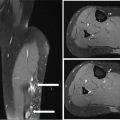
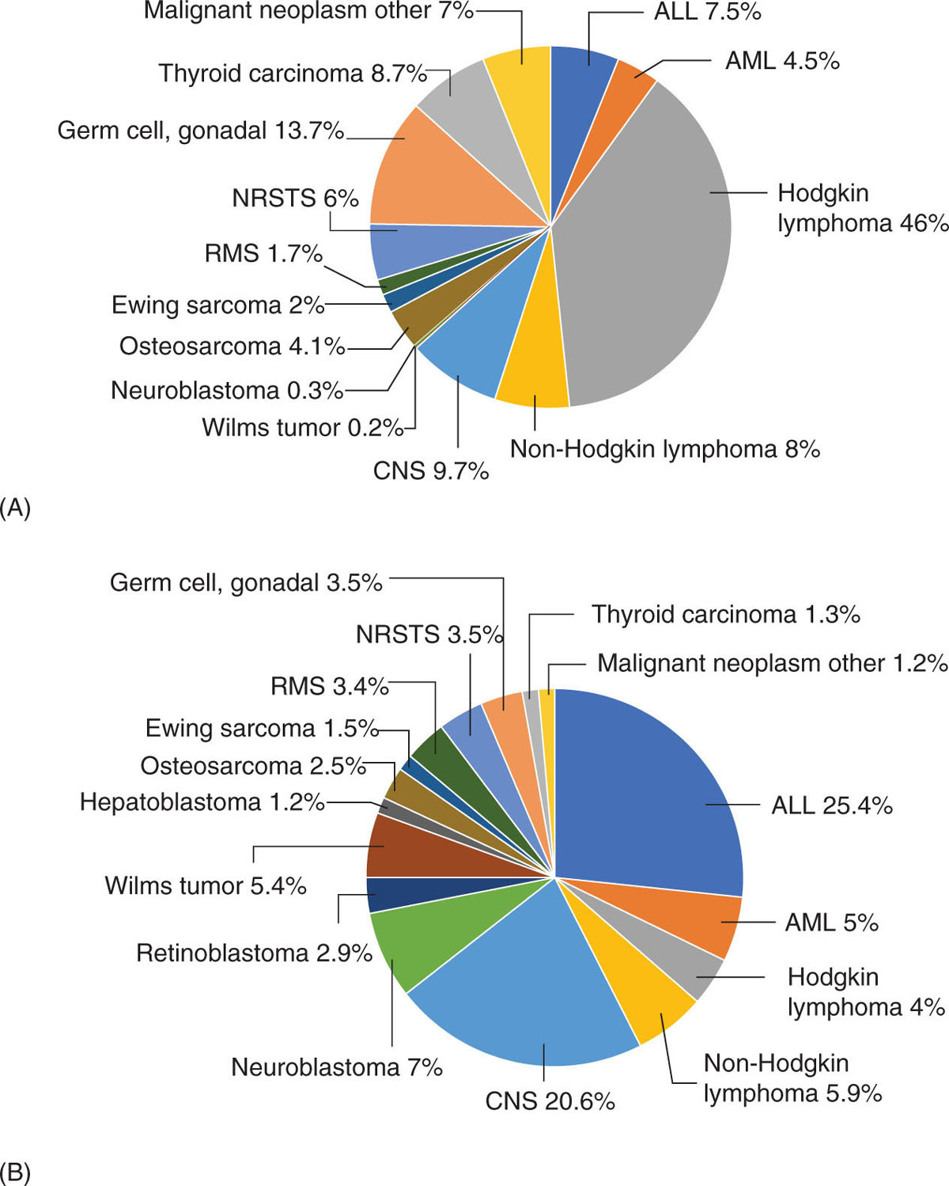
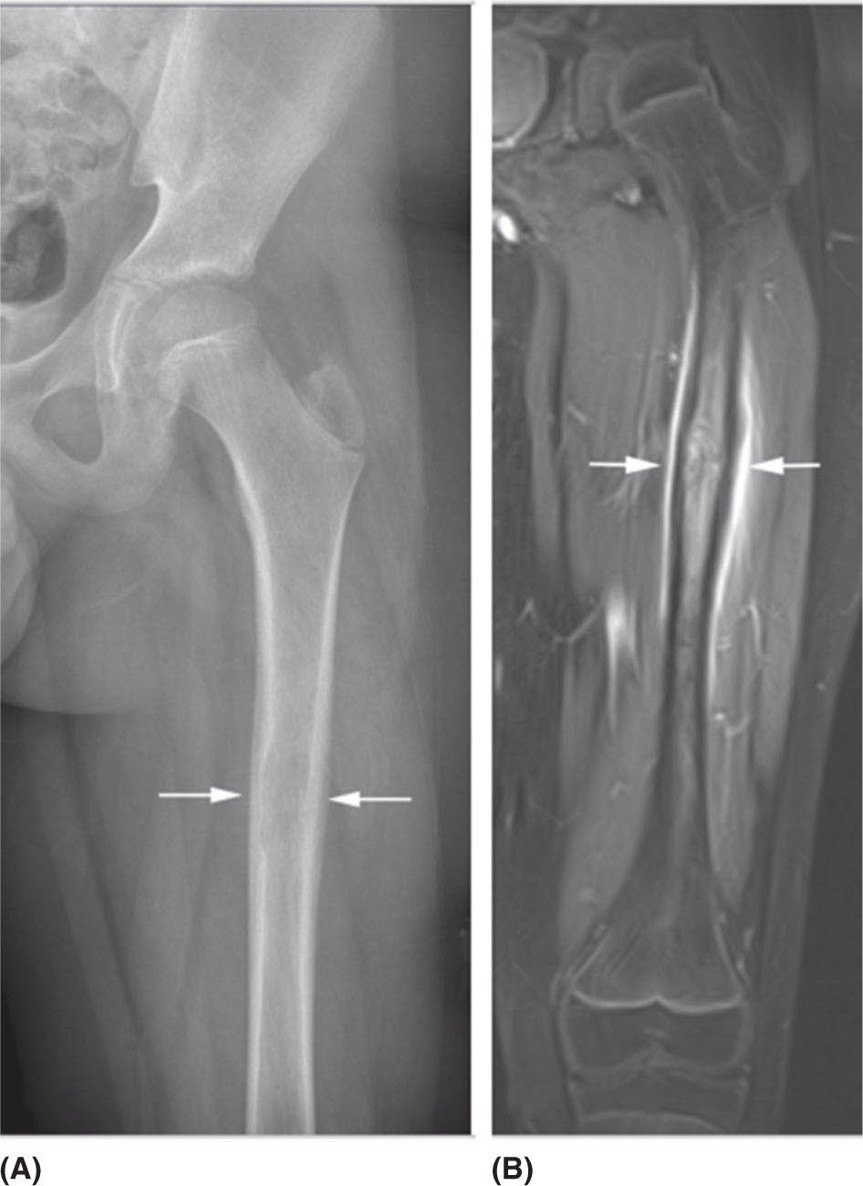
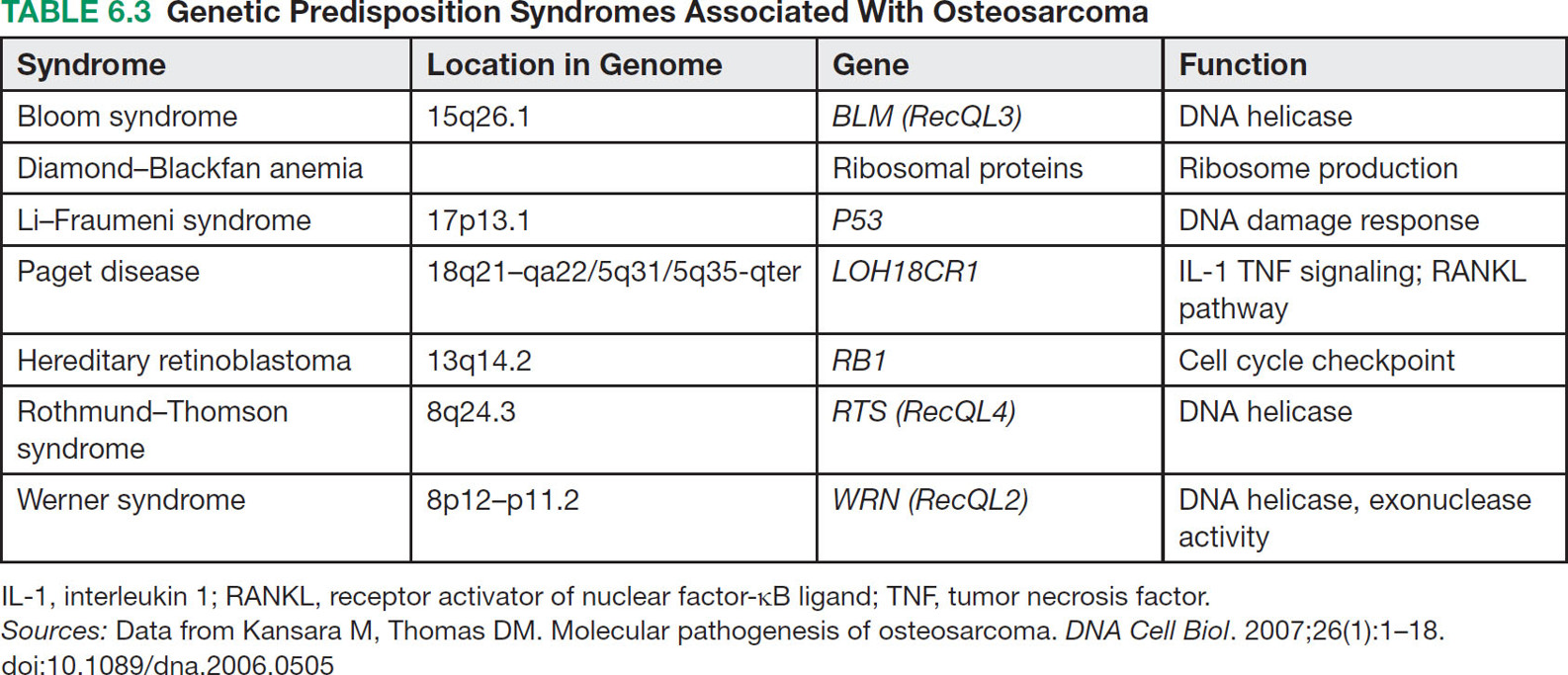
676 Pediatric Oncology Pediatric sarcomas are a heterogeneous group of diseases that can arise in bone or soft tissue and account for approximately 12% of all cancers in patients younger than 20 years of age. New insight into the biology of these tumors has led to the development of more precise risk-based classifications that directly impact treatment. The two most common pediatric bone sarcomas are osteosarcoma (OS) and Ewing sarcoma (EWS). OS accounts for about 3% of all childhood cancers, whereas EWS accounts for only about 2% of all cases. Clinical outcomes for these two sarcomas have dramatically improved over time, with the survival rates exceeding 70% for those with localized disease. Pediatric soft tissue sarcomas include two main types: rhabdomyosarcoma, which accounts for about 40% of all cases of soft tissue sarcomas in children, and the so-called non-rhabdomyosarcomatous soft tissue sarcomas, which encompass many different histologic types including synovial sarcoma, malignant peripheral nerve sheath tumor, and fibrosarcoma. This chapter describes the salient clinical and biologic features of these tumors and summarizes the current diagnostic and treatment strategies for these pediatric sarcomas. Pediatric Sarcoma, Ewing Sarcoma, Osteosarcoma, Alveolar Rhabdomyosarcoma, Embryonal Rhabdomyosarcoma, Non-Rhabdomyosarcoma Soft Tissue Sarcoma diagnostic strategies, ewing sarcoma, fibrosarcoma, osteosarcoma, pediatric sarcomas, rhabdomyosarcoma, treatment strategies Diagnosis, Fibrosarcoma, Osteosarcoma, Pediatrics, Rhabdomyosarcoma, Sarcoma, Ewing, Therapeutics INTRODUCTION Pediatric sarcomas are a heterogeneous group of diseases that can arise in bone or soft tissue and account for approximately 12% of all cancers in patients younger than 20 years of age (see Figure 6.1).1 New insight into the biology of these tumors has led to the development of more precise risk-based classifications that directly impact treatment. The two most common pediatric bone sarcomas are osteosarcoma (OS) and Ewing sarcoma (EWS). OS accounts for about 3% of all childhood cancers, whereas EWS accounts for only about 2% of all cases. Clinical outcomes for these two sarcomas have dramatically improved over time, with the survival rates exceeding 70% for those with localized disease. Pediatric soft tissue sarcomas include two main types: rhabdomyosarcoma (RMS), which accounts for about 40% of all cases of soft tissue sarcomas in children, and the so-called non-rhabdomyosarcomatous soft tissue sarcomas (NRSTSs), which encompass many different histologic types including synovial sarcoma, malignant peripheral nerve sheath tumor, and fibrosarcoma. This chapter describes the salient clinical and biologic features of these tumors and summarizes the current diagnostic and treatment strategies for these pediatric sarcomas. EWING SARCOMA This tumor can arise in the bone and soft tissue and was previously called peripheral primitive neuroectodermal tumor, Askin’s tumor (EWS of the chest wall), and extraosseous EWS. However, all of these terms reflect the same tumor entity, which is characterized by recurrent translocations that most commonly involve the ERWSR1 gene (Table 6.1).2 Current 5-year overall survival rate for patients with localized disease at diagnosis ranges from 65% to 75%, whereas patients with metastatic disease at diagnosis have survival rates of <30%.3 Epidemiology EWS is the second most common tumor in children and adolescents with approximately 200 cases diagnosed annually in the United States and an annual incidence of approximately one case per million each year.4,5 This tumor more commonly affects male Caucasian adolescents and young adults with a median age at presentation of 15 years and shows male predominance compared to females, with a ratio of 1.5:1.6 Caucasians have a ninefold increased incidence of disease compared to Africans.5 The differences in racial distribution among those with African heritage have been attributed in part to the presence of fewer Alu elements in intron 6 near the EWSR1 breakpoint, theoretically decreasing the sites for gene recombination.7 Another group of investigators found two small-nucleotide polymorphisms (SNPs) associated with EWS on chromosomes 1 and 10 (TARDBP and EGR2, respectively), which are less prevalent in Africans, suggesting that they may contribute to the differences in incidence in the latter population.8 A third observation that may account for differences in the incidence if EWS in Caucasian and African populations involves