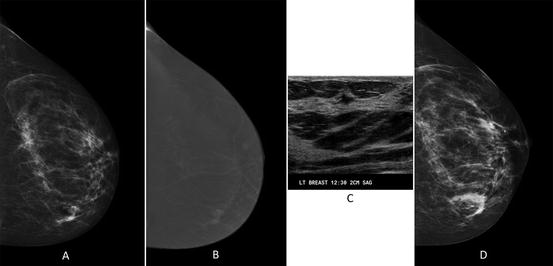
Fig. 1.1
Breast tissue patterns according to the fifth edition of the BIRADS lexicon. (a) Predominantly fatty, (b) scattered fibroglandular, (c) heterogeneously dense, and (d) extremely dense
At present, although controversy persists surrounding the optimal screening interval, mammography remains the only modality that has been proven to reduce mortality from breast cancer by up to 30% [6]. The controversy surrounding screening stems from concerns over the number of false positives, especially in younger women with dense tissue, and the potential of overdiagnosis of ductal carcinoma in situ (DCIS) . Both of these concerns do warrant an informed discussion with every woman, but given that there is no other proven test for early detection of breast cancer, nearly all women still opt to participate in screening. In addition, given the mortality benefit, many major medical organizations continue to recommend annual screening mammography starting at the age of 40 years for the average-risk woman and earlier if the patient is deemed to be at elevated risk.
With sensitivity of approximately 90% and specificity of 89% for the average breast (scattered fibroglandular tissue), the presence of dense breast tissue (heterogeneously dense or extremely dense) lowers the sensitivity of mammography to as low as 62–68% on film-screen but this increases to approximately 83% with digital techniques [7–9]. As mammographic sensitivity is lower in women with dense breast tissue, practice is evolving on how best to screen these women, as approximately 40–50% of the US population is known to have dense breast tissue. A recent change in the BIRADS lexicon on how breast density is characterized also likely will lead to an even greater percentage of women being characterized as “dense” as the current edition recommends that any patch of dense tissue be classified as “dense” [5]. In addition, it is important to recognize that in most practices, breast density is subjectively assessed and that there is considerable inter-reader variability, especially for a majority of women (those who either have scattered fibroglandular tissue or heterogeneously dense tissue) [7, 10–12]. Breast density can also be affected by patient age, parity, hormone replacement therapy, body mass index, and genetic predisposition [13–15]. For women with dense tissue, the multicenter prospective DMIST study of 49,528 women showed that screening with digital mammography increased cancer detection by women with heterogeneously dense or extremely dense breasts on mammography (difference, 0.11; 95% confidence interval, 0.04–0.18; P = 0.003) [16] (Fig. 1.2). Consequently, women with dense tissue ideally should be imaged using digital equipment whenever possible. Fortunately, in 2016, greater than 95% of accredited imaging facilities have digital technology [17].
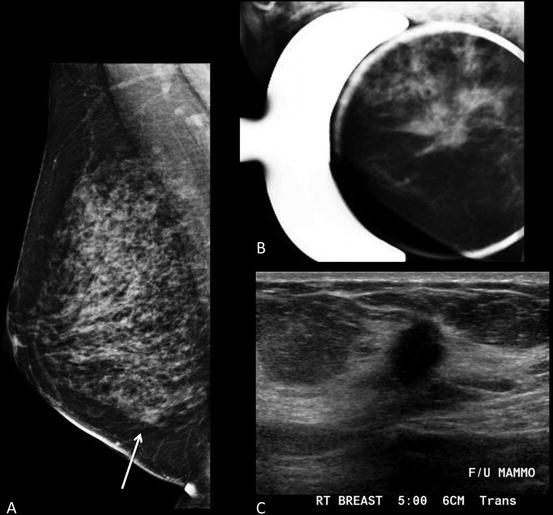

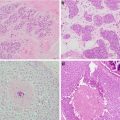
Fig. 1.2
Forty-six-year-old woman with 2 cm mass in the lower inner right breast on screening mammography . (a) MLO view shows an asymmetry inferiorly. (b) Spot compression MLO view confirms an irregular mass with distortion. (c) Ultrasound shows an irregular hypoechoic shadowing mass. Biopsy revealed grade 2 invasive lobular carcinoma
However, mammography has other limitations. Given that up to 10% of women may be recalled for additional imaging for a majority of findings that ultimately are dismissed or are benign, there is a continued need to improve this technology, that is, increase cancer detection while decrease the recall rate from screening. At present, with conventional mammographic imaging, superimposed or overlapping breast tissue on one of the 2D images is the leading cause for recall from screening, which is more common in women with dense breasts [18, 19].
