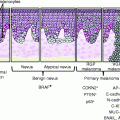
A. Melanocytic nevi
E. Atypical melanocytic tumors
1. Lentigo simplex
1. Atypical Spitz/Reed tumors
2. Junctional, compound, and dermal nevus
F. Atypical dermal melanocytosis
3. Congenital nevi
1. Pigmented epithelioid melanocytoma
(i) Proliferative nodules in congenital nevus
2. Dermal melanocytic tumors of uncertain malignant potential (MELTUMP)
(ii) Superficial atypical melanocytic proliferation
G. Melanoma
4. Nevi, rare variants
1. Precancerous melanosis (in situ melanoma)
(i) Halo nevus
(i) Lentigo maligna
(ii) Balloon cell nevus
2. Superficial spreading melanoma
(iii) Recurrent melanocytic nevus
3. Lentigo maligna melanoma
5. Nevi of special sites
4. Acral lentiginous melanoma
(i) Genital nevi
5. Mucosal lentiginous melanoma
(ii) Acral nevus
6. Nodular melanoma
B. Spindle and epithelioid cell nevi
7. Melanoma, rare variants
1. Pigmented spindle cell nevus (Reed nevus)
(i) Melanoma in congenital nevus
2. Epithelioid and spindle cell nevus (Spitz nevus)
(ii) Desmoplastic melanoma
C. Dermal melanocytosis
(iii) Neurotropic melanoma
1. Blue nevus
(iv) Nevoid melanoma and minimal deviation melanoma
2. Cellular blue nevus
(v) Malignant blue nevus
3. Desmoplastic nevus
(vi) Others
4. Deep penetrating nevus
D. Dysplastic nevus
Melanocytic Nevi
Lentigo Simplex
Lentigo simplex is a small well-defined localized area of hyperpigmentation that is associated with proliferation of melanocytes along the dermoepidermal junction in contiguity (Fig. 7.1). This lesion characteristically is not associated with the nesting phenomenon of the nevus cells, and there are elongated retia, melanophages, and sparse dermal chronic inflammatory infiltrate. The main variants of lentigo simplex include nevus spilus which histologically may be indistinguishable from lentigo simplex or may show the features of lentigo simplex with occasional junctional nesting (jentigo). The principal differential diagnosis of lentigo simplex includes freckle, junctional nevus, or dysplastic nevus. The freckle is a nonproliferative lesion of melanocytes which is associated with hyperpigmentation of the basal skin layer. It usually is not associated with hyperplasia of the rete ridges. If junctional nests are present in a lentigo, it is impossible to exclude that the lesion represents an early junctional nevus. According to Hafner [4] the absence of BRAF, FGFR3, and PIK3CA mutations differentiates lentigo simplex from melanocytic nevus and solar lentigo. The dysplastic nevus characteristically is associated with a proliferation of nevocytes along the dermoepidermal junction but with cytologic atypia. The nests in lentigo simplex when present are small and lie at the tips of the rete ridges; the nests in dysplastic nevi vary in size, are associated with discohesion of nevocytes, and lie variously along the lateral side of the rete ridges and even between them in the superpapillary epidermis. Furthermore, there are stromal changes including increased vascularity and prominent inflammation associated with striking lamellations of collagen and often concentric eosinophilic fibrosis around the rete ridges. Senile lentigo shows irregular rete hyperplasia with often irregular shapes at the base of the rete ridges. The most common shape is a “footlike” array with dense hyperpigmentation without melanocytic hyperplasia. Lentigo maligna is more strikingly different than lentigo simplex/senile but must be included because of the prominent single cell proliferation along the epidermis which may appear benign in early lesions. Lentigo maligna differs with a strikingly atrophic epidermis and sun-damaged dermis and often with a band-like or lichenoid inflammatory infiltrate admixed with melanophages. Furthermore in lentigo maligna the proliferation of melanocytes extends along the external root sheath of the hair follicles, even to the base of the hair follicle. Mucosal lentigines (labial, vulvar, penile) and acral lentigines may closely resemble lentigo simplex with increased number of intraepidermal basal pigmented melanocytes with dendritic morphology. Lentigines may be associated with systemic syndrome (LAMB, LEOPARD, Carney complex) [5].
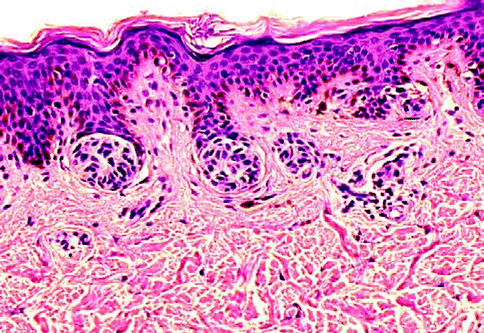