greater diversity in GGAA motifs at certain microsatellite loci in African populations, which may negatively impact the expression of EWS–FLI.9 Molecular Pathology EWS is characterized by a EWS–ETS fusion (Table 6.1), which in part functions as a rogue transcription factor that drives oncogenesis through deregulation of gene expression.10 EWS can bind multiple members of the ETS family of transcription factors listed in Table 6.1, all of which are associated with EWS.2 Sequencing studies have demonstrated that EWS has a low mutational burden. The most commonly mutated gene seen in about 17% of cases is STAG2; other less frequently mutated genes include TP53, EZH2, BCOR, and ZMYM.11 Homozygous CDKN2A deletions are seen in about 13% of the cases,12 and the presence of STAG2 and TP53 mutations has been associated with poor clinical outcomes.11 Similarly, another group of investigators have found that TP53 and 16/p14ARF mutations are associated with poor response to therapy.13 While EWS is not one of the more common pediatric cancers historically seen in familial cancer syndromes, it has recently been reported that about 10% of patients with EWS have germline mutations in a cancer predisposition gene such as TP53, RET, and PMS2.14 FIGURE 6.1 Percent incidence by age and type of pediatric cancer. (A) Cancer by type for patients 15–19 years of age. (B) Cancer by type for patients <15 years of age. ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; CNS, central nervous system; NRSTS, non-rhabdomyosarcomatous soft tissue sarcomas; RMS, rhabdomyosarcoma. Source: Adapted from Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2010. Bethesda, MD: National Cancer Institute. https://seer.cancer.gov/archive/csr/1975_2010. TABLE 6.1 Translocations in ESFT Across All Age Groups ESFT, Ewing sarcoma family of tumors. Source: Adapted from Pinto A, Dickman P, Parham D. Pathobiologic markers of the Ewing sarcoma family of tumors: state of the art and prediction of behaviour. Sarcoma. 2011;2011:856190. doi:10.1155/2011/856190. 69Clinical Presentation Patients usually present with a palpable mass and pain that can be of several months’ duration. In one study, the median time to diagnosis was 70 days, and longer time to diagnosis was more commonly seen in older patients with tumors in the pelvis and extremities; however, the interval to diagnosis did not influence survival.15 Systemic symptoms, such as fever, were more commonly seen in EWS, when compared to the most common bone tumor in this age group, OS. A German retrospective study found that patients younger than 9 years of age usually presented with less metastatic disease (23.8% compared to 32.3%, p = .009) and sites of the primary tumor differed with a decreased incidence of pelvic primary tumors in this younger cohort.16 Overall, in this German cohort across ages, lungs were the most common site of metastatic disease (ranging from 56.4% to 74.6%) and extrapulmonary metastases increased with age for both bony and soft tissue metastases. Older patients have an increased incidence of extraskeletal EWS, with the average age at presentation being 19.5 years. The extraskeletal location most commonly occurs in females (63.3% of extraskeletal EWS patients and 53.4% of skeletal EWS patients are females) and non-whites.17 Diagnosis and Imaging Initial evaluation should include a complete history with physical exam and laboratory evaluation including a complete blood count (CBC) with differential, blood chemistry tests, and lactate dehydrogenase (LDH). On plain film, EWS of the bone may have a permeative appearance and laminated periosteal reaction of the bone that is commonly referred to as onion skin appearance. The tumor may cause the periosteum to separate from the bone (Codman triangle18; see Figure 6.2). EWS and OS may have similar appearances on plain films, and many times, location is the hint to the diagnosis, with EWS primary tumors more commonly found in the diaphysis of long bones compared to the metaphysis in OS. Additional imaging should include an MRI of the primary site including the whole extremity when indicated. The biopsy should involve the surgeon who will be performing surgery when definitive local control is needed. For patients having closed biopsies, several core biopsies may be required for obtaining adequate tissue for pathologic diagnosis.19 Following confirmation of diagnosis, a thorough staging assessment is required prior to starting therapy. Traditionally, this involves evaluation of common metastatic sites for EWS, including the chest, bone marrow, and other bony sites. A chest CT is required to assess pulmonary disease and bone scintigraphy to detect bony