LRR (10 years)
Any first recurrence (10 years)
Breast cancer mortality (20 years)
No RT
RT
Abs
No RT
RT
Abs
No RT
RT
Abs
N = 1314, 1–3+ ALN
20.3
3.8
16.5
45.7
42.3
3.4
50.2
42.3
7.9
N = 1133, 1–3+ ALN
21.0
4.3
16.7
45.5
33.8
11.7
49.4
41.5
7.9
N = 177, ≥ 4+ ALN
32.1
13
19
75.1
66.3
8.8
80.0
70.7
9.3
The absolute difference of any first recurrence that radiation provides in case of these patients with chemotherapy was larger than the larger subset of 1–3 pN+ patients, also suggesting that the effect of local-regional radiation impacting distant disease may work in conjunction with patients receiving chemotherapy . Although the data for PMRT in 1–3 LN+ patients is convincing, there is currently no consensus statement to recommend radiation treatment for all patients who fall within this subset, especially patients with solitary micrometastatic disease (Table10.2).
Table 10.2
Anatomic borders ofbreast nodal volumes
– | Superior | Inferior | Anterior | Posterior | Lateral | Medial |
|---|---|---|---|---|---|---|
Supraclavicular | Inferior border of the cricoid cartilage | Head of the clavicle | Sternocleidomastoid (SCM) muscle | Anterior aspect of the scalene muscle | Superior: lateral edge of SCM inferior: junction of the first rib and clavicle | Lateral border of thyroid and trachea |
Axillary Lv. III | Insertion of pec. minor in the coracoid process of scapula | Axillary artery crosses medial edge of pec. minor | Posterior surface of pec. major | Ribs and intercostal muscles | Medial border of pec. minor | Thoracic inlet |
Axillary Lv. II | Axillary artery crosses medial edge of pec. minor | Axillary artery crosses lateral edge of pec. minor | Posterior surface of pec. major (volume includes all of pec. minor) | Ribs and intercostal muscles | Lateral border of pec. minor | Medial border of pec. minor |
Axillary Lv. I | Axillary artery crosses lateral edge of pec. minor | Insertion of pec. major into ribs | Plane defined by the anterior surface of pec. major and lat. dorsi | Anterior surface of subscapularis m | Medial border of lat. dorsi m | Lateral border of pec. minor m |
Internal mammary | Superior aspect of the 1st rib | Superior aspect of the 4th rib | Encompasses both IM artery and vein | |||
However, patients who have high-risk features, especially triple negative disease, have been shown by prospective randomized evidence to have a substantial RFS and OS benefit with adjuvant therapy and regional nodal radiation therapy after mastectomy compared with adjuvant chemotherapy after mastectomy alone without a significant change in the toxicity profile [54]. After a median follow-up of 86.5 months, 5-year RFS rates were 88.3% vs. 74.6% for adjuvant chemotherapy plus radiation and adjuvant chemotherapy alone, respectively (HR 0.77,p = 0.02). Five-year OS also improved 90.4% vs. 78.7% in favor of radiation (HR 0.79,p = 0.03).
Neoadjuvant Chemotherapy and Adjuvant Radiation
For patients with non-resectable and advanced primary tumors, neoadjuvant chemotherapy use has increased in the recent years because of the potential to downstage disease. Nearly 80% of patients on neoadjuvant chemotherapy experience a decrease in tumor size [55]. Further, the subset of these patients who obtain a complete pathological response also experiences the lowest risk of local-regional failure, substantiating the idea that pathologic response to chemotherapy can also influence the use of adjuvant and regional nodal irradiation.
Two NSABP trials (B-18 and B-27) addressed these issues by randomizing patients with stages T1-3 and N0-1 breast cancer to either neoadjuvant or postoperative chemotherapy [56]. In NSABP B-18, neoadjuvant doxorubicin and cyclophosphamide (AC) demonstrated an objective clinical response in 79% of patients, of which 43% was a clinical partial response (cPR) and 36% was a clinical complete response (cCR). Thirteen percent had a pathologic complete response (pCR). Preoperative chemotherapy patients also experienced an increased incidence of pathologically negative axillary nodes compared with postoperative chemotherapy patients (58% vs. 42%, respectively;p < 0.0001). NSABP B-27 showed that the addition of docetaxel to AC preoperatively increased clinical response rates from 86 to 91% (p < 0.001), cCR from 40 to 63% (p < 0.001), and pCR from 13 to 26% (p < 0.001) [57].
Although neither study showed a statistically significant survival advantage with either arms, certain subsets of patients benefit from neoadjuvant chemotherapy. Patients who experience a pCR, for example, experience significant OS (HR = 0.32,p < 0.0001) and DFS (HR = 0.47,p < 0.0001) benefits compared to those who have a partial or no response. This is in part due to cPR patients having the lowest risk of local-regional recurrence. Similarly, pathologic nodal status by number of lymph nodes involved at the time of surgery was also an excellent indicator of OS and DFS. Node-positive patients who received neoadjuvant chemotherapy followed by mastectomy and found have residual disease exhibit recurrence rates from 11 to 22%. Node-positive patients who have a cPR have a local-regional recurrence rate of 0%.
Regarding age, women less than 50 years of age seemed to benefit the most from preoperative chemotherapy. This is likely because younger women are more likely to have receptor-negative disease, which has been shown to have a better pathologic response to early initiation of adjuvant systemic therapy than receptor-positive tumors [58]. A small series has shown that patients whose tumors were ER negative were more likely to achieve a pCR than patients who were ER positive (21.6% vs. 8.1%,p < 0.001) [59]. Women who are 50 years of age or older, however, had better outcomes with postoperative chemotherapy. This is most likely because the delay in delivering hormonal therapy with receptor positive tumors negatively impacts survival (Figs.10.1 and10.2).
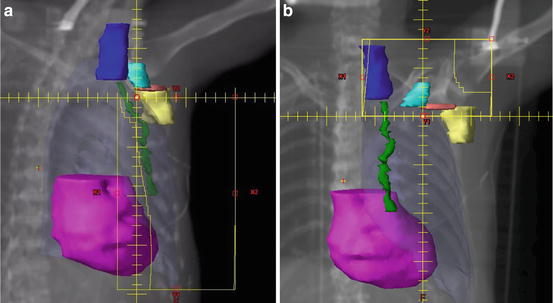
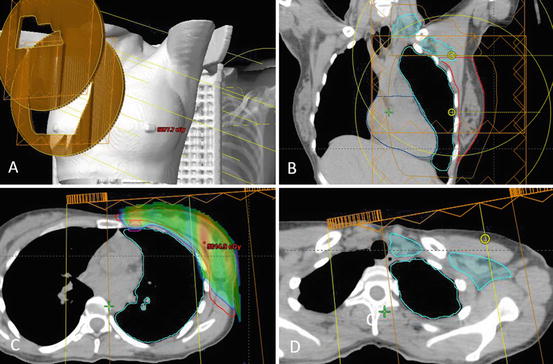

Fig. 10.1
(a,b) Comprehensive nodal irradiation involves coverage of the axillary level I (yellow), level II (pink), level III (teal), supraclavicular (dark blue), and internal mammary (green) lymph nodes typically with a three-field technique. The first two tangential fields are half beam blocked to avoid overlap with the third supraclavicular field. Field angling and multi-leaf collimators are used to minimize heart (magenta), lung (pale blue), spinal cord, and humeral head doses

Fig. 10.2
Comprehensive nodal irradiation with proton therapy involves a supraclavicular field and a primary field (a,b), which cover the PTV breast (red), internal mammary nodes (magenta) (c), and supraclavicular and axillary lymph nodes I–III (teal) (d). The dose delivered spares the majority of the lung and heart while providing adequate PTV coverage. The dose wash border represents 95% of total dose
Unfortunately, the data that are available for treating locally advanced breast cancer after neoadjuvant chemotherapy are mostly small single-arm institutional analyses. Huang et al. conducted a pooled analysis of 542 patients treated on six consecutive institutional prospective trials with neoadjuvant chemotherapy and showed that PMRT reduced local-regional recurrence for patients with clinical T3 or T4 tumors, pathological tumor size >2 cm, or four or more positive LNs [60]. Similarly, PMRT improved cause-specific survival (CSS) patients with stage ≥ IIIB disease, clinical T4 tumors, and seven or more positive nodes. Among the 20% of patients who achieved a pCR, PMRT still had a significant impact on LRR rates, with 10-year LRR rates improving from 33 to 3% (p = 0.006). On multivariate analysis, the lack of radiation had the largest 10-year LRR hazard of 4.68 (p < 0.0001), more so than stage ≥IIIB (HR = 2.38,p = 0.001) or minimal or worse clinical response to chemotherapy (HR = 1.88,p = 0.021).
Therefore, adjuvant radiation may be beneficial especially for locally advanced tumors with high nodal burden. Currently, randomized phase III studies are being conducted to determine the need for PMRT and regional nodal irradiation specifically in early-stage pathologic node-positive breast cancers. NSABP B-51 is currently enrolling those with a complete nodal response to neoadjuvant chemotherapy to either WBI with RNI vs. WBI without RNI if patients underwent alumpectomy or PMRT with RNI vs. no additional radiation if patients underwent a mastectomy. In patients who remain node positive on intraoperative SLNB , the Alliance A011202 trial randomizes similar patients to either ALND and RNI (without RT to the dissected axilla) or RNI covering all lymph node basins. Both trials are currently enrolling patients and the data is not currently available .
Conclusion
Nodal management in breast cancer patients has rapidly evolved over the last decade to include minimally invasive diagnostic procedures such as SLNB instead of the more morbid ALND as well as comprehensive nodal irradiation. While nodal irradiation in patients with 1-3 lymph nodes is controversial in the post-mastectomy, high-risk node-positive patients have been shown to benefit from comprehensive nodal irradiation including the internal mammary nodes.
References
1.
2.
3.
Hughes KS, Schnaper LA, Bellon JR, Cirrincione CT, Berry DA, McCormick B, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31(19):2382–7.CrossrefPubMedPubMedCentral
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree