Country
Physical layout
Human resources
Financial support
Collaborations
Ethiopia (sub-Saharan Africa) (low-income economy)
Dedicated ward in the pediatric unit of a general hospital, largest referral hospital in country for all pediatric cancer cases
Few pediatricians, no oncologist. Nurses not trained in pediatrics or oncology. Pathology poorly developed
Government hospital with minimal outside financial support
International: INCTR/Georgetown twinning program. Fellowship in pediatric oncology started 2013. Training for pediatric oncology nurses and pharmacists started
Rwanda (sub-Saharan Africa) (low-income economy) (two examples)
1. A separate wing in the pediatric department of the main referral hospital where patients are hospitalized for diagnosis, surgeries, and if there are any complications during chemotherapy
2. A hospitalization ward within a district hospital supported by an NGO 2.5 h away from the central hospital where patients receive chemotherapy and are seen for follow-up
Nurses with no specific training in pediatrics or oncology
General nurses with some training in oncology
Government hospital with no external financial support
International: unofficial consultations obtained with pediatric oncologists in the USA, South Africa
International: consultations with a US institution
Pakistan (South Asia) (lower-middle-income economy) (four examples)
1. Freestanding children’s cancer hospital in a major city (1 of 3 pediatric cancer units in the city). Most consultative services available in the community
2. Large pediatric unit in government hospital
3. Separate wing in the pediatric department of University Teaching Hospital with well-developed adult cancer program. Research facilities available
4. Pediatric unit in the only comprehensive cancer center in the country. Subspecialty support at the cancer center and in the community. Research facilities available
Trained pediatric oncologists, pediatric nurses, pharmacist
Trained pediatricians and nurses
Trained pediatric oncologists, nurses, and pharmacists. All subspecialty support including radiation oncology on-site available
Trained pediatric oncologist, nurses, pharmacists
Totally financed by philanthropy
Government hospital with local philanthropic support
Fee for service
Philanthropy and fee for service
Several international affiliations, local network through Pakistan Society of Pediatric Oncology (PSPO)
Local support from PSPO
International and local collaborations
International and local collaborations
Guatemala (Latin America and Caribbean) (lower-middle-income economy)
Independent 3-story building adjacent and connecting to a general pediatrics hospital
The unit has dedicated intensive care, procedure area, urgent/emergent care area, pharmacy, data management, inpatient and outpatient pediatric oncology areas
Pediatric oncology trained physicians and nurse educators
Began a fellowship program in 2004
Some services (pathology, radiology, surgery, etc.) are contracted out to general pediatrics hospital or other institutions
Not-for-profit organization and public partnership (the organization fundraises for the PCU and the ministry of health provides an annual sum for care of oncology patients)
Regional: collaboration with 6 other countries in the region for development and monitoring of standardized treatment guidelines by disease (AHOPCA—Association of Central American Pediatric Hematologists and Oncologists)
International: twinning with St. Jude Children’s Research Hospital International Outreach Program
Costa Rica (Latin America and Caribbean) (upper-middle-income economy)
Pediatric oncology ward in a general pediatrics hospital
Has dedicated inpatient and outpatient pediatric oncology areas, others shared with pediatric hospital
Pediatric oncology trained physicians and nurse educators
All other services are provided through the general pediatric hospital
Universal coverage for children
National healthcare system absorbs all treatment costs
A non-for-profit organization supports the hospital
Regional: collaboration with AHOPCA as detailed for Guatemala
International: support from St. Jude Children’s Research Hospital International Outreach Program
Colombia (Latin America and Caribbean) (upper-middle-income economy)
Pediatric oncology ward in a national cancer institute (primarily an adult cancer institute)
The unit has dedicated inpatient and outpatients pediatric oncology area, others shared with adult institute
Pediatric oncology trained physicians and nurse educators
Most ancillary services are provided through the cancer institute, some are subcontracted to neighboring pediatric hospitals
Universal coverage for children
Ministry subcontracts with private insurances to serve as intermediaries with contributive and subsidized policies
International: twinning with Dana-Farber/Boston Children’s Global Health Initiative
What Are the Benefits to Building Pediatric Cancer Units?