metastases; however, some centers have opted to use fluorodeoxyglucose (FDG)-PET scan to evaluate bony and soft tissue metastases. The latter modality is preferred at many centers because it may offer improved specificity for identifying bony metastases in EWS when compared to bone scintigraphy.20 Finally, bilateral bone marrow aspirates and biopsies should always be performed to exclude disease in the bone marrow. It is expected that these additional diagnostic procedures will identify patients with metastases in about 20% to 25% of the cases.19 TREATMENT EWS is treated with a multimodal approach that uses chemotherapy and local control with surgery, radiation therapy (RT), or both. Prior to the introduction of chemotherapy, only 10% of patients with EWS survived. In fact, in the first Intergroup Ewing Sarcoma Study (IESS-I), one of the randomized arms included chemotherapy versus observation following surgery, but this randomization was quickly discontinued when two of three patients who were randomized to no chemotherapy relapsed.21 With the use of combined modality therapy, over 75% of patients with localized disease are expected to survive; however, patients who present with metastatic disease continue to have poor outcomes with <30% expected to survive long term.19,22,23 The Intergroup trials for localized disease conducted in the United States over the past several decades are summarized in Table 6.2. The first two studies demonstrated the importance of adding doxorubicin to a standard vincristine, actinomycin D, and cyclophosphamide (VAC) backbone and the advantage of giving more intense high-dose intermittent therapy.21,24 The third Intergroup trial randomized patients to a standard VAC doxorubicin backbone with or without the drug pair ifosfamide and etoposide (IE). The 5-year event-free survival (EFS) rate for those receiving IE was 69% compared to 54% in the standard arm, and median overall survival was also significantly better among patients who received IE (72% vs. 61%).25 This trial established the five-drug combination as the standard of care for patients with EWS in the United States. A subsequent trial failed to demonstrate any benefit of intensifying the total dose of cyclophosphamide.26 More recently, the Children’s Oncology Group (COG) conducted a randomized trial in which dose intensification was achieved through interval compression by decreasing the period between cycles from 21 to 14 days. This regimen proved to be feasible and the 5-year EFS rates were significantly better for the patients who were randomized to an interval-compressed schedule (73% vs. 65%).22 This regimen has now become the standard of care for EWS in the United States. The most recent COG study investigated the value of adding another drug pair, cyclophosphamide and topotecan, to this backbone, but the results of this trial are not currently available. The outcomes for patients with metastatic disease remain poor, and several studies have failed to impact the outcomes of these patients.23 Based on some preliminary evidence of the modest activity of insulin-like growth factor-1 receptor (IGF1R) inhibitors in EWS,27,28 the COG is evaluating the addition of IGF1R monoclonal antibody, ganitumab, to chemotherapy in patients with disseminated disease. The Euro-Ewing 99 trial attempted to further optimize therapy by randomizing patients with metastatic disease and high-risk localized disease to either standard chemotherapy (with pulmonary radiation therapy [RT] for metastatic patients) or high-dose chemotherapy with stem cell rescue. The results of this study were recently published, with the high-dose chemotherapy with stem cell rescue improving EFS compared to the standard chemotherapy with pulmonary RT at 8 years from randomization (see Table 6.2).29,30 FIGURE 6.2 An 11-year-old boy with Ewing sarcoma. (A) Anterior–posterior x-ray study showing smooth periosteal reaction and subtle permeative lucency in the femoral diaphysis (arrows). (B) Post-contrast T1-weighted coronal MRI showing permeative lesion in the diaphysis with smooth surrounding periosteal reaction (arrows). 71TABLE 6.2 Historical Trials and Outcomes in Ewing Sarcoma A, actinomycin D; C, cyclophosphamide; D, doxorubicin; E, etoposide; EFS, event-free survival; HD Bu-Mel, high-dose busulfan-melphalan; I, ifosfamide; RFS, relapse-free survival; RT, radiation therapy; V, vincristine. Sources: Data from Burgert EO Jr, Nesbit ME, Garnsey LA, et al. Multimodal therapy for the management of nonpelvic, localized Ewing’s sarcoma of bone: intergroup study IESS-II. J Clin Oncol. 