Digital breast tomosynthesis (DBT) is a newer modality that can overcome some of the current limitations of conventional digital mammography [19]. First approved by the Food and Drug Administration (FDA) in 2011, this technology acquires both an arc of projection images that are reconstructed into a pseudo-3D dataset and conventional images (either as a separate acquisition or reconstructed from the 3D dataset). When acquired as a separate acquisition, combining DBT with conventional digital mammography comes with at least two times the radiation dose to the patient as compared to digital mammography alone. However, DBT has substantial advantages as the interpreting radiologist can scroll through the 3D dataset almost entirely eliminating overlapping breast tissue [20–22]. In addition, in several recent studies, when combined with digital mammography, digital breast tomosynthesis has been shown to decrease recall rates and improve detection of invasive breast cancers as compared to digital mammography alone (Fig. 1.3) [23–26]. A recent observational study by Rose et al. where DBT was integrated into a screening practice resulted in significant decreases in recall rates from 8.7 to 5.5% (p < 0.001) with increase in detection of invasive cancer from 2.8 to 4.3 per 1000 screening examinations and an increase in the positive predictive value for recalls from 4.7 to 10.1% (p < 0.001) [24]. Sharpe et al. evaluated 85,852 patients in a prospective study and demonstrated that DBT was associated with a 54.3% increase in the cancer detection rate compared with 2D mammography (3.5–5.4 per 1000; absolute change, +1.9 per 1000; relative change, +54.3%; P < 0.018) [18].
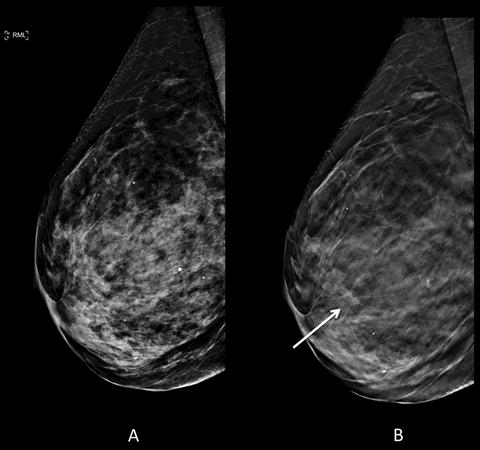

Fig. 1.3
Seventy-six-year-old woman with dense breast parenchyma for annual full-field digital mammogram with digital breast tomosynthesis (DBT) . (a) Conventional 2D lateral view is negative for malignancy. (b) Architectural distortion (arrow) is identified only on DBT image. A mass was seen on subsequent ultrasound (not shown) with subsequent biopsy pathology of grade 1 invasive ductal carcinoma
Although DBT clearly adds value in the screening setting, it also is helpful in the diagnostic work-up of patients with suspicious findings on clinical exam or those recalled from screening. This is particularly true in women with scattered fibroglandular tissue or heterogeneously dense breast tissue, as DBT can detect additional suspicious areas in women with newly diagnosed cancer or provide reassurance that the area of concern is benign or not clinically significant, resulting in fewer biopsies and specifically, fewer false-positive biopsies [27–30]. Digital breast tomosynthesis is particularly useful in characterizing margins of masses that are otherwise obscured by overlapping breast tissue on conventional imaging [31, 32]. Consequently, imaging protocols continue to evolve. For example, in most centers where a majority of women undergo screening using both digital mammography and DBT, if a circumscribed mass is seen on DBT, the patient is recalled for ultrasound only, thereby frequently avoiding any further diagnostic mammographic views and associated radiation (Fig. 1.4). While DBT considerably improves cancer detection and lowers recall rates, it remains an imperfect modality, particularly for women with extremely dense tissue. In order to detect malignancy, DBT relies on interfaces between the glandular tissue and fat , which is essentially non-existent in this subgroup of women [12].