Fig. 7.1
Junctional nevus
Junctional, Compound, and Dermal Nevus
Junctional melanocytic nevi are focal pigmented lesions that by definition are flat to slight raised and exhibit intraepidermal nesting of nevomelanocytes usually localized to the tip of slightly hyperplastic rete ridges (Fig. 7.2). A nest of nevomelanocytes is considered to be five or more cells in a single cluster. Characteristically, the cells in the nests of junctional nevi are round to oval and lie contiguously together with scattered, usually coarse, melanin granules in their cytoplasm. The nucleus of the junctional nevus cells is larger than the normal nevomelanocyte and is round to oval, and usually a delicate nucleolus is evident. While the melanocytes in a nest are clumped together, because of separation artifact they usually are clearly separate from the adjacent keratinocytes with a space separating the nests from the keratinocytes. Often the nevus nests compress the adjacent keratinocytes that become elongated and fusiform with oval nuclei. No necrosis or apoptosis of the epidermal cells is present. At low-power magnification, one appreciates a well-circumscribed lesion that usually has hyperplasia of rete ridges with lentiginous proliferation of nevomelanocytes along the dermoepidermal junction but with nesting. The nevocytes have round to fusiform nuclei with tiny dot-like blue nucleoli. Their cytoplasm is clear and contains rather coarse melanin granules. Dendritic processes are not obvious although stubby small dendrites may be observed by high-power examination or with immunohistochemical stains (HMB45). Occasionally the lesions, especially in acral sites, may not be associated with a lentiginous proliferation of melanocytes but with junctional nests. In such instances there may be trans-epidermal elimination of nests with their presence being noted even in the stratum corneum. The variants in junctional nevi include the nevi of childhood in which there may be prominence of single cells in the epidermis even to the level of the granular cell layer (pagetoid pattern), confined to the central part of the nevus (Fig. 7.3). Such intraepidermal proliferation, however, retains the benign characteristics of the cells in lentiginous array and in nests. Congenital junctional nevi are histologically indistinguishable from acquired nevi in small biopsies except that the predominance of deep dermal or periadnexal nests is more common in the congenital nevus than in the acquired nevus. Acral junctional nevi are often associated with a proliferation of single cells in the stratum spinosum characteristically overlying the junctional nests. The lentiginous junctional dysplastic nevus shows variation in morphology of the single cells and also in the size of the nests. Likewise, rather than showing a cohesive aggregate of nevus cells in the nests, the dysplastic nevocytes are characteristically discohesive in nests and show irregularities in nuclear size and shape in individual nests. Superficial spreading melanoma may show a prominent nesting pattern, but the cells are large and have prominent eosinophilic nucleoli and large cytoplasm filled with fine, dustlike, melanin granules; there is less retraction from keratinocytes of the nests; and it presents an “aggressive” proliferation with keratinocytes apoptosis (Fig. 7.4). Finally, the pigmented junctional spindle cell nevus is composed of a uniform population of spindle cells in well-defined and oriented nests, perpendicular or parallel to the skin surface. A compound melanocytic nevus refers to a lesion in which there is a proliferation of nevus cells in nests both in the epidermis and in the dermis. Low-power examination reveals a well-circumscribed symmetrical lesion. The intraepidermal component usually is associated with well-developed nests present at the tips of the rete ridges with a regular and repetitive pattern. A lentiginous proliferation, similar to dysplastic nevus, is variably present in the compound nevus and in particular in the central area of congenital compound nevi (Fig. 7.5). Usually the intraepidermal component does not extend beyond the dermal component but if present, the extent beyond the dermal component is symmetrical on both sides of the lesion. The junctional nests are identical to those described under the junctional nevus. Intraepidermal junctional nevus cells have been described by the designation type A cells. These A cells are large round nevus cells with coarse melanin granules within them. In the dermal component of the lesion the picture is quite variable. In early lesions there are small nests of cells, many similar to type A cells, with large round nuclei present in the papillary dermis. These cells as they increase in number with lesion aging become smaller and round without pigment. They exhibit tiny nucleoli and are associated with fine fibroblast-like cells surrounding the nest. As the lesion ages, these cells are associated with a spindle-shaped cell that has been designated a type C cell at the base of the lesion. The type C cells are associated with increase in stroma with eosinophilic ground substance, an increased reticulum fibers present separating the type C cells from one another. The type C cells can become arranged in highly complex patterns resembling neuroid structures or neurofibromas. Type A cells express S100 protein and HMB45. Type B cells may express either S100 or the Schwann cell-associated antigen but they are HMB45 negative. This variation in morphology and immunochemical findings is considered to be a maturation phenomenon of the nevus cells in their dermal component (Fig. 7.6). If the dermal component extends deeply into the dermis, in particular along adnexal structure, one must consider a congenital nevus. The differential diagnosis of the compound nevus includes nevus spilus. Some variants of nevus spilus have dermal nests and therefore represent variants of compound nevus. The dysplastic nevus usually is a type of compound nevus but with an irregular proliferation of atypical nevomelanocytes