1990;8(9):1514–1524. doi:10.1200/JCO.1990.8.9.1514; Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348(8):694–701. doi:10.1056/NEJMoa020890; Juergens C, Weston C, Lewis I, et al. Safety assessment of intensive induction with vincristine, ifosfamide, doxorubicin, and etoposide (VIDE) in the treatment of Ewing tumors in the EURO-E.W.I.N.G. 99 clinical trial. Pediatr Blood Cancer. 2006;47(1):22–29. doi:10.1002/pbc.20820; Nesbit ME Jr, Perez CA, Tefft M, et al. Multimodal therapy for the management of primary, nonmetastatic Ewing’s sarcoma of bone: an intergroup Study. Natl Cancer Inst Monogr. 1981(56):255–262. PMID:7029293; Womer RB, West DC, Krailo MD, et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30(33):4148–4154. doi:10.1200/JCO.2011.41.5703. Radiation is universally recommended in the case of a positive margin and for patients in whom surgery is not advised; however, in Europe, patients who have a poor histologic response and a negative margin also receive RT.31 Local control is usually performed at week 12 and is achieved with the use of surgery, RT, or both. A recent review of 1,031 patients treated for EWS demonstrated that patients who received RT alone had decreased overall survival rates at 5 years when compared to those who were treated with surgery alone or surgery plus RT (52.5% vs. 77.2% vs. 64.7%, p < .001), although the underlying prognostic factors related to the choice of treatment modality likely contribute to these differences.32 Radiation to metastatic sites is recommended after completion of chemotherapy; stereotactic body radiation therapy (SBRT) and whole lung irradiation (15 Gy) are used for patients who present with pulmonary metastases. 72Sequelae of Therapy Improved outcomes have resulted in higher numbers of EWS survivors and, therefore, a higher population at risk for developing long-term sequelae after therapy. In one study from the Childhood Cancer Survivor Study (CCSS), the cumulative mortality rate at 25 years following entry into the study was 25%.33 The most common cause of death was disease recurrence, followed by subsequent malignant neoplasms, cardiac disease, other medical causes, and pulmonary disease. It is recommended that patients be followed with routine imaging scans and laboratory work (CBC with differential and blood chemistry tests) every 3 to 4 months typically in the first year from completion of treatment, followed thereafter by increasing intervals of every 4 to 6 months for 2 to 4 years after finishing therapy and annually thereafter. Treatment of Recurrence Patients who experience a recurrence have poor long-term survival rates of about 20%.34 The most commonly used regimens for relapse include cyclophosphamide with topotecan and temozolomide with irinotecan, with or without vincristine. These regimens have response rates in the range of 32% to 68%.35,36 High-dose chemotherapy with stem cell rescue has also been used in the setting of recurrent disease to consolidate patients who experience a response to therapy, but its role in this setting remains controversial.3,37 EWS has elevated levels of expression of SLFN11, a direct transcriptional target of EWS–FLI and a determinant of response to several drugs such as camptothecins and poly ADP ribose polymerase (PARP) inhibitors.38 Several ongoing trials are currently using PARP inhibitors in combination with irinotecan and temozolomide. EWS also has high expression of LSD1, and preclinical studies show that LSD1 inhibition blocks the function of EWS–ETS proteins, therefore making this enzyme an attractive target for treating EWS.39 Another group is investigating a small-molecule inhibitor called YK-4-279, which is involved in blocking RNA helicase A that partners with EWS–FLI1.10,40 Finally, several trials using drugs targeting IGF1R have demonstrated modest activity with single agents and how these drugs are being used in combination with chemotherapy in frontline COG trials for patients with metastatic disease.3,27,28 OSTEOSARCOMA Epidemiology OS is the most common bone tumor in children and adolescents, with an age-adjusted incidence rate of 5.4 per million in patients younger than 20 years of age and a slight male predominance compared to females in this age group (1.34:1).4,41 OS has a bimodal age distribution, peaking in adolescence and after the age of 60 years.41,42 Although mostly sporadic, OS can be associated with various genetic predisposition syndromes (Table 6.3).43,44 For example, survivors of retinoblastoma are at the highest risk for developing OS with a standardized incidence ratio of 41.45 In one study, nearly 10% of younger patients with OS had a Li–Fraumeni-associated TP53 mutation or a rare exonic TP53 variant.46 73Biology OS is characterized by multiple chromosomal aberrations including copy number