In low-income countries (LIC) when children with cancer are referred to tertiary institutions, they are often grouped with children with other diagnoses, amidst adults with cancer, or both; wards may be overcrowded and/or have substandard hygienic facilities (lacking hand washing stations, hand sanitizers, individual toilets, etc.). Knowing that in the absence of reliable and accessible laboratory services most children with leukemia will die of infection before leukemia is diagnosed and that in the absence of chemotherapy or radiotherapy, solid tumors are exclusively surgical diseases, the absence of separate pediatric oncology wards in some LIC is no surprise. Furthermore, when standards of living are poor and health systems are fragmented or inadequate, pediatric cancer may either not seem to occur (because they are not diagnosed), present to medical attention for surgical interventions only (due to presence of a mass), or present to medical attention severely ill (due to advanced disease or sepsis). The reasons overcrowded settings prevail in LIC are likely multifactorial and variable from one place to another. Lack of space, staff, drugs, or expertise, as well as fatalism or a sense of futility of treatment, among others, may serve as explanations. It is through improvement of standards of living and health care infrastructure that children with cancer are able to reach medical care, be diagnosed with cancer, and stimulate the mobilization of resources towards the offering of cancer-directed therapy. Once the need to address pediatric cancer is acknowledged and more patients are diagnosed, expertise is built, and fundamental barriers to oncology care such as access to chemotherapy and safe handling of chemotherapy are addressed, clinicians building a PCU and looking to improve pediatric cancer outcomes must devise strategies for expanding towards the effective delivery of multimodal therapy (treatment that incorporates medical, psychosocial, supportive, surgical, and/or radiation oncology services).
PCUs often aim to cohort children with cancer. The justification for grouping children with cancer together and separating them from patients with other diagnoses, stems primarily from the desire to protect them [1]. However, this practice also creates several important opportunities that deserve to emphasized. From an organizational stand point, the introduction of chemotherapy, the infectious risks imposed by it, the need for close monitoring of toxicities, and the need for safe handling of chemotherapy and waste products often triggers the desire to separate cancer patients from others. Therefore, chemotherapy availability and administration can become the catalyst for physical creation of pediatric oncology wards. However, as mentioned before, a PCU is more than its infrastructure or physical space and can look differently in different parts of the world. Therefore, conceptualizing, creating, restructuring, or building a PCU creates opportunities for development of expertise by nurses, physicians, and surgeons (among other staff); standardization of care; education of dedicated staff in safe handling of chemotherapy; tracking of outcomes; prevention of infections; prevention or early management of toxicity; building of care teams; and provision of multidisciplinary patient-centered care (Fig. 5.1).


Fig. 5.1
Opportunities provided with the development of pediatric cancer units
In the absence of dedicated space and staff, the quality of care provided to children with cancer can suffer [1, 2]. Some practical examples include the following. Without specialized PCUs, general physicians and nurses may not be able to build the expertise to guide development and implementation of treatment guidelines (or protocols). Without standardization of care, outcomes cannot be reliably measured and the need or areas to improve may be overlooked or neglected. When cancer patients need to share rooms or beds with other patients, they may be exposed to infectious diseases (respiratory, diarrheal, or other) that are mild to moderate in the normal host but can lead to death in them due to their immunosuppressed status. Without specialized nursing (generated by experience or formal curriculum), toxic effects of chemotherapy and complications may not be recognized and managed promptly, leading to morbidity or death. Furthermore, if standard procedures to protect staff during administration of chemotherapy and to handle and dispose of potentially dangerous materials are not developed, health professionals and family members are unnecessarily exposed to potentially toxic substances. Finally, in the absence of a sense of unity, respect, and common purpose among providers, decision making about care and communication with patients and families can be heavily fragmented.