in the epidermis overlying the dermal component. There is no symmetry in this lesion so that the dermal component lies eccentrically in relationship to the epidermal component. Also in the compound nevus there is no atypia. Nodular melanoma can be differentiated from the compound nevus by the fact that there is, in addition to intraepidermal nesting, usually pagetoid aggressive spread of malignant melanocytes at the shoulder. Furthermore the characteristic maturation of type A to B to C cells is absent. In melanoma the deeper cells frequently exhibit finely granulated pigment in their cytoplasm, an atypical and rare finding in compound nevi. Also, the deeper component is not associated with a pushing border or expansive dermal nodule formation in nevi; this change is characteristic of melanoma. Mitotic activity in the dermal component is extremely rare in compound and dermal nevi, whereas it is common in melanomas [6]. Finally, compound nevi very rarely have a striking inflammatory response, whereas melanoma is commonly, especially below the intraepidermal component, associated with a lymphocytic host response. The term dermal melanocytic nevus refers to a lesion in which the nevus cells are completely confined to the dermal component with no intraepidermal involvement. The epidermis may be normal or flatted because of effacement of the rete ridges. There is no melanocytic proliferation or nesting in the typical dermal nevus. Occasionally, scattered single large nevomelanocytes are present overlying a dermal nevus but they are not considered significant unless there is a striking contiguous proliferation of them. The papillary dermis is unremarkable although occasionally there is some fibrosis of the papillary dermis. The nevic component of the dermal nevus exhibits nests of cells and sheets of cells that extend into the deep widened papillary dermis. As the cells reach the papillary-reticular dermal junction, they frequently are noted to infiltrate as single cells into the superficial reticular dermis. The presence of nests of cells in the reticular dermis in an otherwise banal appearing dermal nevus is considered an abnormality of maturation (see section “Proliferative Nodules in Congenital Nevi”, pag. 119). The characteristic lesion shows single cells that often have a fibroblast-like appearance. These cells are confined to the upper reticular dermis but presence in the deep reticular dermis suggests a congenital nevus. There is no evidence of preferential expansile growth in the form of expansile nodules in the deep component of a dermal nevus. The dermal nevus cells are usually type B and type C in nature. The superficial portions may be associated with nests of type B cells, while the deeper portions show the fibroblast-like changes associated with type C cells. Occasionally in the very superficial portions of the dermal component, type A cells as large round pigmented cells may be present within the upper papillary dermis. As the type C cells proliferate as S-shaped fibroblast-like cells with increasing ground substance, they resemble neurofibromas. Extensive organized stromal aggregates may resemble Wagner-Meissner corpuscles. Lesions containing prominent type C cell proliferation with extensive ground substance are dubbed “neuronevi” by Masson. In most such lesions one can still observe evidence of melanocytic proliferation, specifically nests of nevic cells. However, in some lesions there is no evidence of melanocytic activity. To differentiate such lesions from neurofibromas, one must observe the adventitial dermis of hair follicles and vessels. Neurofibromas infiltrate the adventitial dermis up the basement membrane of the external root sheath. Neuronevi on the whole respect the adventitial dermis. Mitoses do not occur in benign dermal nevi nor is the reevidence of expansile nodule formation deep in the nevus. “Aging” of dermal nevi has been associated with senescent changes which have been likened to the “ancient change” of schwannomas. Balloon cell changes also represent a possible senescent phenomenon due to degeneration of melanosomes. Sclerosis and infiltration by fat cells is related to involuting phase of the nevus. In such lesions there are hyperchromasia of nuclei, multinucleate giant cell formation, and large blue nucleoli; however, mitoses are absent. More than three mitoses in one section suggest to perform multiple levels to exclude a nevoid melanoma. In dermal nevus there is no evidence of necrosis and expansile clonal nodule formation and the nuclei do not contain the bright eosinophilic nucleoli of melanoma cells. One interesting change that occurs in dermal nevi is the presence of multiple large spaces lined by nevus cells. These spaces resemble vascular structures. The most important differential diagnostic consideration of the dermal nevus is melanoma. In melanoma there is no evidence of maturation of type B to type C cells. Rather, the lesion is composed of a uniform clonal population (single or multiple) of pleomorphic cells. In the depth of the lesion, often cells are larger rather than smaller in melanoma in contrast to the maturation of nevi. Mitoses are present throughout the lesion, in particular in the proliferative marginal areas, whereas in dermal nevi no mitotic activity is observed. A useful method in differentiating compound and dermal nevi from melanoma is to use the immunocytochemical stain with HMB45 and p16 antibodies. HMB45 usually does not stain the dermal deep component of ordinary acquired nevi. However, up to 30–40 % of the dermal component of dysplastic nevi can stain with HMB45 and all the dermal melanocytosis are positive. In such cases the histology of the lesion allows for the correct diagnosis. p16 is strong positive both in the nuclei and the cytoplasm of the nevi and negative in the vertical phase of the melanoma.
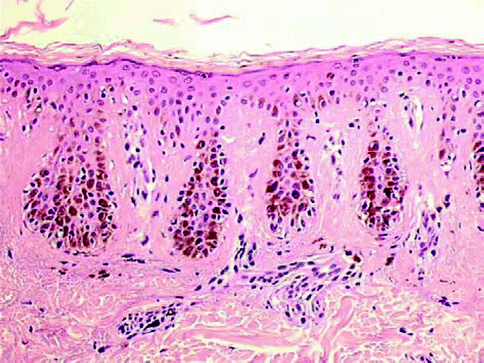
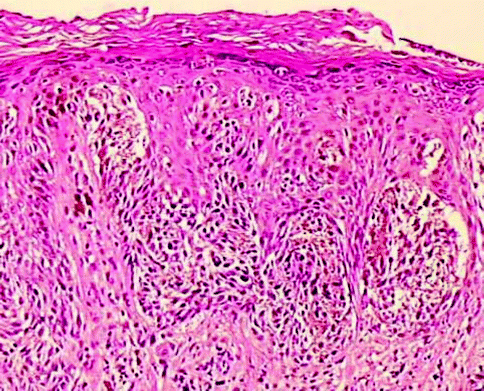
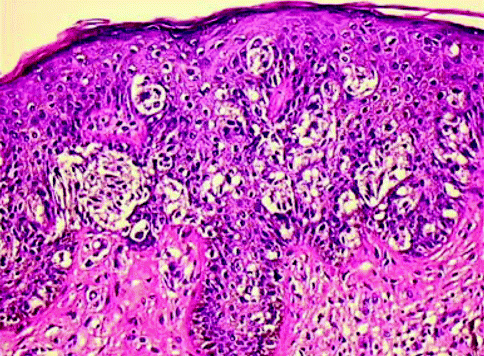
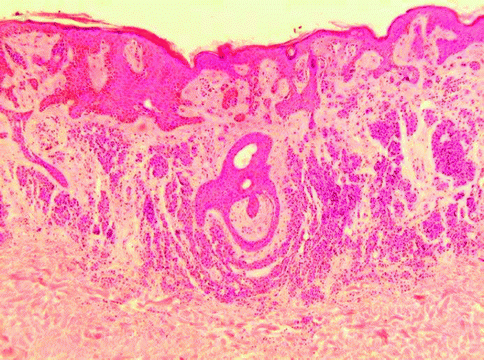
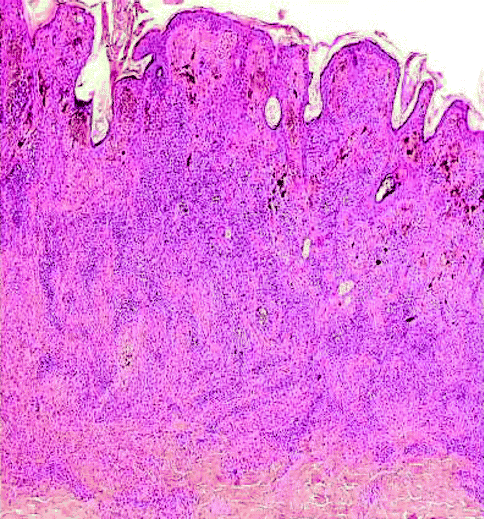