variations and structural variations. Molecular profiling of this tumor has revealed TP53 pathway aberrations in virtually all of the tumors, either as a result of sequence mutations or structural variations that more commonly involve translocations within the first intron of the gene.47 In addition, a distinct phenomenon of hypermutation known as kataegis has been reported in up to 50% of the samples analyzed. Other recurrently mutated genes include RB1, ATRX, and DLG2. Pathway analysis has identified the phosphoinositide 3-kinase (PI3K)/mechanistic target of rapamycin (mTOR) pathway as a potential therapeutic vulnerability.48 Clinical Presentation OS usually presents with pain and swelling of the affected area. It most commonly arises in long bones and about 50% of the cases originate around the knee. The next most common site is the proximal humerus, which is involved in about 10% of the cases.49 In North America, the median duration of symptoms prior to establishing a diagnosis is 2 to 4 months.50 Most patients present with localized disease, but about 20% have metastases most commonly confined to the lungs.49 Diagnosis and Imaging For all pediatric sarcomas, the initial evaluation is similar, involving imaging of the primary tumor and evaluation for the presence of metastatic disease. After physical exam and history, laboratory studies should be obtained, which include a CBC with differential and blood chemistry tests including LDH and alkaline phosphatase (the latter might be increased in patients with primary bone tumors or metastases). The primary tumor is best imaged with an MRI and plain films. Primary anatomic imaging should include the whole limb to look for skip metastases (Figure 6.3). Chest CT should be obtained as the lungs are the most common site of metastases in 85% of the cases of metastatic disease, and a bone scan or PET should be performed to evaluate for the presence of distant bone metastases.49,51 Biopsy should be carefully planned by the primary surgical treatment team, because the biopsy tract in an extremity primary site can affect local control approaches. Some institutions may conduct a biopsy or remove suspicious lung nodules to confirm the diagnosis. FIGURE 6.3 A 6-year-old boy with osteosarcoma. (A) Anterior–posterior x-ray study shows typical aggressive bone-forming tumor (arrows) with sunburst periosteal reaction, calcified soft tissue mass, and Codman triangle. (B) Post-contrast T1-weighted coronal MRI shows a large soft tissue mass (arrows) surrounding the tumor involving the marrow of the distal femur. 74Treatment Prior to the use of combined modality therapy, which includes chemotherapy and surgery, the relapse-free survival rates for patients with OS were <20%.52 The landmark study of Link (see Table 6.4) in which patients with localized OS were randomized to receive surgery alone or adjuvant chemotherapy with methotrexate, cisplatin, doxorubicin (MAP), bleomycin, and actinomycin D demonstrated that the 2-year relapse-free survival rates were significantly improved for those patients who were randomized to adjuvant chemotherapy when compared to those who were treated with surgery alone (66% vs. 17%).52 This study established the definitive role of chemotherapy in this disease. Other studies during the same time period demonstrated that the timing of chemotherapy either prior to or after resection of the primary tumor did not appear to affect survival, although the use of preoperative chemotherapy could potentially allow for a less morbid surgical procedure with limb preservation and good function.53–55 To confirm this, the Pediatric Oncology Group (POG) conducted a randomized trial to evaluate the outcomes for patients receiving adjuvant versus neoadjuvant chemotherapy. Another landmark study conducted between 1986 and 1993 established the effectiveness of presurgical chemotherapy followed by limb-sparing surgery (LSS) in patients with localized OS.56 In this trial, 100 patients were randomized to immediate chemotherapy or immediate surgery. At 5 years, the EFS was 65% for immediate surgery patients and 61% for those assigned to presurgical chemotherapy. Thus, these two studies provided the basis for the current standard of treatment for OS in the United States, which includes preoperative chemotherapy with MAP followed by local control with LSS at around week 10 and additional postoperative chemotherapy for a total of 32 weeks of therapy. Other studies have evaluated the addition of other agents to the MAP backbone in an effort to improve outcomes for patients with the highest risk, such as those with a poor histologic response (as defined as <90% tumor necrosis) after preoperative