What Are the Key Components of a Pediatric Cancer Unit?
Key Components, Human Resources: The spectrum of human resources needed at the PCU depends on the diagnoses being treated, the volume of patients seen at the center, and its economic supports. As adapted from the St. Jude Children’s Research Hospital International Outreach Program in their “Guide to Establishing a Pediatric Oncology Twinning Program” [3], ideally human resources at the PCU include a medical director, other physicians (generalists, pediatricians, pediatric oncologists) trained and designated to care of children with cancer, nurses and nurse educator, data managers, and administrators, along with specialists in infectious disease and infection control, pathology, radiology, radiation oncology, surgery (general or pediatric oncologic, orthopedic, and neurologic), pharmacy, pain, palliative and psychosocial services, child life, nutrition, and rehabilitation. Some staff, such as physicians and nurses, are ideally dedicated to the PCU and do not work at other locations. Other staff is likely shared with other departments or institutions depending on the volume of patients at the PCU.
Key Components, Other: In conceptualizing a PCU, the natural reaction is to focus on diagnostics, therapeutics, and supportive care. However, a multitude of biologic `and nonbiologic factors likely influence pediatric cancer outcomes at the PCU (see Fig. 5.2). Biologic factors include genetics, exposures, clinical characteristics, and molecular characteristics of the cancer. However, by the time the patient arrives to the PCU and is diagnosed, biologic factors are for the most part not modifiable. Nonbiologic factors include social and economic context, oversight and support, referral and surveillance capacities, infrastructure availability, and the functionality of the care team at the PCU. In LMC, nonbiologic factors and their influence on outcomes need to be studied in more detail because nonbiologic factors are likely most influential, prevalent, and actionable among barriers to improvement of pediatric cancer outcomes. A comprehensive linear and ecologic model of nonbiologic factors that influence pediatric cancer outcomes in a PCU is presented in Fig. 5.3.
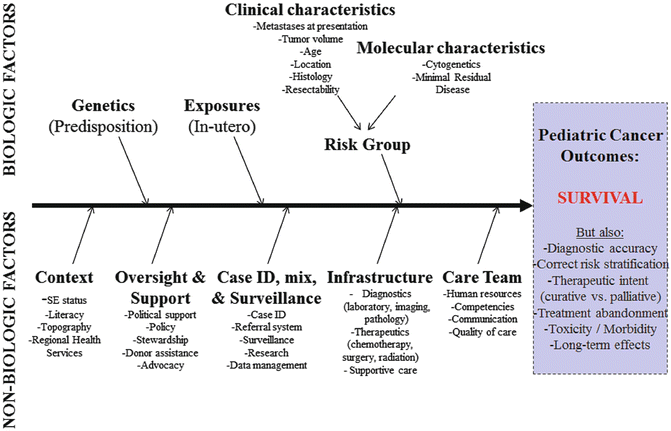
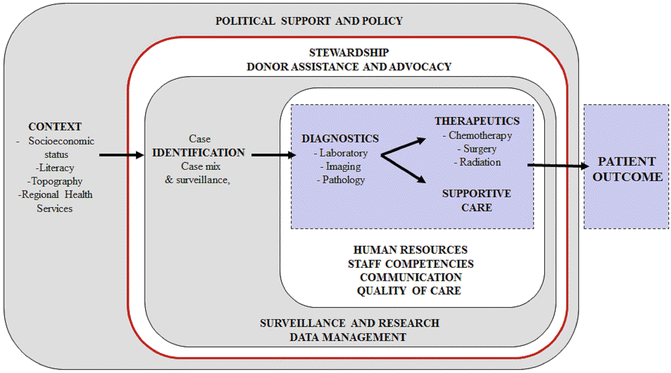

Fig. 5.2
Development of a pediatric cancer unit—conceptual model of factors that influence pediatric cancer outcomes

Fig. 5.3
Development of a pediatric cancer unit—linear and ecological conceptual model of nonbiologic factors that influence pediatric cancer outcomes
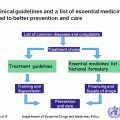
International Society of Pediatric Oncology and International Network for Cancer Treatment and Research (SIOP and INCTR) Recommendations: In 1991, the SIOP Working Committee on Standards of Care and Training and Working Committee on Psychosocial Issues in Pediatric Oncology put forward recommendations for the organization of a PCU [4]. This consensus document recommended that all children with cancer should be offered child-oriented diagnosis, treatment, aftercare, and follow-up and gave basic guidelines for the development of PCUs. With progressive improvement in the standard of care in many countries resulting in improved survival, it was recognized that the guidelines for development of a PCU needed to be updated. In 2008, the first revised document was presented at the SIOP Pediatric Oncology in Developing Countries (PODC) meeting, which was further revised at consecutive meetings, and consensus was obtained. In 2010 the SIOP guidelines were further modified by the members of the INCTR Pediatric Oncology Working Group, many of whom were active SIOP PODC members. Table 5.2 shows the joint SIOP/INCTR recommendations for the development of a PCU [58].