Fig. 7.2
Lentigo simplex

Fig. 7.3
Childhood junctional nevus

Fig. 7.4
Melanoma, pagetoid spread

Fig. 7.5
Congenital compound nevus, lentiginous proliferation-like dysplastic nevus

Fig. 7.6
Compound nevus: maturation
Congenital Nevi
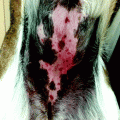
Congenital nevi, by definition, are pigmented skin lesions present at birth. These lesions are divided in small (up to 1.5 cm in size), intermediate, and giant congenital nevi. Intermediate lesions are greater than 1.5 cm but can be removed by simple excision. Giant lesions, on the other hand, require often multiple staged excisions for removal. The histopathologic picture of a congenital nevus may be indistinguishable from an acquired nevus. According to Mark and Mihm [7], the following patterns are diagnostic of congenital origin: (1) intramural or subendothelial nesting of nevus cells in small– to medium–size arteries, veins, and lymphatic vessels; (2) nevus cell nests in the papilla of the hair follicle; (3) nevus cells scattered in single cell array throughout the lower reticular dermis and subcutaneous fat; (4) nevus cells in arrector pili muscles; and (5) nevus cell in tight perivascular array mimicking an inflammatory infiltrate throughout the reticular dermis. Thus, a congenital nevus may show areas of lentigo, areas of junctional nevus, areas of compound nevus, or areas of dermal nevus with limitation to the papillary-reticular dermal junction of the nevus cells. However, in many congenital nevi in striking contrast to acquired nevi, the nevocytes extend into the lower third of the dermis and may even involve the subcutaneous fat. The presence of this change is often also associated with involvement of skin appendages, vessels, and nerves. The dermal involvement in the lower reticular dermis and fat is predominantly composed of single cell array resembling the so-called Indian file. Involvement of the hair follicle includes nests in the lower two-third of the external root sheath, nests in sebaceous glands, or even nests of nevic cells in the papillae. Similarly, nests may occur in eccrine ducts or in eccrine glands. Subendothelial deposits of nevocytes may be observed in both the walls of arteries and veins. Finally, protrusion of nevus cells, delimitated by endothelial cells, into the lymphatics in the superficial and deep dermis may also be noted and distinguished by true lymphatic invasion by a melanoma (Fig. 7.7). Less specific is a pattern of nevic cells surrounding the appendages in the adventitial dermis and extending out into the collagen of the reticular dermis. At times the reticular dermal collagen may be strikingly abnormal in the congenital nevus. Thus, the collagen is infiltrated by the nevic cells, but the fibers of collagen are small in diameter, do not show striking interlacing as normal reticular dermal collagen fibers, and often lie parallel to the long axis of the epidermis. This type of reticular dermal change is more common in adult nevi in our experience than in the children’s congenital nevi. High magnification reveals the cells to be of the type B cell in the superficial dermis but more of a type C cell appearance in the deeper dermis having a more fusiform aspect. Thus, the cells infiltrate singly amidst collagen fibers in the subcutaneous fat, have a rather fusiform appearance. However, at times throughout the entire dermis and into the subcutaneous fat, type B nevus cells may be noted. The giant congenital nevus [8], on the other hand, more commonly has extensive proliferation of nevus cells that are present throughout the entire dermis and into subcutaneous fat and may even be present in fascia and muscle beneath the cutaneous lesion. The histological alterations in these lesions, while composed of type B and type C cells or fusiform cells, are much more extensive and the changes involving appendages much more easily observed in the giant congenital nevus than in the small congenital nevi. Likewise in the large congenital nevi there are often quite prominent complex neuroid structures, areas of neurofibroma-like differentiation associated with chondroid and cartilage. Areas of cellular blue nevi and blue nevi may be observed in the giant congenital nevus as well as areas of spindle and epithelioid cell nevi. Frequently, when one is dealing with a congenital nevus that is extensive and deep, it is necessary to perform S100 stain to identify the depth margin of the lesion. If a congenital nevus is removed regardless of reason, the fascia and muscle below the skin must always be observed for the possibility of residual nevi nests.
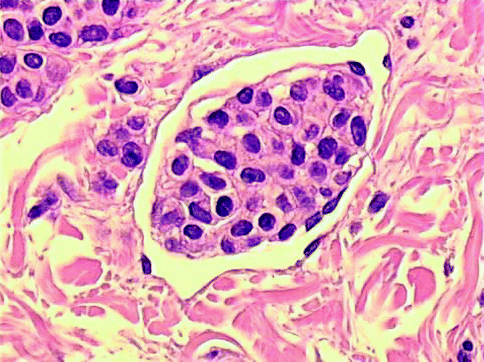

