BACKGROUND
Since the last edition of this book, several biomedical milestones have been achieved, substantially advancing pediatric oncology. The human genome project has made breakthroughs possible in our understanding of the molecular basis of cancer and has allowed us to distinguish among phenotypically similar entities and develop more rational treatment strategies.
1 Powerful genomic techniques have not only improved nosologic classification but also revealed new prognostic markers and potential targets for more closely targeted therapies.
2 Drug discovery has been facilitated by high-throughput screening that can interrogate thousands of compounds in a relatively short period of time.
3 During the coming decade, integration of these approaches into clinical pediatric oncology should help reduce both cancer mortality and the long-term side effects of conventional cancer treatments. However, the benefits we describe will be fully accessible only to selected patients who reside in affluent parts of the world. Meaningful access to effective therapies for all children remains a major challenge in pediatric oncology.
Each year, approximately 200,000 new cases of cancer are diagnosed worldwide in children and adolescents. Countries with large populations of children less than 15 years of age and a low general life expectancy have the highest percentage of cases of pediatric versus adult cancer.
4 In fact, nearly 80% of children with cancer reside in low- to mid-income countries (LMIC).
5 However, pediatric cancer is not among the main causes of childhood mortality in LMIC and is not included in the national health care agendas of most such countries. Although noncommunicable diseases, led by adult cancer, have become an increasingly important priority for the World Health Organization (WHO),
6 pediatric cancer care in LMIC has emerged through the isolated initiatives of institutions and/or individuals. In many cases, the parents of children who received cancer treatment in a high-income country have developed philanthropic initiatives to assist local children with cancer. In these arrangements, public hospitals cover the costs of a specific number of beds for pediatric cancer and the salaries of a limited number of health care professionals, while families or philanthropic organizations pay for diagnostic procedures, medications, and other expenses. Sources of financial coverage of treatment vary among countries, ranging from complete national coverage to essentially no coverage at all. In the latter cases, only a few children receive treatment. In many LMIC, there is a combination of financial models that combine government, private, and philanthropic sources.
7 However, despite such arrangements, the rate of abandonment of therapy is very high and adherence to treatment is poor owing to factors such as the families being unable to bear the cost of travel, not allocating time away from work, having to care for their other children, and having to create financial provision for other necessities.
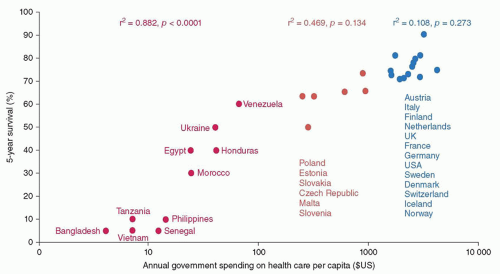
Countries with populations greater than 30,000 are economically classified by the World Bank on the basis of gross national income (GNI) per capita. Of the 34 countries listed as low-income, 26 (76%) are in sub-Saharan Africa and 17 have a GNI per capita less than US$630, including Malawi ($270) and Burundi ($280) at the low end. Unfortunately, the gap between the richest and poorest countries continues to widen. Nine of the top 15 countries, with a GNI per capita greater than $50,000, are in Western Europe, while 16 (80%) of the 20 countries with half their population below the international poverty line (U.S. $1.25/day) are in sub-Saharan Africa. Other countries with more than half the population below the poverty level include Honduras (64.5%), Haiti (58.7%), Guatemala (53.7%), and Mexico (52.3%). Strengthening the overall health care systems of LMIC should improve the survival of children with cancer as well as the general quality of health care.
In this chapter, we discuss the status of pediatric oncology worldwide and review various strategies to extend cancer care to the largest possible number of children.
EPIDEMIOLOGY OF PEDIATRIC CANCER WORLDWIDE
Pediatric cancer is rare, accounting for 0.5% to 10% of cases, depending on the age distribution in various countries.
8,9,10 Several pediatric cancers show substantial geographic and ethnic differences in their incidence.