chemotherapy.57 A randomized trial conducted by Meyers et al. assigned patients to receive MAP chemotherapy with or without ifosfamide and with or without muramyl tripeptide.57 This trial achieved a 3-year EFS of 68%. The addition of ifosfamide to cisplatin, doxorubicin, and methotrexate did not improve EFS or overall survival, but the addition of muramyl tripeptide significantly improved survival.58 Despite the results of this trial, this agent is currently not available in the United States.59 The most recent randomized trial, EUROAMOS, attempted to answer several questions in patients with localized disease by modifying therapy based on histologic response. Patients who had >90% tumor necrosis (defined as a “good” response) were randomized to receive either standard MAP chemotherapy or MAP with pegylated interferon alfa-2b.60 No advantage was seen with the addition of interferon, with the 3-year EFS rates being 77% for the MAP arm and 80% for the MAP plus interferon arm. In addition, 23% of patients randomized to the interferon arm never received it and 39% of those who received interferon stopped it early.60 In this trial, the remaining 618 patients who had a poor histologic response to preoperative chemotherapy defined as ≥10% viable tumor were randomized to receive MAP chemotherapy alone or MAP with three postoperative cycles of IE and two additional cycles of ifosfamide (MAP/IE). The 3-year EFS was 55% for MAP and 53% for MAP/IE. In addition, MAP/IE was associated with more grade 4 nonhematologic toxicity.61–67 TABLE 6.4 Historical Osteosarcoma Chemotherapy Regimens a For randomized patients. EFS, event-free survival; I, ifosfamide; MAP, methotrexate, cisplatin, doxorubicin; MIOS, Multi-Institutional Osteosarcoma Study; RR, relapse rate. Sources: Data from Goorin AM, Schwartzentruber DJ, Devidas M, et al. Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651. J Clin Oncol. 2003;21(8):1574–1580. doi:10.1200/JCO.2003.08.165; Meyers PA, Schwartz CL, Krailo M, et al. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol. 2005;23(9):2004–2011. doi:10.1200/JCO.2005.06.031; Cortes EP, Holland JF, Wang JJ, et al. Doxorubicin in disseminated. JAMA. 1972;221(10):1132–1138. doi:10.1001/jama.1972.03200230020005; Cortes EP, Holland JF, Wang JJ, et al. Amputation and adriamycin in primary osteosarcoma. N Engl J Med. 1974;291(19):998–1000. doi:10.1056/NEJM197411072911903; Jaffe N, Frei E 3rd, Traggis D, Bishop Y. Adjuvant methotrexate and citrovorum-factor treatment of osteogenic sarcoma. N Engl J Med. 1974;291(19):994–997. doi:10.1056/NEJM197411072911902; Marti C, Kroner T, Remagen W, et al. High-dose ifosfamide in advanced osteosarcoma. Cancer Treat Rep. 1985;69(1):115–117. PMID:3855382; Link MP, Goorin AM, Miser AW, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986;314(25):1600–1606. doi:10.1056/NEJM198606193142502; Ochs JJ, Freeman AI, Douglass HO Jr, et al. Cis-Dichlorodiammineplatinum (II) in advanced osteogenic sarcoma. Cancer Treat Rep. 1978;62(2):239–245. 75Local control measures should be determined by an experienced orthopedic oncologist for most cases of OS involving extremity tumors. Currently, about 80% of patients with extremity OS can be treated with LSS for local control.68 The role of radiation is limited in this disease to axial lesions that are not resectable or that locally recur (in conjunction with surgery when possible); high radiation doses delivered with specialized techniques such as protons are necessary.69 The importance of complete surgical resection of all sites of resectable OS is key to long-term control and cure.70 Historically amputation was the only option for many patients, and this was the preferred method to ensure complete resection of the tumor, with local sites being the most common location for relapse. With the use of preoperative chemotherapy, many resections were made easier for surgeons to allow for limb-sparing procedures that historically would have resulted in an amputation. Patients who present with pulmonary metastatic disease should have their lesions resected in order to potentially achieve cure.71 Typically, this procedure is performed in a staged fashion with local control of the primary site occurring first. Prognostic factors associated with inferior clinical outcomes in the presence of pulmonary metastatic disease include the number of metastases at diagnosis and macroscopically incomplete resection of all sites of disease.71 Sequelae of Therapy Survivors of OS are at increased risk of developing therapy-related complications. In a study by the CCSS, patients who survived 5 years from their diagnosis had an 88.6% probability of being alive for 20 years from their original diagnosis.72 However, 87% of survivors experienced at least one chronic medical issue and over half experienced two or more chronic health conditions. The most common cause of death was attributed to tumor recurrence, followed by subsequent malignant neoplasms, cardiac complications, and pulmonary complications. Tinnitus/vertigo and hearing loss requiring a hearing aid were reported in 14% and 7% of survivors, respectively. About 10% of survivors reported adverse general health and 29% reported a physical limitation. Finally, survivors of OS were less likely to be married or to graduate from high school. Based on these findings, survivors of OS require lifetime follow-up after completion of treatment. Patients should be followed with routine scans and lab work (CBC with differential and blood chemistry tests) every 3 to 4 months during the first year from completion of therapy with routine subspecialty care that may involve specialists from cardiology, nephrology, fertility, and rehabilitation, depending on the needs of the individual. The patient will also require cardiac evaluations with echocardiograms to assess the heart function, depending on the total dose of doxorubicin. Interval assessments after therapy completion can be spread out to every 4 to 6 months for 2 to 4 years from therapy and annually after 5 years, depending on the institutional standards. Treatment of Recurrence Clinical outcome following relapse of OS is poor. In one study by the Cooperative Osteosarcoma Study Group, the overall survival rate at 5 years was 23%.73 Patients with early relapses and a higher disease burden fared worse, as well as those who did not achieve a surgical complete remission. Administration of chemotherapy appeared to correlate with better survival in those who did not achieve a second complete remission.73 Similarly, the COG found that survival after recurrence was poor, with 17% of patients being alive at 5 years. Outcome was better for those who were <10 years of age at the time of initial 76treatment and presented with localized disease, had a longer interval to time of first relapse (>2 years), and had isolated lung or bone relapse.74 About 50% of all relapses occur within 18 months from the end of initial treatment and another 5% of patients will have a relapse after 5 years of therapy. As previously mentioned, patients who relapse early (<2 years from the initial diagnosis) have worse outcomes than those who recur later.75 Regardless of the site of recurrence, surgical resection is key to disease control, with 5-year survival rates being 20% to 45% after resection of lung recurrences and 20% at other sites.76,77 Patients who experience isolated lung recurrences should undergo complete resection of recurrent disease. Complete resection can be successful for patients with isolated lung disease without requiring chemotherapy or further treatment.76–79 A retrospective review of patients treated at major centers showed that chemotherapy following metastasectomy did not improve the long-term survival compared to surgery alone.80 For patients who require chemotherapy, multiple regimens have been used, including ifosfamide with etoposide, gemcitabine with docetaxel, and sorafenib with everolimus. A recent randomized, placebo-controlled, Phase 2 trial demonstrated that regorafenib increased the median progression-free survival and overall survival of patients with recurrent OS.81 Because responses to chemotherapy using the Response Evaluation Criteria in Solid Tumors (RECIST) are infrequent and do not appear to accurately predict survival,82 the COG embarked on a retrospective analysis assessing the outcome of recurrent OS in patients who were treated in seven Phase 2 trials. They observed a 4-month progression-free survival rate of 12%, which is now used as a benchmark for EFS outcomes that can be used to assess the efficacy of novel agents.83 Using this model to assess the efficacy of new agents, the COG has evaluated several new agents in the Phase 2 setting, such as eribulin, without evidence of clinical activity.84 For patients with multiple painful bony metastatic sites at the time of recurrence, samarium-153-ethylene-tetramethylene-phosphonate (153Sm-EDTMP; with or without stem cell support) may help with pain symptoms.85 NON-RHABDOMYOSARCOMA SOFT TISSUE SARCOMAS Epidemiology NRSTSs encompass a large group of tumor types that have a presumed mesenchymal origin and account for about 4% of all childhood malignancies.86 There is a slight male predominance in childhood, and the histology distribution of these tumors is dramatically different from the histology seen in adults. In the pediatric population, synovial sarcoma, malignant