Fig. 7.7
Congenital compound nevus: subendothelial deposits of nevus cells (pseudo-vascular invasion)
Proliferative Nodules in Congenital Nevi
Congenital nevi can exhibit expansile nodular proliferations of nevus cells which are a cause for concern and present difficulties in the histopathologic diagnosis [9]. The more common nodule is composed of nevocytes that are type B in character; it is benign and shows no mitotic activity (Fig. 7.8). Any expansile aggregate of melanocytic origin must be carefully evaluated for features of malignancy which include severe nuclear atypia, the presence of necrosis of nevocytes with or without ulceration, the presence of an expansile destructive behavior of the nodule with deformity and obliteration of adjacent structures, and the presence of mitoses. Very importantly, there is usually a striking and abrupt border between the benign nevus cells and the atypical melanocytic cells in the malignant lesions. Benign lesions show cells that tend to blend with the nevus cells in the adjacent congenital nevus. Thus, there appears to be a maturation from those cells in the midst of the lesion to the surrounding nevic cells. Malignant nodules are usually greater than 5.0 mm. in size. Lesions that show some mitotic activity (less than 7/mm2), without necrosis, without ulceration, and without destructive deforming architectural features are considered as atypical and diagnosed the lesion as an atypical nevomelanocytic proliferation in a congenital nevus (see section “Atypical Melanocytic Tumors”, pag. 139). The cellular components of these nodules may include populations of type B nevus cells or population of spindle and epithelioid cells. The histochemical stain for reticular fibers (silver impregnation method) can be useful in the differential diagnosis with melanoma; the positive fibers surround with a dense network every individual cell in the proliferative nodule; on the contrary, they are pushed to the periphery in the nodule of melanoma vertical growth. The congenital nevus of the scalp in the occipital region has been associated with intracranial nevocytic proliferations. Meningeal proliferations occur as well as proliferations of nevus cells along penetrating arteries of the brain substance. Rarely, proliferations in the cisterna have led to internal hydrocephalus. The combination of an occipital congenital nevus and internal hydrocephalus is known as Touraine’s syndrome. The principle differential diagnostic considerations include tumors of neural and fibroblastic origin. The common presence of neuro-differentiation resembling neurofibromas and schwannomas in giant congenital nevi may lead to an erroneous diagnosis if the entire clinical picture is not evident. We have found that in cases of peripheral nerve sheath tumors occurring in the setting of giant congenital nevi, one may find striking involvement of nerves by nevus cells in such cases. This change may help to identify the origin of the peripheral neural proliferation in a congenital nevus. However, taken along the peripheral nerve sheath, tumors can be indistinguishable from spontaneous neurofibromas or schwannomas and the clinical history may be necessary to identify the correct pathogenesis of the lesions. The dermatofibrosarcoma protuberans, especially the Bednar tumor or pigmented variant, can sometimes be confused with a fibroblastic variant of the congenital nevus. In congenital nevi, however, one does not find a storiform pattern. Also, the dermatofibrosarcoma protuberans is a large deforming nodule which displaces appendages. The fibroblastic variant of the congenital nevus is associated with a linear deposition of collagen fibers parallel to the long axis of the epidermis frequently, does not act as a deforming nodule, and hence leaves appendages unaltered.
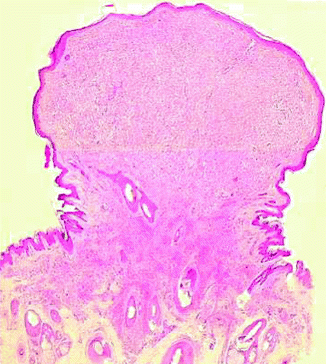

Fig. 7.8
Proliferative nodule in congenital nevus
Superficial Atypical Melanocytic Proliferation in Congenital Nevi
Another difficult histologic interpretation is the observation of striking epithelioid cells in the epidermis and superficial dermis of congenital nevi, especially in young patients that may simulate a superficial spreading melanoma. The nevus cells may show prominent nuclei with evident nucleoli and finely granular melanin in their cytoplasm. A careful evaluation of these proliferations reveals that the nuclei show very delicate and regular chromatin and that nuclear cytoplasmic ratios are small. Another important pattern is the absence of pagetoid spread at the nevus shoulder. Also the dermal component shows a propensity to mature and blend with the underlying dermal nevus cells.
Nevi, Rare Variants
Halo Nevus
The halo nevus is defined as a pigmented lesion with usually benign clinical characteristics surrounded by a zone of depigmentation that is symmetrically disposed [10]. This rim of depigmentation gives rise to the name halo. Histologically there is a compound nevus usually associated to a very striking, band-like, infiltrative lymphocyte response that is present among the dermal component of the nevus (Fig. 7.9). In addition there is loss of melanin with tagging of lymphocytes along the dermoepidermal junction in the depigmented area. The halo nevus has also been known as Sutton’s nevus or leukoderma acquisitum centrifugum. The halo is usually round or oval and the pigmented lesion is symmetrically disposed in the center of the lesion. The type of pigmented lesion that may give rise to a halo nevus includes ordinary acquired compound, junctional compound and dermal nevi, compound nevi of Spitz, dysplastic nevi, and rarely congenital nevi. Halo blue nevi rarely occur. Characteristically the cells that infiltrate the nevus are T cell in type. Halo nevi are most commonly associated with compound nevus that is symmetrical, well circumscribed, and composed of intraepidermal nesting with type B cells and C cells in the dermis. At times one may see lymphocytes migrating into the junctional nests with lymphocyte nevus cell satellitosis. Lymphocyte nevus cell satellitosis is commonly observed in the dermal component. Altered nevus cells have an eosinophilic appearance to their cytoplasm and many binucleate with prominent nuclei forms are noted. On the whole most of the dermal nevus cells are larger than normal nevus cells. Mitotic figures are only rarely found in halo nevi and should alert suspicion of possibly an atypical proliferative lesion leading to examination of multiple sections. As far as pigmentation is concerned, the nevus cells may show residual melanin pigment. Occasionally scattered melanophages are noted around the inflamed dermal component as well as below the adjacent epidermis. Halo nevi must be distinguished from halo dysplastic nevi or from melanoma with vertical growth phase. In halo dysplastic nevi in addition to the brisk infiltrate, one observes a proliferation of atypical nevomelanocytes in the epidermis well away from the dermal component. These cells are present in lentiginous array and show irregular nesting. One must be aware, however, that most dysplastic nevi have some host response associated with them. The host response is usually not associated with lymphocyte nevus cell satellitosis of either the intraepidermal or the dermal component of the nevus or with the presence of lymphocytes along the dermoepidermal junction with lymphocyte nevus cell satellitosis. Melanoma can be differentiated histologically on the basis of the atypia of the cells, the presence of an expansile nodule growth in the dermal component and aggressive infiltration of the epidermis with pagetoid spread, and the presence of scattered mitoses with or without ulceration and necrosis. As far as the cytology of the individual cells is concerned, the halo nevus cells may be larger than normal nevus cells but show benign nuclear characteristics, whereas the melanoma cells show very pleomorphic nuclei with irregular chromatin patterns and with very prominent eosinophilic nucleoli. Likewise in the halo nevus, pagetoid spread is usually absent or minimal. Furthermore the intraepidermal component is a well-defined nested pattern as opposed to the melanoma which shows both irregular-sized nests and pagetoid spread. Nevus cells may be very difficult to discern in the midst of the inflammatory infiltrate. The S100 and particularly p16 stain may be helpful in finding the nevus cells in a suspect lesion.
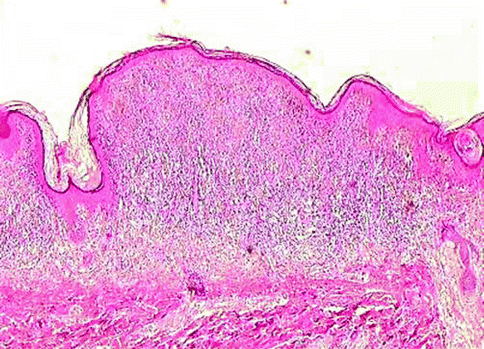