Table 5.2
SIOP/INCTR recommendations for the development of a PCU
Introduction | ||
1. All children, adolescents, and young adults deserve age-appropriate diagnosis, treatment, aftercare, and long-term follow-up. This is best offered in a dedicated pediatric cancer unit (PCU) | ||
2. A PCU should be situated in or have an affiliation with a general hospital, pediatric departments, or cancer center | ||
3. Depending on the extent of services offered, all PCUs can be divided into Level I, II, or III | ||
General guidelines for all levels of PCUs | ||
1. The age range of patients served in a PCU can range from infancy through adolescence or even early adulthood (i.e., up until the age of 21 years) | ||
2. Basic infection control practices (e.g., good hand-washing policies, isolation of infectious cases) should be incorporated into all levels of PCUs | ||
3. All PCUs should be staffed by pediatric oncologists or pediatricians who have had some formal training in pediatric oncology | ||
4. All physicians involved in the diagnosis and care of cancer patients should have a working knowledge of palliative care including pain management | ||
Level I PCU | Level II PCU | Level III PCU or center of excellence |
Definition | ||
1. A Level I PCU is defined as a unit capable of diagnosing and delivering care for the most curable and least complicated pediatric oncology diagnoses (i.e., Hodgkin’s disease, non-Hodgkin’s lymphoma, ALL, and Wilms’ tumor) | 1. Level II PCUs should be able to provide all the services of a Level I PCU and most/many of the services of a Level III PCU | 1. A Level III PCU should be a special unit within a large general hospital, cancer center, pediatric department, or children’s hospital capable of treating all forms of pediatric cancers |
2. This PCU should also have the capability of diagnosing and stabilizing patients with more complicated cancer diagnoses prior to transfer to a higher level PCU | 2. Level II PCUs should work in close collaboration with a Level III PCU locally or nationally | 2. Should have a minimum of 50 or more new diagnoses of pediatric cancer/year |
3. Level II PCUs could be involved in the training of pediatric oncology specialists, but the main responsibility/direction of such training efforts would remain with the Level III units | ||
4. Under the direction of the Level III PCUs, these units could participate in clinical trials and research | ||
Facility and infrastructure | ||
1. Level I PCUs could be located in large cities (as one of several units) or small cities and towns (outside major metropolitan areas) where the PCU might be the only center for treatment for noncomplicated cancers | 1. Level II PCUs should be able to provide all the services of a Level I PCU and most/many of the services of a Level III PCU | 1. Inpatient facilities in an advanced PCU should include a dedicated pediatric cancer unit with isolation rooms, some hepa-filtered rooms, and a separate procedure room |
2. Be located within or associated with a general hospital | 2. Level II PCUs should work in close collaboration with a Level III PCU locally or nationally | 2. Outpatient facilities should include the following |
Day clinic or infusion center (for short-term infusion chemotherapy and blood products) | ||
Area for routine chemotherapy (i.e., vincristine by IV push) and antibiotics | ||
Procedure area with capacity for conscious sedation | ||
Rooms for physical exam | ||
Designated areas for blood draws, including management of central lines | ||
Chemotherapy safety protocol and procedures to avoid chemotherapy errors and to manage chemotherapy accidents (i.e., spills) | ||
3. Have capacity for both inpatient and outpatient care (ability to deliver chemotherapy for abovementioned tumors, provide antibiotics for fever and neutropenia, and transfusions) | 3. Have at least a hospital-based tumor registry | |
4. Have the ability to communicate with all ancillary services (e.g., telephone or pager) | 4. Have the resources and capability to be the training center for pediatric oncology specialists (including, but not limited to, doctors, nurses, advanced practice nurses and/or physician assistants, pharmacists, and social workers) | |