peripheral nerve sheath tumors, and fibroblastic tumors predominate, while most of these malignancies are seen during the second decade of life. Some genetic predisposition syndromes have been associated with an increased risk for developing particular NRSTS (see Table 6.5)87–98 and these children should be followed closely for their lifetime risk of developing these cancers. Biology The majority of pediatric NRSTSs have unique chromosomal translocations (Table 6.6), and some of them, such as tumors characterized by NTRK fusions, are amenable to targeted therapy.99 Owing to the rarity of these individual tumors, they have been grouped together for treatment purposes, though as seen in Table 6.6, the identified pathways involved are often completely unrelated, which has made studying many of these tumors difficult.100–102 Clinical Presentation, Diagnosis, and Imaging Children usually present with an enlarging soft tissue mass, which is usually painless. Systemic symptoms are uncommon and other symptoms are related to the anatomic location of the tumor. Diagnosis is made in most cases by excisional biopsy, core-needle biopsy, and rarely by complete surgical resection of the mass at the time of presentation. Laboratory studies should include a CBC with differential and a chemistry panel including LDH and urinalysis. An MRI of the primary tumor should be obtained, as well as a CT of the chest with FDG-PET to search for distant metastases. Treatment Treatment for this broad group of tumors involves surgery, with or without chemotherapy and with or without radiation. This group of tumors was poorly studied in multi-institutional National Cancer Institute (NCI)-sponsored trials until the most recent trial named ARST0332. ARST0332 was the first trial to use a risk-based approach to assign therapy (see Figure 6.4). In addition, this study incorporated for the first time the French Federation of Comprehensive Cancer Centers (Fédération Nationale des Centres de Lutte Contre le Cancer; FNCLCC) grading system in addition to the POG system to assign histologic grade.103 Spunt and colleagues compared these two systems in a cohort of 130 patients from three POG historical trials (8653, 8654, and 9553) and showed they had a correlative value.104 More importantly, their data showed that mitotic index may have the highest correlative value for pediatric NRSTS. They found 44 patients had discrepant grades between the two systems; but when they further looked at mitotic index using 10 mitotic figures per high-power field (HPF) as a cutoff, these patients’ outcomes followed the trend with patients who had <10 mitotic figures per HPF having an EFS close to 70% compared to those with >10 figures per HPF having an EFS of around 15%. These outcomes matched up with those patients who had correlative grades as had been previously said by the two systems. The recently completed ARST0332 study analyzes data using the two systems to determine which one better applies to the pediatric population prospectively. 77TABLE 6.5 Genetic Predispositions in NRSTS
Translocation
Fusion Gene
Percent Positive
t(11;22)(q24;q12)
EWS–FLI1
>85
t(21;22)(q22;q12)
EWS–ERG
5–10
t(19;der)inv(21;22)
EWS–ERG
<1
t(7;22)(p22;q12)
EWS–ETV1
<1
t(17;22)(q12;q12)
EWS–ETV4
<1
t(2;22)(q33;q12)
EWS–FEV
<1
t(16;21)(p11;q22)
FUS–ERG
<1
t(2;16)(q35;p11)
FUS–FEV
<1
Study
Observed Results
Local disease
IESS-I (n = 342)
VAC versus
VACD versus
VAC + lung RT
5-y RFS
24%
60%
44%
IESS-II (n = 214)
VACD q3 weeks
versus
moderate-dose chemo weekly
5-y RFS
73%
56%
p = .04
INT-0091 (n = 518)
VDC
versus
VDC + IE
5-y EFS
54%
69%
p = .005
INT0154/POG9354 (n = 518)
VDC + IE (standard)
versus
VDC + IE (high dose)
5-y EFS
72.1%
70.1%
p = .005
COG
AEWS0031
(n = 568)
VDC + IE (3 weeks)
versus
VDC + IE (2 weeks)
5-y EFS
65%
73%
p = .05
Metastatic disease
INT-0091
VDC
versus
VDC + IE
5-y EFS
22%
22%
(NS)
EE99
VIDE + VAC then HD Bu-Mel w/rescue
versus
VIDE + VAI then VAI + lung RT
8-y EFS
60.7%
46.3%
p = .032

Investigator, Year
Protocol/Therapy
RR or EFS (Duration)
Cortes, 1972, 1974 (n = 13 and n = 21)
Doxorubicin
45% (1–32 mo)
Jaffe, 1974 (n = 20)
Methotrexate with vincristine
80% (2–23 mo)
Ochs, 1978 (n = 8)
Cisplatin
63% RR
Marti, 1985 (n = 18)
Ifosfamide (9 g/m2 over 5 days)
33% RR
Link, 1986 (n = 36 randomized, 77 declined) — MIOS trial
Observation
versus
bleomycin, cyclophosphamide, actinomycin D, methotrexate, doxorubicin, cisplatin
17% (2 y)a
66% (2 y)a
Goorin, 2003
MIOS chemotherapy-neoadjuvant
versus
MIOS chemotherapy-adjuvant
61% (2 y)
69% (2 y)
Meyers, 2005
MAP
versus
MAP + I
65% (4 y)
66% (4 y)
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Pediatric Oncology
Sara E. Helmig and Alberto Pappo