Fig. 7.9
Halo nevus
Balloon Cell Nevus
In balloon cell nevus more than 50 % of dermal nevomelanocytes manifest abundant clear, finely vacuolated cytoplasm and small hyperchromatic nuclei with a scalloped contour (Fig. 7.10) [11]. Multinucleation may be observed. The main differential diagnosis is that of balloon cell melanoma, based on the recognition of a malignant neoplastic cytology; in balloon cell melanoma, the majority of cells manifest pleomorphism, the nuclei appearing large with irregularly distributed chromatin, mitoses, and necrosis.
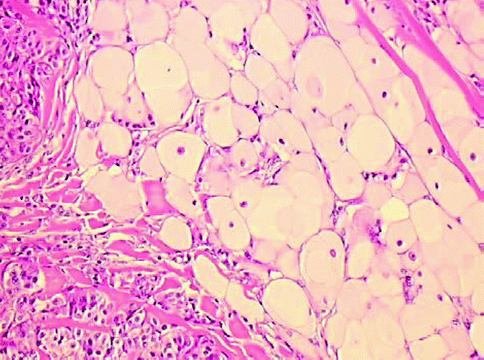

Fig. 7.10
Balloon cell nevus
Recurrent Melanocytic Nevus
Usually the recurrent nevus is a compound nevus but in 32 % of cases it is a dermal nevus and a junctional nevus in 5 % of cases. Less than 10 % of recurrent nevi are dysplastic nevi. The recurrent nevus describes the appearance of pigmentation at the site of a previously removed benign (Fig. 7.11) lesion usually incompletely affected by shave biopsy [12]. The histological problem is frequently an intraepidermal melanocytic growth of atypical melanocytes in lentiginous array over the previous scar that suggests pagetoid melanoma [13]. Clinically, the recurrence occurs very rapidly, usually in 1–2 months. This speed of recurrence is helpful in distinguishing a recurrent nevus from a recurrent melanoma which usually takes a much more protracted period of time even up to years for the recurrence to appear. The recurrent nevus presents a broad intraepidermal proliferation of melanocytes, sometimes greater than 6.0 mm in width with a combination of intraepidermal nests and single cells. The pagetoid spread of both nests and single cells is frequently observed. However, involvement in the stratum corneum does not commonly occur. Mitoses are characteristically absent in the intraepidermal component and in the superficial dermal component where single cells sometimes infiltrate the scar (Fig. 7.12). Nests predominate over single cells in this proliferation. The intraepidermal cells often have an epithelioid or round appearance with scattered melanin in their cytoplasm. This change resembles superficial spreading melanoma cells but the nuclear picture is that of a benign process with finely dispersed nuclear chromatin and small blue nucleoli. In the dermis beneath the proliferated lesion, there may be single round cells of a melanocytic nature but there are often numerous melanophages with scattered inflammation. Admixed with the depth of the scar or beneath it, one will find at the base of the scar nests of type B or type C nevus cells evidence of the residual dermal component of the lesion. Recurrent spindle and epithelioid cell nevi are one variant which may cause difficulty because of the propensity of the nevic cells to infiltrate the collagen of the scar. However, the spindle and epithelioid cell recurrences do not form expansile nodules but rather wedge-shaped proliferations with a mimicry of the inverted triangle of the benign spindle and epithelioid cell nevus. The recurrent dysplastic nevus is associated with epidermal atypia of a more marked degree. We have rarely seen epidermal hyperplasia with elongate rete in recurrent dysplastic nevi overlying the scar. The recurrent blue nevus shows characteristic dendritic cells that lie adjacent to the dermal fibrotic zone of the scar. Recurrent melanoma is the most significant differential diagnostic consideration in which one finds pagetoid spread of severely atypical epithelioid cells in the area adjacent to the scar in addition to the area overlying the scar. Likewise, if there be a dermal component, it is composed of an expansile nodule or vertical growth phase equivalent. One must always remember that recurrent nevi may be associated with single cell infiltration of the scar; this change should not be interpreted as melanoma. Review of prior material is essential when available.
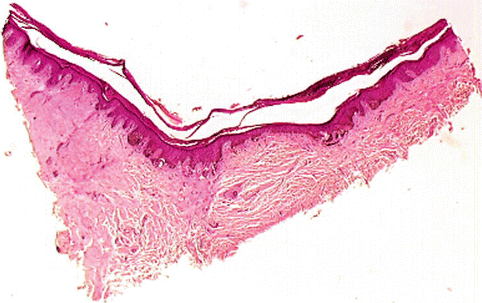

Fig. 7.11
Recurrent nevus and scar

Fig. 7.12
Recurrent nevus
Nevi of Special Site
The nevi of particular body sites (skin of genital, breast, acral, and belt area) and in physiologic states such as old or young age or pregnancy may show junctional or dermal proliferation that simulates dysplastic nevi and melanoma. This relatively frequent, recently recognized, and growing group of nevi has been termed, generically, nevi of special sites.
Nevi of Genital Skin
These nevi commonly affect the vulva of young women but occasionally occur on the male genitalia as well. Low-power examination reveals usually a well-circumscribed lesion with nevus cells in nests both intraepidermally and dermally. There is often easily visible a single cell basal proliferation which may extend on both sides of the dermal component of the lesion when present; pagetoid spread, but of benign melanocytes, is found in approximately two-thirds of the patients. Keratinocytic hyperplasia is variable and does not show the regular rete ridge elongation of the dysplastic nevus [14]. Beneath the epidermis there is a coarse fibrosis without pattern that is distinctly different than the lamellar fibroplasia of the dysplastic nevus. The dermal component is usually confined to the upper half of the reticular dermis (Fig. 7.13) and consists of nests of nevus cells. High-power magnification usually shows a lentiginous and nested proliferation of nevomelanocytes but often spindle or epithelioid forms. These cells form oval discohesive theques that are present at both sides of the tips of the rete ridges and sometimes span the supra-papillary epidermis. Fusion of adjacent rete at their tips and even confluence of junctional nests in a plaque-like configuration may be noted. Extension along the basal layer of appendageal structures, especially the outer root sheath of the hair follicles, may be a striking feature. The high-power examination reveals that the spindle cells resemble some constituents of the compound nevus of Spitz, but these are smaller than the Spitz nevus cells and have less evident nuclear membrane and less prominent nucleoli. Chromatin however is evenly dispersed through the nucleus and there is a thin chromatin rim. Multinucleate giant cells in the intraepidermal component are a frequent finding. Often there are nests of pigmented epithelioid cells in the epidermis that are variably pigmented. These cells have small nuclei but often prominent nucleoli. As stated above, pagetoid spread may be observed but the pagetoid cells are not malignant in appearance and have a nuclear morphology similar to those in the intraepithelial and intradermal nests. The lesions tend to be symmetrical; if they have lateral extent, it appears symmetrically on both sides of the dermal component. Cells in the dermal component are disposed in nests which become smaller both in the size of the nests and in the size of the cells as they infiltrate into the papillary-reticular dermal junction and deeper into the reticular dermis. However, in some lesions one observes small nests of pigmented epithelioid cells entrapped in the collagen. The deeper cells mature into ordinary type B nevus cells. The lesion most commonly mistaken for the vulvar nevus is superficial spreading melanoma. One confidently can exclude superficial spreading melanoma by appreciating the characteristic spindle and epithelioid morphology of the intraepidermal component, the lack of cytologic features of malignancy including pleomorphism and prominent eosinophilic nucleoli, as well as an absence of mitotic activity. The second lesion to be considered is the dysplastic nevus. Dysplastic nevi may occur on vulvar skin as well as on male genital skin but they are very rare. The genital nevi are usually isolated lesions and are not associated with evidence of dysplastic nevi elsewhere.
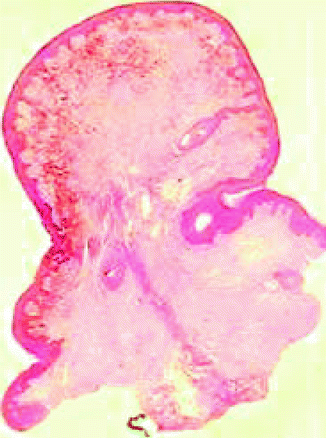

Fig. 7.13
Vulvar nevus: superficial dermal component
Acral Nevus
The main morphological features distinguishing the acral lentiginous nevi from other acral non-lentiginous nevi are: elongation of rete ridges; continuous proliferation of melanocytes at the dermoepidermal junction; presence of single scattered melanocytes, or less commonly small clusters, within the upper epidermis; poor or absent lateral circumscription; melanocytes with abundant pale cytoplasm and round to oval, sometimes hyperchromatic, nuclei; and prominent nucleoli present at the dermoepidermal junction (Fig. 7.14). Some histological features of acral lentiginous nevi are similar to those of dysplastic nevi; however, anastomosing rete ridges, cytological atypia, and well-formed lamellar fibroplasia are usually absent. The histopathological criteria to distinguish these nevi from melanoma are the lack of pagetoid lateral spread, the absence of mitotic activity in the deep dermal component, and the evidence of dermal nevocytic differentiation. The identification of this benign acral nevus, which we have identified as the benign counterpart of acral lentiginous melanoma, is important in order to avoid misdiagnoses and consequent under- or over-treatment of doubtful pigmented lesions of acral skin [15].
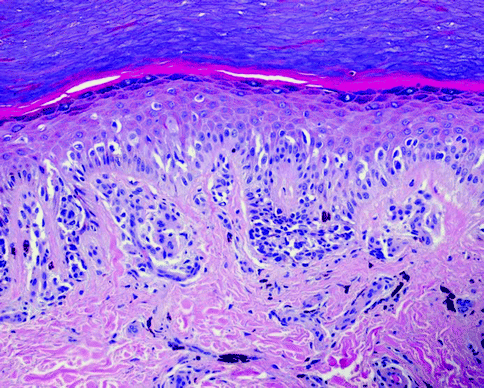

