with T1 and T2 invasive ductal, lobular, and tubular breast cancers, there was 96% complete tumor ablation [28]. One patient (4%) had a full-thickness skin burn. Our institution’s feasibility study of more homogenous tumor pathology in 21 invasive cancers ≤2 cm (19 ductal histologies), there was complete ablation in all cases with no complications [29]. Limitations include the inability for real-time monitoring of the ablation edge to confirm complete ablation of the lesion with an adequate margin without damaging the skin.
To improve RFA temperature distribution and possible skin damage, Manenti et al. [31] utilized a cool-tip system in 34 patients with IDC ≤2 cm. Patients were evaluated with mammogram, breast ultrasound, and 3.0-T MRI before and after cool-tip RFA. Complete ablation occurred in 97% of tumors on histologic analysis, and post-ablation MRI showed no suspicious enhancement in 31 cases. Cosmesis was excellent in 28 patients, and there was a mild superficial skin burn in one patient with a tumor <1 cm to the skin edge. However, the cool-tip system also does not provide real-time monitoring.
RFA may also have a role in treating breast cancer patients who are elderly or not optimal surgical candidates [32, 33]. A recent study reported incorporating preoperative endocrine therapy in elderly patients followed by RFA. In 21 patients older than 70 years who had taken neoadjuvant endocrine therapy 6 months for cancers ≤3 cm, 1-year follow-up after RFA had one local failure [34]. None of the patients received irradiation. At 5-year follow-up of ten patients, three patients had local failures (two of the three patients had cancers with lobular histology) for an overall local recurrence rate of 19%. Four patients (19%) had skin burns, and there were four deaths during the study duration (two from cancer-related causes and two from non-cancer-related causes). Combining this study with others using preoperative endocrine therapy before RFA to evaluate outcomes of 104 patients but shorter follow-up (15–29 months), there was one local recurrence [30]. Future studies evaluating neoadjuvant endocrine therapy followed by RFA compared to endocrine therapy only in this select patient population is needed.
Additionally, a feasibility study by Klimberg et al. [35] evaluated RFA in treating the margins after vacuum-assisted excisional biopsy of breast cancer. Vacuum-assisted excisional biopsy with an 8-gauge probe has been reported to oftentimes completely remove the cancer. Leveraging the ability of large-gauge biopsies to remove the majority of a lesion, RFA (15 patients) or laser ablation (3 patients) was utilized to treat the cavity for possible residual disease at the margins. MRI was incorporated pre-procedure and after vacuum-assisted excisional biopsy. If residual or multicentric disease was noted on MRI, then patients underwent standard of care and were not included in the study. Otherwise, patients underwent excision as part of the study. Laser ablation was discontinued given unpredictability to create a consistent ablation area. Of the 15 patients undergoing RFA, all had successful complete ablation except one patient who had a 1-mm focus of atypia/DCIS distant to the immediate ablation field. Seven patients had no residual cancer on segmental mastectomy after vacuum-assisted excisional biopsy, and the other eight patients had nonviable tumor remaining at the tumor site.
Limitations of RFA
Due to the high variability of breast tissue density, the use of RFA has proven to be challenging. Oftentimes, the tissue surrounding breast masses is not homogenous and can cause differences in conductivity. Tissue impedance is affected leading to variation in treatment times to achieve total tumor ablation. This variation can lead to uncertainty in assessing the extent of tumor ablation [30].
The distance between the tumor, the skin, and chest wall should be at least 1 cm as RFA can cause skin or pectoralis muscle burns. In cases with borderline skin and chest wall distances, caution can be taken by subcutaneously injecting sterile water to avoid energy transmission to adjacent structures, applying lateral compression of the breast during the ablation procedure or ice cooling [29, 36]. Patients who undergo RFA must be counseled that a postprocedure palpable mass may remain even if they did not present with a palpable mass [31, 34]. A review of RFA studies reports a histologically confirmed successful ablation in 76–100% of studies with 1% pneumothorax, 5% skin burns, and 5% pectoralis muscle burns [30].