5. Have a chemotherapy safety protocol and procedures to avoid chemotherapy errors and manage chemotherapy accidents (i.e., spills) | 5. Have an organized medical record keeping system in place with data managers | |
6. Have a hospital policy for the procurement, storage and accountability of drugs (usage, expiration dates, availability of stock), and other essential supplies needed for chemotherapy preparation and administration. A link with a central procurement agency may be needed in some countries | 6. Have multidisciplinary clinics for bone tumors, brain tumors, and retinoblastoma | |
7. Pediatric ICU capable of taking care of all oncological emergencies | ||
8. Access to an accredited blood bank with capability to supply all components of blood (PRBC, platelets, plasma, cryoprecipitate, factor concentrates) | ||
9. A hospital infection control team | ||
10. Hand-washing facilities on all inpatient and outpatient units, dirty and clean utility areas, and a waste disposal system | ||
11. Have a hospital policy for the procurement, storage and accountability of drugs (usage, expiration dates, availability of stock), and other essential supplies needed for chemotherapy preparation and administration | ||
12. A link with a central procurement agency may be needed in some countries | ||
All drugs listed in the WHO Essential Drug List should ideally be available in the hospital pharmacy | ||
13. All chemotherapy drugs and antibiotics needed for the execution of treatment protocols should be available in the hospital pharmacy | ||
Staff | ||
1. Pediatric oncologists or pediatricians with basic training in pediatric oncology | 1. Level II PCUs should be able to provide all the services of a Level I PCU and most/many of the services of a Level III PCU | 1. Medical personnel who have specialized training in pediatric oncology (pediatric oncologists, pediatric oncology nurses, pediatric oncology pharmacists, pediatric oncology social workers and/or psychologists, etc.) |
2. Nurses trained in basic pediatric care and care of the immunocompromised patient | 2. Level II PCUs should work in close collaboration with a Level III PCU locally or nationally | 2. “Round the clock” access to a pediatric oncologist/pediatrician, nursing, social worker, and chaplain should be available 7 days a week |
3. Availability of a pathologist or interventional radiologist for fine needle aspirate/biopsy | 3. Dedicated oncology pharmacy with laminar hoods. An oncology pharmacist would be ideal; however, a pharmacist trained in oncology, working within a general pharmacy setting would be acceptable | |
4. Availability of a surgeon—preferably pediatric surgeon | 4. Availability and access to other essential subspecialty services | |
Pediatric surgery, neurosurgery, orthopedic surgery, and ophthalmology | ||
Radiation oncology | ||
Cardiology, renal (dialysis), infectious diseases, gastroenterology | ||
5. Nursing staff should have a working knowledge of chemotherapy administration, i.e., placement of IVs, administration of fluids, and basic chemotherapy (i.e., vincristine, cyclophosphamide) | ||
6. Pediatricians should have a basic, working knowledge of chemotherapy; its administration; and side effects. This includes intrathecal chemotherapy | ||
7. Pediatricians should be trained to do procedures (bone marrow aspiration/biopsy, spinal taps with intrathecal chemotherapy) and the interpretation of the results | ||
8. “Round the clock” access to a pediatric oncologist/pediatrician and nursing should be available 7 days a week | ||
Diagnostic facilities | ||
1. Access to a basic laboratory (crossmatch, basic chemistry, bone marrow stain, and CBC) | 1. Level II PCUs should be able to provide all the services of a Level I PCU and most/many of the services of a Level III PCU | 1. Radiology services should include availability of CT, MRI, nuclear medicine services including PET, MIBG, and bone scan |