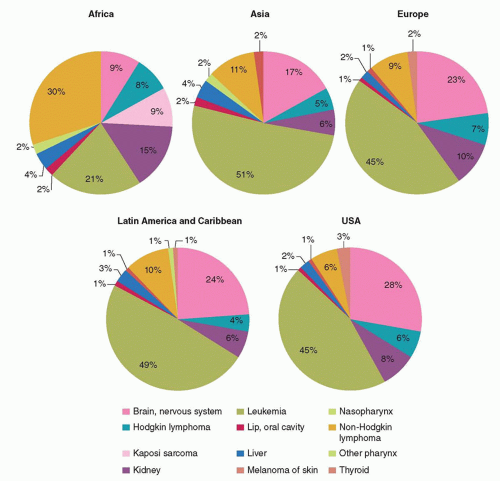
11,12 The distribution of various cancers within a geographic area (
Fig. 54.1) reflects the interaction between constitutional/genetic and environmental factors that have been established over thousands of years as populations have expanded, adapted to their environment, and adopted occupational and lifestyle characteristics. While lifestyle factors such as cigarette smoking, occupation, and nutrition play an important role in cancer predisposition in adults, infection-related factors appear to be the strongest environmental determinants in the uneven geographic distribution of pediatric cancers.
13 The contribution of Epstein-Barr virus to the pathogenesis of Burkitt lymphoma, Hodgkin lymphoma, extranodal NK-cell lymphoma of nasal type, and nasopharyngeal carcinoma is well established,
14,15 as is the association between hepatitis B virus (with or without exposure to aflatoxin) and hepatocellular carcinoma.
16 More recently, it has become clear that the prevalence of cervical carcinoma in adolescent females in developing countries is a consequence of infection with human papilloma virus, especially serotypes 16 and 18.
17,18 Finally, the HIV/AIDS epidemic in sub-Saharan Africa has led to an increase in associated cancers, especially Kaposi sarcoma, which is associated with human herpes virus 8 (HHV8), and non-Hodgkin lymphoma (NHL),
19,20 demonstrating how infection can alter the incidence of certain tumors over a relatively brief period and can play a crucial role in pediatric tumorigenesis.
Pediatric Burkitt lymphoma is the classic example of multiple, complex interactions between genetic and environmental factors.
21,22 Although cytogenetic studies consistently show translocation of the MYC oncogene to one of the genes encoding the immunoglobulin chains, the incidence, presenting clinical features, and response to chemotherapy of this cancer vary geographically. The high incidence of childhood Burkitt lymphoma in sub-Saharan Africa is associated with equatorial climate
characteristics, including infection with mosquito-borne arboviruses, Epstein-Barr virus, and malaria.
23,24 In the United States and Europe, Burkitt lymphoma is associated with none of these factors,
25 whereas in some Latin American regions it is associated with Epstein-Barr virus infection and equatorial climate but not with malaria. There is limited evidence showing that prevention of malaria reduces the incidence of Burkitt lymphoma, as does the presence of protective antibodies to
Plasmodium falciparum.26,27 People of African descent born in the United States are not predisposed to Burkitt lymphoma, corroborating the impact of environmental factors; in fact, the incidence of Burkitt lymphoma in Black children is half that in non-Hispanic White children in the United States.
Genetic factors also influence the incidence of pediatric cancers in diverse geographic regions. The expansion of the African population that arrived in the United States during the 1700s and the relative absence of racial mixing resulted in a large number of Black individuals residing in different environments. Analysis of population-based registries has shown that acute lymphoblastic leukemia (ALL), the most common pediatric cancer, is significantly less common in Black than in White children.
28 Remarkably, this difference can be explained by the absence of the peak in ALL incidence between ages 2 and 4 years that is typical in White children in affluent countries. The relatively recent immigration of Hispanic people to the United States, who now comprise about 17% of the country’s population (52 million people), has also provided insight into the impact of genetic ancestry on pediatric cancer incidence. An analysis of cases of acute leukemia in 17 population-based cancer registry areas of the Surveillance, Epidemiology and End Results (SEER) Program during 2001 to 2007 revealed that Hispanics had a significantly higher incidence of progenitor B-cell ALL (incidence ratio rate [IRR] = 1.64) and acute promyelocytic leukemia (IRR = 1.28) than did non-Hispanic Whites, while Blacks had a lower incidence of all acute leukemia subtypes investigated.
29 Recent genome-wide association studies have suggested that inherited variations in the genes
ARID5B,
IKZF1,
CEBPE,
CDKN2A, and
BMI1-PIP4K2A are associated with childhood predisposition to ALL.
30 One of these variants, the
ARID5B single nucleotide polymorphism (SNP) 10821936, has been found to increase the risk of ALL across racial, ethnic, and geographic groups.
31 Geographic and racial/ethnic differences in the incidence of pediatric cancer offer opportunities to investigate the basic mechanisms of tumorigenesis and, most importantly, provide a strong rationale for individualized approaches to implementing national pediatric cancer programs, depending on the country or continent.