Percutaneous Microwave Coagulation
Percutaneous microwave coagulation (PMC ) for the treatment of uterine fibroids and liver tumors [37, 38] led to the limited evaluation of percutaneous microwave coagulation (PMC) for the treatment of early breast cancers. PMC offers shorter ablation times, higher temperatures, and preferential heating of the cancer compared to normal breast tissue given that cancer has a higher water content. In a study of 41 patients with invasive ductal cancer clinically estimated to measure ≤3 cm, 95% of cases had complete coagulation on surgical pathology [39]. However, mean tumor volume was 5 cm3 with a range of 0.09–14.14 cm3. The mean coagulation time was 4.48 min. In three patients, a small epidermal burn or slight thermal injury to the pectoralis muscle occurred. Multi-institutional studies with long-term recurrence outcomes are not available.
High-Intensity Focused Ultrasound
There is less experience with high-intensity focused ultrasound (HIFU) utilizing ultrasound or MRI guidance for breast cancer ablation. HIFU has also been used in liver, prostate, kidney, and brain cancers [40]. An ultrasound beam transmits a pressure wave that produces temperature increases resulting in targeted coagulation necrosis and protein denaturation without harming adjacent tissue. In a meta-analysis of seven studies each having 6–28 patients, complete ablation was found in 46% of patients and near complete ablation (defined as less than 10% residual tumor) in 30% of patients. Treatment times were long, ranging from 78 to 171 min. Complications included pain (40%), skin burns (4%), and residual edema at the cancer site (17%). Further work to improve HIFU’s ablation on histology and time are needed before becoming an option for routine clinical use.
Laser Ablation
Laser ablation is performed under local anesthetic by inserting the laser needle and a temperature sensing probe. The laser power is increased until the temperature probe reaches 60 °C. In a study of 54 patients (50 invasive and 4 in situ) [41], complete ablation was 70% with treatment time ranging 5–30 min with a median of 5900 J of laser energy used for median tumor size of 13 mm. These results included an initial learning and implementation period. After the learning period, the last 28 patients had 93–100% complete ablation. Continuous monitoring of the temperature was used to determine when adequate ablation was achieved as real-time imaging does not show tumor ablation changes. No significant complications were noted. Patients reported temporary sensitivity at the ablation site.
In a smaller study of 14 patients with T1 and T2 tumors [42], laser completely ablated 50% of tumors determined by NAD staining. Tumors less than 2 cm had higher complete ablation (88%). There was one skin burn and one small pneumothorax treated conservatively. Laser ablation’s limitations and results from these and other small studies have limited further widespread evaluation of it as an option for percutaneous treatment of breast cancer.
The Role of Breast MRI in Percutaneous Ablation
In addition to mammogram and ultrasound , breast MRI has been utilized to determine patient eligibility by evaluating disease extent and the presence of multicentric disease and also to determine residual disease after ablation treatment [7, 19]. In a small study of 14 patients, Vilar et al. [43] compared tumor dimension on breast ultrasound with MRI before RFA and also correlated surgical pathology to a post-ablation MRI. Pre-RFA MRI measurements showed a statistically significant larger tumor volume compared to ultrasound in 80% of cases. After RFA, MRI accurately demonstrated no residual disease in all cases. In those cases with residual tumor remaining after RFA, MRI accurately measured the tumor dimensions compared to surgical pathology dimensions. In a similar study of 15 patients with invasive ductal cancer ≤15 mm [16], cryoablation was successful in 85% of the cases. MRI was performed with mammogram and ultrasound before ablation, and a repeat MRI was done 25–40 days after ablation, followed by surgical excision. MRI did not detect residual cancer at the ablation site in all three patients (sensitivity 0%) and had a specificity of 88%. The NPV was 83%.
In ACOSOG Z1072 , tumor size ≤1 cm was associated with no residual MRI enhancement in 100% of cryoablation cases. However, for size >1 cm, 77% had complete ablation with no residual enhancement on MRI. MRI had a similar NPV of 81% for detecting residual disease after cryoablation [19]. Improvements in image detection of multifocal disease and extensive intraductal component are still needed.
Additional Considerations
Histopathologic confirmation with receptor subtyping should be obtained by core needle biopsy prior to ablation. Percutaneous ablation has not been adequately studied and should be used under study in patients who received neoadjuvant chemotherapy as viable tumor cells may still be present beyond the tumor compromising the ability to obtain local control. Percutaneous ablation techniques are also contraindicated in patients with extensive DCIS or lobular carcinoma. Physicians performing percutaneous ablation of breast cancers should have adequate experience in performing image-guided procedures [22, 30].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree