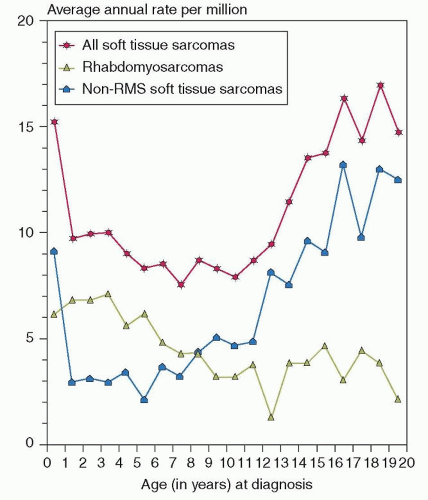
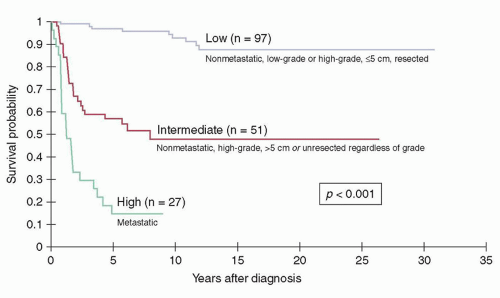
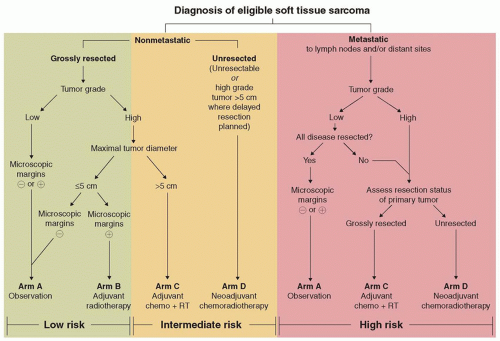
Diagnosis |
Key Clinical Features |
Treatment |
Outcome |
Intermediate, Locally Aggressive |
Desmoid fibromatosis |
Most common in adolescents
Usually arises in extremities/body wall
May be multifocal
Develops in 10% of patients with FAP |
Observation, since some spontaneously resolve
Surgical resection
Systemic chemotherapy when symptomatic and resection not feasible |
Generally favorable although recurrent head/neck and visceral tumors may have a more aggressive clinic course |
Giant cell fibroblastoma |
Predilection for males
Dermal or subcutaneous location typical |
Surgical resection |
Good, although may recur locally |
Kaposiform hemangioendothelioma |
Most common in infancy
Usually arises in head/neck and extremities
Large and deep tumors may be associated with Kasabach-Merritt syndrome |
Surgical resection when feasible
Steroids for enlarging, unresectable tumors
Steroids plus vincristine for unresectable tumors associated with Kasabach-Merritt syndrome |
Generally favorable |
Lipofibromatosis |
Most common in infants and young children
Male predominance
Usually arises in extremity or body wall
15% of cases are congenital |
Surgical resection |
Generally favorable |
Myofibroma |
Most occur in infancy
May be congenital
Rare familial cases reported
Solitary form: male predominance, head/neck or trunk location
Multifocal form: female predominance, visceral involvement in 25% |
Observation, since some spontaneously resolve
Surgical resection
Systemic therapy for those with extensive visceral involvement |
Generally favorable, although deaths reported in infants with extensive visceral involvement |
Intermediate, Rarely Metastasizing |
Angiomatoid fibrous histiocytoma |
Slight female predominance
Usually arises in subcutaneous tissues of extremities/trunk, often near lymph nodes
Systemic symptoms (fever, anemia, malaise) have been reported |
Surgical resection |
Generally favorable |
DFSP |
Significantly more common in blacks
Usually arises in subcutaneous tissues
Most common in extremities and body wall |
Surgical resection (consider Mohs surgery)
Imatinib mesylate for unresectable or metastatic disease; consider for microscopic residual disease after maximal surgery
RT effective for microscopic disease after maximal surgery, but may be more associated with more side effects than imatinib mesylate therapy |
Generally favorable |
GIST |
Strong female predominance
Associated with Carney triad, Carney-Stratakis dyad, NF-1
Some cases are familial
Usually arises in stomach or small intestine and may be multifocal
Metastasizes to liver and peritoneal surfaces |
Surgical resection with lymph node sampling if feasible
Observation for unresectable/metastatic tumors if minimally symptomatic; surgical debulking or tyrosine kinase inhibitor therapy if treatment required |
Depends on extent of disease and resectability |
Infantile fibrosarcoma |
Nearly always presents before 2 years of age
May be congenital
Usually arises in deep tissues of the extremities or trunk
Often grows very rapidly |
Surgical resection if feasible
Vincristine/actinomycin D chemotherapy and consider delayed surgical resection for unresectable or metastatic tumors |
Generally favorable, although rapid tumor growth may produce morbidity and mortality |
IMT |
Occurs throughout childhood
Usually arises in soft tissues of body cavities and viscera
20% have associate syndrome of fever, weight loss, malaise, growth failure, anemia, thrombocytosis, elevated erythrocyte sedimentation rate, and polyclonal hypergammaglobulinemia |
Surgical resection when feasible
Consider ALK inhibitors for ALK-rearranged tumors
Anecdotal responses to nonsteroidal anti-inflammatory agents and steroids |
Limited data on outcome |
Low-grade myofibroblastic sarcoma |
Most commonly arises in the head and neck region |
Surgical resection |
Generally favorable, although may recur locally |
Myoepithelioma |
Most commonly arises in the extremities and limb girdles |
Surgical resection
Cytotoxic chemotherapy for unresectable or metastatic tumors |
Limited data on outcome |
Myxoinflammatory fibroblastic sarcoma |
Predilection for the distal extremities |
Surgical resection |
Limited data on outcome |
Plexiform fibrohistiocytic tumor |
Occurs throughout childhood, including in infancy
Usually involves superficial tissues
Most commonly arises in the upper extremity or shoulder |
Surgical resection |
Generally favorable, although may recur locally |
Solitary fibrous tumor |
Most commonly arises in the head and neck in childhood |
Surgical resection |
Limited data on outcome |
Malignant |
ASPS |
Female predominance
No cases reported in children under 2 years of age
Most commonly arises in the extremities
Metastasizes to the lung most commonly
Clinical course often indolent; even those with extensive lung metastases may survive for decades |
Surgical resection
RT for microscopic residual disease
Resistant to cytotoxic chemotherapy; agents targeting angiogenic pathways may be useful |
Good for those with nonmetastatic disease. Outcome poor for metastatic disease, although clinical course is often indolent |
Angiosarcoma |
Typically presents as rapidly enlarging mass, often with overlying ulceration
May arise in soft tissues or viscera; can be multifocal |
Surgical resection, consider lymph node sampling
If lymph nodes involved, lymph node dissection
RT for microscopic or gross residual disease after maximal surgery
Chemotherapy for unresectable or metastatic disease |
Generally poor, except for nonmetastatic resectable tumors |
Clear cell sarcoma of soft tissue |
Male predilection in childhood
Most commonly arises in extremities
May be painful
Propensity for regional nodal spread
Distant metastases usually involve the lung |
Surgical resection, including lymph node sampling
If lymph nodes involved, lymph node dissection
RT for residual disease after maximal surgery
Chemotherapy only for unresectable tumor to facilitate surgery |
Generally poor, except for nonmetastatic, resectable tumors |
DSRCT |
Most common in adolescents
Strong male predominance
Commonly arises in the abdomen or pelvis, with invasion into solid organs and serosal dissemination
Metastasizes to regional lymph nodes, liver, kidney, lung, bone, and bone marrow |
Surgical resection, either at initial diagnosis or after induction chemotherapy
Chemotherapy
RT
Autologous stem cell transplantation may lengthen survival
Consider hyperthermic peritoneal perfusion with cisplatin chemotherapy for peritoneal metastases |
Generally poor, except for nonmetastatic, resectable tumors |
Embryonal sarcoma of the liver |
Usually presents with abdominal pain and distension; obstructive jaundice is unusual
May be multifocal
Serum alpha-fetoprotein usually normal or only slightly elevated |
Surgical resection, including liver transplantation when necessary
Chemotherapy |
Generally favorable for nonmetastatic, resectable tumors treated with chemotherapy. Poor for those with metastatic or unresectable disease |
Epithelioid hemangioendothelioma |
Most common in older children and adolescents
Most commonly arises in the deep soft tissues of the extremity and liver
Most hepatic tumors are multifocal
Metastasizes to regional lymph nodes, lung, liver, and bone; metastases may develop many years after the diagnosis |
Surgical resection, including liver transplantation when necessary
Transarterial chemoembolization may control disease while awaiting liver transplant
Rare responses to systemic therapy (doxorubicin, 5-fluorouracil, interferon α2b, bevacizumab, sorafenib) |