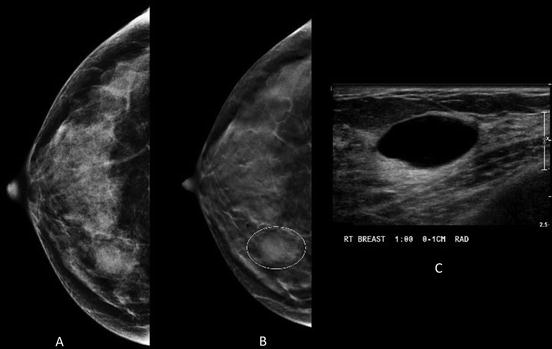

Fig. 1.4
Forty-three-year-old woman with obscured mass in the inner right breast on conventional MLO view (a) Tomosynthesis reveals circumscribed margins (b) Instead of additional diagnostic mammographic views to better characterize the margins, the patient was recalled to undergo ultrasound that revealed a simple cyst (c)
Ultrasound and Elastography
Ultrasound uses sound waves to image the breast tissue and therefore comes with no radiation exposure to the patient. In most circumstances, breast ultrasound serves to complement mammography as a diagnostic tool for characterization of breast findings on both clinical exam and imaging. Breast ultrasound is mainly used to differentiate cystic from solid masses and can often classify benign from malignant masses for some women [33]. Ultrasound guidance for percutaneous core needle biopsy expedites the diagnosis of breast malignancy or confirms a benign diagnosis. In addition, image-guided biopsy streamlines the management of women eventually diagnosed with breast cancer by providing improved preoperative planning with diagnostic accuracy comparable to an open surgical biopsy [34].
One limitation of breast ultrasound, however, is the inconsistent differentiation of benign from malignant lesions. While not widely available, some breast imaging practices are beginning to incorporate breast elastography into diagnostic imaging. This technique can be performed at the time of the diagnostic breast ultrasound and provides information about tissue stiffness [35–37]. Typically, invasive cancers are “stiffer” or less elastic than normal breast tissue or even benign breast lesions [36, 38]. By incorporating this technique into diagnostic imaging, it has the potential to improve ultrasound specificity and decrease the number of benign breast biopsies (Fig. 1.5) [37–39].
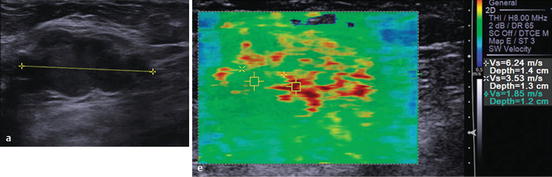

Fig. 1.5
Elastography showing that the solid macrolobulated mass seen on gray-scale ultrasound (left image) has high velocities highly suspicious for malignancy (right image). Biopsy confirmed invasive ductal carcinoma (Reprinted with permission from Ref. [80], Richard G. Barr, Breast Elastography. Thieme Publishers Inc. 2014)
More recent studies suggest that ultrasound of the whole breast can be performed to screen high-risk women and women with dense breast tissue with several studies showing that ultrasound can detect mammographically occult malignancy [40–42]. A study of 13,547 women undergoing screening mammography and ultrasound showed that with the addition of screening ultrasound, for all women, sensitivity for breast cancer detection increased from 74.7 to 97.3%, and in the cohort of women with extremely dense breast tissue, the sensitivity increased from 47.6 to 76.1% [8]. As a result of this data, some advocate for supplemental ultrasound screening of all women with dense breast tissue. However, the incremental cancer detection is 0.3–14.0 cancers/1000 women screened depending on the patient’s risk status (being closer to 4–7/1000 if high risk and closer to 2/1000 if low or average risk) [43]. In addition, supplemental screening with ultrasound leads to substantial false positives with positive biopsy rates less than 10% [41–44]. Therefore, not all clinicians advocate for screening with this modality. In fact, based on current evidence and weighing the risks and benefits of the various available modalities, if a patient is considered high risk, MRI is the preferred supplemental screening modality (discussed in further detail below), whereas if the patient is low or average risk, digital mammography with DBT is advisable [45].