2. Plain X-ray facility—optimally ultrasound capability | 2. Level II PCUs should work in close collaboration with a Level III PCU locally or nationally | 2. Availability or access to pathology services, including hematopathology, flow cytometry, cytogenetics, and molecular genetics |
3. Have a close link to a higher level PCU which could accept patients with more advanced disease, confirm diagnoses, and recommend treatment | 3. Specialized laboratory services with the ability to do all chemistries, special tests, and microbiology | |
4. Availability of pathology services for diagnosis | ||
5. Access to an accredited blood bank | ||
Support services | ||
1. Emergency transportation for patients between institutions or for home | 1. Level II PCUs should be able to provide all the services of a Level I PCU and most/many of the services of a Level III PCU | 1. Nutrition |
Availability of a dietitian or nutritionist | ||
2. Basic resuscitation equipment on-site including wall or portable oxygen | 2. Level II PCUs should work in close collaboration with a Level III PCU locally or nationally | 2. Rehabilitation services |
Occupational therapy, physical therapy, speech therapy | ||
3. Adequate hand-washing facilities and sinks | 3. Psychosocial services for patient and families | |
Social work, child life, pediatric psychology/psychiatry, pastoral care, and teachers | ||
Neuropsychologist—if possible | ||
4. Transportation for patient-related activities | 4. Resources for placement and maintenance of central lines, administration of high-dose chemotherapy, and management of anticipated complications | |
5. Long-term follow-up/cancer survivorship program | ||
6. Palliative care program | ||
Inpatient and outpatient services with access to home care and hospice | ||
Staffed by dedicated personnel familiar with pain and symptom management and end-of-life care | ||
7. Bone marrow/stem cell transplantation program | ||
Advanced PCUs may, but would not, necessarily need to have an “on-site” BMT/Stem Cell Transplant Program | ||
8. Parent support group | ||
9. Availability of a guest house, food, subsidized payment structure, transportation, and housing | ||
Academic activities | ||
1. Have a close link to a higher level PCU, which could accept patients with more advanced disease, confirm diagnoses, and recommend treatment | 1. Level II PCUs should be able to provide all the services of a Level I PCU and most/many of the services of a Level III PCU | 1. All Level III PCUs should be linked to a national and/or international multidisciplinary pediatric oncology organization |
2. Have the ability to follow simple therapeutic protocols | 2. Level II PCUs could be involved in the training of pediatric oncology specialists, but the main responsibility/direction of such training efforts would remain with the Level III units | 2. Academic affiliation |
3. Continuing education for all physicians and, as appropriate, for other members of the pediatric oncology team. Must attend at least one local/national/international conference or formal meeting devoted to pediatric oncology annually | 3. Academic activities | |
IRB, Ethics Committee | ||
Tumor board | ||
Multidisciplinary conferences | ||
Participate in a “twinning program” with another center of excellence | ||
Be directly involved in or have the ability to conduct clinical trials and research | ||
Be the center for CME conferences, training of residents, fellows, nurses, pharmacists, and social workers | ||
Additional recommendations | ||
1. Access to microbiology facilities | ||
2. Only utilize established treatment protocols | ||
Certification or Needs Assessment Program for PCUs: No consensus exists regarding the need for certification or periodic assessment of a PCU. In the SIOP/INCTR recommendations, it was suggested that programs that had international collaborations or twinning programs could use those resources for guidance and improvement. However, there was consensus that a process of certification, though voluntary, could be used to obtain support and additional services from governmental agencies or supporting NGOs.