Generally favorable for those with resectable disease |
Epithelioid sarcoma |
Strong male predominance
Occurs from infancy through adolescence
Predilection for the distal extremities
Metastasizes to regional lymph nodes and lung |
Surgical resection and lymph node sampling
If lymph nodes involved, lymph node dissection RT for microscopic residual disease after surgery
Chemotherapy for unresectable or metastatic disease |
Generally favorable for small tumors that are grossly excised. Poor outcome for those with large tumors and those with unresectable or metastatic disease |
Fibrosarcoma (adult-type) |
Low-grade fibromyxoid sarcoma is the most likely subtype to be seen in children
Arises most commonly in extremities, body wall, and head/neck region
The lung is the most frequent site of metastasis |
Surgical resection
RT for microscopic residual disease after maximal surgery, although RT may be avoided in low-grade tumors
Chemotherapy, with or without RT, for unresectable or metastatic disease |
Outcome depends on tumor grade, size, presence or absence of metastases, and extent of surgery |
Leiomyosarcoma |
May occur in immunosuppressed patients (AIDS, solid organ or bone marrow transplant), in whom it may be multifocal
May arise in virtually any anatomic location
Most common site of metastasis is the lung |
Surgical resection
RT for microscopic residual disease after maximal surgery
Consider chemotherapy and RT for unresectable or metastatic disease |
Outcome depends on tumor grade, size, presence or absence of metastases, and extent of surgery |
Liposarcoma |
More common in females and adolescents
Most commonly arises in the deep soft tissues of the extremities
Lymph node and distant metastases are uncommon at initial diagnosis |
Surgical resection
RT for microscopic residual disease after maximal surgery
Consider chemotherapy and RT for unresectable or metastatic disease |
Outcome depends on tumor grade, size, presence or absence of metastases, and extent of surgery |
MPNST |
25% of cases associated with NF-1
Often arises in association with major nerve trunks, so may be painful
Most commonly arises in the trunk or extremities
The lung is the most common site of metastasis |
Surgical resection
RT for microscopic residual disease after maximal surgery
Consider ifosfamide-containing chemotherapy and RT for unresectable or metastatic disease |
Outcome depends on tumor grade, size, presence or absence of metastases, and extent of surgery |
Malignant rhabdoid tumor |
Most common in infancy and early childhood
Male predominance
Associated with germline SMARCB1 mutation in about one-third of cases
Arises most commonly in the kidney; visceral and head/neck sites predominate
May be multifocal
Most frequent sites of metastasis are lymph nodes and lung |
Surgical resection
RT
Chemotherapy |
Generally poor, especially in fetal and neonatal cases |
Synovial sarcoma |
Occurs from infancy through adulthood
Arises most frequently in extremities (lower more often than upper)
Lymph node metastases are uncommon
The lung is the most common site of metastasis |
Surgical resection
RT for microscopic residual disease after maximal surgery and for unresectable disease
Chemotherapy for large, high-grade tumors and for those with unresectable or metastatic disease |
Outcome depends on tumor grade, size, presence or absence of metastases, and extent of surgery |
Undifferentiated sarcoma |
Occurs throughout childhood
Arises most commonly in the soft tissues of the body wall |
Surgical resection
RT for microscopic residual disease after maximal surgery and for unresectable disease
Chemotherapy for large, high-grade tumors and for those with unresectable or metastatic disease |
Outcome depends on tumor grade, size, presence or absence of metastases, and extent of surgery |