Breast Magnetic Resonance Imaging
Magnetic resonance imaging of the breast is an imaging technique that uses a high-field magnet to image the breast tissue and demonstrates high sensitivity and specificity for breast cancer [46]. During the study, which is most commonly performed on a 1.5-Tesla magnet, the patient lies prone with the breasts pendent within a dedicated imaging coil. After acquiring some non-contrast images, intravenous contrast is administered (unless the study is only being performed to assess silicone implant integrity) to highlight the neo-vascularity of the tissue, and dynamic imaging is done for approximately 5–6 min after contrast injection. Since MRI highlights contrast enhancement, breast tissue density is less relevant in limiting sensitivity, as compared to mammography. In order to minimize background parenchymal enhancement (marked background enhancement does lower detection of DCIS and invasive lobular cancers) and minimize false positives (highest in perimenopausal women), the ideal time for breast MRI is between days 5 and 15 of the menstrual cycle, as the uptake of intravenous contrast is affected by hormonal fluctuations. However, in newly diagnosed cancer patients, it is not always possible to time the study accordingly. Therefore, when patients are imaged outside of this window, there is a greater chance of a false positive. Given that MRI does carry the risk of false positives, it is important to obtain histopathologic confirmation via MRI-guided breast biopsy for any finding prior to finalizing management recommendations.
Currently, breast MRI is utilized for screening as well as breast cancer diagnosis and staging. Clinical trials from the USA and Europe have demonstrated that MRI can significantly improve cancer detection that is otherwise clinically, mammographically, and sonographically occult [42, 47, 48], particularly in women at elevated lifetime risk for breast cancer. According to the American College of Radiology (ACR) Appropriateness Criteria, current indications for breast MRI include evaluation of silicone implant integrity, screening of high-risk patients (defined as women with greater than 20% lifetime risk), defining extent of disease in women with newly diagnosed breast cancer or women undergoing neoadjuvant chemotherapy, evaluation for recurrence, identification of an unknown primary in woman with metastatic disease, and problem solving of incompletely characterized imaging findings on other modalities [49].
Several studies, primarily in high-risk populations, have demonstrated that MRI detects an additional 3.5–28.6 cancers/1000 screened as compared to screening mammography alone [42, 50–59]. One of the larger studies, ACRIN 6666, which included women who were of intermediate to high risk and some of whom had dense breast tissue yielded 14.7 additional cancers per 1000 women screened [42].
Breast MRI can also be useful in defining extent of disease, including the presence of multifocal and multicentric disease in patients with known malignancy, especially in those with dense tissue. Multiple clinical studies have shown that on average, MRI reveals occult disease in the ipsilateral breast in approximately 15% (range 12–27%) and disease in the contralateral breast in 4% (range 3–24%) [60, 61]. MRI has also been shown to be particularly useful in determining the extent of disease in women with invasive lobular cancer, as this subtype is often underestimated by mammography and physical examination [62, 63]. Finally, as breast MRI visualizes the chest wall, surrounding tissues, and axilla, it also can assess chest wall invasion and axillary nodal involvement [60, 61].
Another potential indication for breast MRI is in the evaluation of residual disease in patients who have close or positive pathologic margins prior to re-excision. In patients with locally advanced breast cancer who undergo neoadjuvant chemotherapy prior to definitive surgical treatment, MRI can assist with assessing response to therapy and at times can help guide the choice of chemotherapeutic regimen (Fig. 1.6) [61, 64]. Multiple studies have shown that MR is more accurate than mammography, ultrasound, or physical examination in determining residual disease after therapy [64]. However, if utilized for this indication, it is critical that MRI be performed prior to the start of chemotherapy and subsequent to therapy, either after completion of part or the entire regimen.