Can We Address Pediatric Cancer in Resource-Limited Settings?
Eighty percent (80 %) of the children with cancer live in low- and middle-income (LMC) [5]. Although regional variations in the incidence of childhood cancers have been reported [6, 7], the high proportion of pediatric cancer in LMC stems primarily from the fact that most of the world’s population under 18 years of age lives in LMC [8]. The etiology of childhood cancers remains predominantly unknown [6, 7] and increases in incidence of childhood cancer in LMC are usually a reflection of improved detection rates [2, 5]. Mortality from childhood cancer on the other hand is significantly higher in LMC compared to HIC, with a survival gap >50 % between some LMC and HIC [2]. Therefore, the highest burden of pediatric cancer in LMC and the centerpiece of cancer disparities between HIC and LMC does not result from high or changing incidence, but from differences in cancer-related mortality. In order to address this burden, PCUs in LMC can focus on reducing the risk of cancer-related death by promoting early diagnosis, delivering effective care, reducing the risk of treatment-related death, and reducing treatment abandonment (Fig. 5.4).
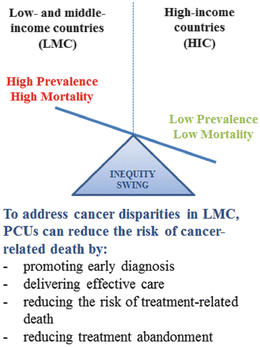

Fig. 5.4
Cancer disparities inequity swing
What about LIC or extreme resource-limited settings (ERLS)? What can clinicians do to address pediatric cancer in these settings? While providing an answer, experts in HIC sometimes struggle with envisioning pediatric cancer care without the full spectrum of technologies routinely used for diagnosis, monitoring, and treatment. In contrast, clinicians in LIC or ERLS struggle with how not to address pediatric cancer despite known human resource scarcity, equipment shortages, and high workloads. Clinicians in LMC are witnesses to how, as standards of living improve and health systems are strengthened, the burden of cancer becomes more noticeable and the need to address it imperative [9].
When addressing pediatric cancer in LMC, clinicians should keep in mind pediatric cancer is a heterogeneous group of diseases. Therefore centers in LMC, even those in ERLS, can make valuable contributions to the burden of childhood cancer in their settings. In this section we summarize four key examples of optimization of resources: the case-by-case approach; protocol development and prioritization; comprehensive integration of pain, palliative, and psychosocial services; and capacity building and education through twinning.
The Case-by-Case Approach: Epidemiologists seek to understand patterns, causes, and consequences of childhood cancer; pediatric oncology collaborative groups seek to standardize care and study and compare best strategies; and public health departments seek to address prevention, screening, and delivery of care at the population level. However, ultimately, cancer occurs at the level of the individual and must be addressed case by case. Even in pediatric oncology centers of excellence in HIC where access and standardization are the norm, the fit between open collaborative trials, standards of care, patient and family’s goals of therapy, and the patient’s diagnosis and clinical situation is made case by case. When making decisions about pediatric oncology treatment, centers in LMC, including ERLS, must consider factors such as the patient’s status, proposed therapeutic plan, resources available, and nonbiologic factors (see Table 5.3).
Table 5.3
Factors to consider when making decisions about pediatric oncology treatment using the case-by-case approach
Patient | Therapeutic plan | Resources | Nonbiologic factors |
|---|---|---|---|
Diagnosis | Goals of therapy | Chemotherapy safety | Direct and indirect costs of therapy |
Extent of disease | Expected toxicity | Supportive care | Transportation, housing, income |
Nutritional status | Expected morbidity (from treatment, surgery, or radiation) | Surgical equipment and postoperative care | Culture and values |
Comorbidities | Resectability (upfront surgery vs. delayed) | Radiation therapy access, planning, and toxicity
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|