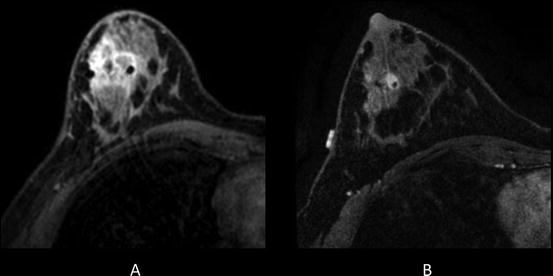

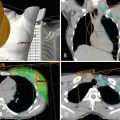
Fig. 1.6
Thirty-one-year-old woman who presented with right breast 3 cm palpable lump. Ultrasound guided biopsy confirmed grade 3 invasive ductal carcinoma. At the time of biopsy, a suspicious axillary node was identified and a fine needle aspiration confirmed metastatic disease. The patient subsequently underwent breast MRI to determine the extent of disease. (a) Pretreatment MRI confirmed extensive disease in the right breast and several suspicious right axillary lymph nodes. The patient then underwent neoadjuvant chemotherapy. (b) Posttreatment MRI showed partial response to therapy
In an effort to better differentiate benign from malignant lesions on MRI, some centers now routinely utilize diffusion-weighted imaging (DWI) , a specialized MR sequence that assesses alterations in water movement across cell membranes [65]. Using this acquisition, a quantitative map of water diffusion can be created (apparent diffusion coefficient (ADC) map) which can be used to help classify an enhancing lesion as more likely benign (high ADC value) or malignant (low ADC value) (Fig. 1.7). Smaller-range region of interest focused on highest signal is optimal for measurements. In recent studies, the threshold range for discriminating benign from malignant is approximately 1.1–1.2 × 10−3 mm2/s with associate sensitivity of 82.8–92.8% and specificity of 80.2–90% [66, 67]. In addition to characterizing focal lesions, the change in ADC value has been shown to correlate with tumor response from neoadjuvant chemotherapy and therefore may aid in guiding therapy [68, 69].
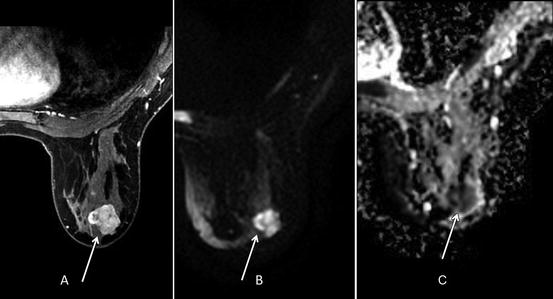

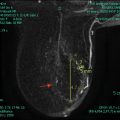
Fig. 1.7
Thirty-nine-year-old woman with biopsy proven right breast retroareolar triple-negative grade 3 invasive ductal carcinoma who underwent MRI to evaluate extent of disease. (a) Post-contrast VIBRANT sequence demonstrates 2.6-cm-irregular-enhancing mass corresponding to known malignancy. (b) Hyperintense signal in mass on diffusion-weighted imaging (DWI). (c) Hypointense signal in mass on apparent diffusion coefficient (ADC) represents restricted diffusion with ADC average of 0.765 × 10−3 mm2/s (less than 1.1–1.2 × 10−3 mm2/s suspicious for malignancy)
Finally, as MR technology continues to advance, it is now possible to perform an ultrafast MR acquisition, which is comprised of high-temporal-resolution and 3D whole-breast images. Performed on a 3-Tesla magnet (not widely available in the USA), this dynamic contrast-enhanced MR protocol consists of standard and contrast-enhanced ultrafast images. A retrospective study of 60 patients with 33 malignancies using this technique revealed statistically significant difference in enhancement rate and kinetic features between benign and malignant lesions [70]. Therefore, this technique has the potential to decrease the false positives of breast MRI thereby reducing the number of benign biopsies in women being imaged with MRI.
Emerging Technologies: Contrast-Enhanced Mammography
Contrast-enhanced mammography (CEM) is a promising tool currently FDA-approved for diagnostic imaging. At a radiation dose similar to a conventional mammogram, this technology acquires a low-energy and high-energy mammographic image centered at the k-edge of iodine following intravenous administration of an iodinated contrast agent. The low-energy acquisition is comparable to a conventional mammogram and subtraction of the two acquisitions highlights tumor neo-vascularity. Early studies in the diagnostic setting have shown that CEM is more sensitive than conventional mammography [71–73] and has a sensitivity of 96–100%, which is comparable to MRI [74, 75]. When used to determine extent of disease in a newly diagnosed cancer patient, CEM is slightly less sensitive than MRI but it comes with fewer false positives, that is, better specificity (Fig. 1.8) [76, 77]. Given these results, it seems reasonable to use this modality for patients with a newly diagnosed cancer who are unable to undergo MRI due to severe claustrophobia, gadolinium allergy, or presence of internal ferromagnetic material in order to determine the extent of disease. In addition, based on an early study of 26 patients with mammographic or clinical findings warranting biopsy, of which 13 ultimately were proven to be invasive cancers, CEM detected all malignancies and accurately identified disease extent [78]. CEM may also have a role in problem solving of incompletely characterized findings on mammography or ultrasound, detection of mammographically occult findings, and assessment of recurrence [76].