Fig. 7.14
Acral nevus
Spindle and Epithelioid Cell Nevi
Pigmented Spindle Cell Nevus (Reed Nevus)
It is a benign tumor characterized by proliferation of spindle nevus cells intensely pigmented that are confined at the dermoepidermal surface or the top portion of the papillary dermis. Described by Reed in 1975 as pigmented spindle cell nevus, “non-Spitz” is also considered a variant of the epithelioid and spindle cell nevus. Its identification has clinical significance. The lesion is usually a patch or plaque or a plaque with a small papule in contrast to the compound nevus of Spitz that is a papule or nodule. The spindle cell nevus is a symmetrical lesion, with sharp margins, characterized by a proliferation of spindle nevus cells, intensely pigmented, with typical superficial location limited to the dermoepidermal junction and sometimes extended to the superficial portion of the papillary dermis (Fig. 7.15). In the typical form the melanocytes are arranged in bundles or nests fairly regular in shape, size, and distribution and oriented in a vertical or horizontal pattern to occupy the junction and supra-basal portion of the epidermis and the superficial papillary dermis [16]. The extension to the dermis is often absent and, when present, is usually in bundles or small sharply demarcated nest and rarely to individual cells or in small groups irregularly shaped that may mimic melanoma in its spindle cell variant. In spindle cell nevus, however, the haphazard growth and pagetoid intraepidermal spread of melanocytes, especially at the lateral shoulder of the lesion, are absent. Pagetoid spread in the central nested area of the lesion is observed. The cell population often occurs uniformly in fusiform shape, with elongated nucleus and dispersed chromatin, small inconspicuous nucleoli, and little cytoplasm, with abundant melanin pigment, often in coarse granules. Epithelioid cells are rarely observed and even rarer is the presence of multinucleated giant cells. Mitosis may be present, even in large number, but at the junction the presence of a high mitotic index with evidence of atypical mitoses and depth should suggest the possibility of melanoma. The epidermal changes are characterized by elongation of the rete ridges and moderate hyperkeratosis. Frequently in the dermis, a lymphocytic infiltrate and numerous melanophages are present. Ulceration and foci of necrosis are usually absent. In addition to the previously described typical pattern, variants of the spindle cell nevus are reported: pigmented spindle cell nevus with prevalent epithelioid cells, atypical pigmented spindle cell nevus, plexiform pigmented spindle cell nevus, and combined spindle cell nevus. Plexiform pigmented spindle cell nevus is characterized by the extension of the melanocytic proliferation in the reticular dermis in the form of bundles of spindle cells intensively pigmented often in intimate association with adnexal structures, nerve or vascular, and interspersed by melanophages, isolated and in aggregates. This variant simulates the deep penetrating nevus, from which it differs, in particular, for the presence of an evident junctional proliferation of spindle cells in nests. The combined pigmented spindle cell nevus variant is characterized by the combination of a spindle cell nevus with another different nevus, more frequently dermal nevus or blue nevus. Although the spindle cell nevus shows cytoarchitectural aspects similar to epithelioid cell nevus (Spitz nevus) can be easily distinguished from this, especially for the presence of abundant melanin pigment and the presence of a junctional proliferation with little or minimal dermal extension. Differential diagnostic problems may arise with melanoma and dysplastic nevus, especially for the atypical variant of spindle cell nevus. The distinction from dysplastic nevus can be difficult when the spindle cell nevus presents cytoarchitectural focal atypia, in which case the marked tendency to arrange themselves in bundles of melanocytes and the general uniformity of the cell population are the main morphological aspects for a correct diagnosis of spindle cell nevus. The absence of a high degree of architectural disorganization and severe cytological atypia; the intraepidermal spread of melanocytes confined mostly to the lower half of the epidermis, with rare isolated melanocytes in the superficial portions, but limited to the central portion of the tumor; and the presence of uniform cell population within the entire lesion represent the essential criteria for distinguishing spindle cell nevus from melanoma. The trans-epidermal migration of isolated cell or small nests is frequently present in pigmented spindle cell nevus and can simulate a pagetoid intraepidermal infiltration; however, the lack of malignant cytologic character, the absence of spread beyond the nests into the normal epidermis, and the absence of destruction of the epidermis are the most important characters in differential diagnosis with melanoma.
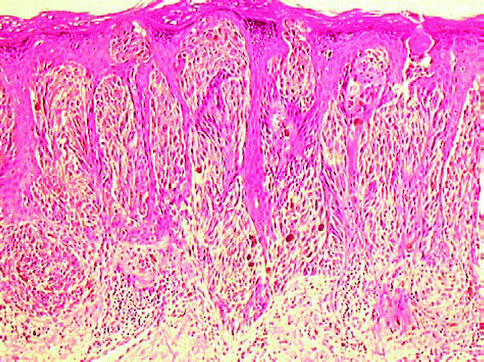

Fig. 7.15
Pigmented spindle cell nevus
Epithelioid and Spindle Cell Nevus (Compound Nevus of Spitz Nevus)
This lesion is a benign tumor composed of spindle-shaped epithelioid melanocytes that occurs predominantly on the skin of the face or limbs in young individuals. It was described for the first time by Sophie Spitz [18] who designated the lesion as juvenile melanoma [17, 18] and, subsequently, as benign juvenile melanoma by Kopf and Andrade [19] or as pseudomelanoma. Today, these terms are to be avoided. In 1960, Kernen and Ackerman introduced the term epithelioid and spindle cell nevus [19], which is now universally accepted. This terminology, highly descriptive, on the one hand underlines the salient histopathological appearance, represented by a benign proliferation of fusiform and epithelioid melanocytes. The spindle and epithelioid cell nevus shows typically a symmetrical appearance often with a triangular pattern with the base in the upper dermis parallel to the long axis of the epidermis and the apex toward the depth (Fig. 7.16). The melanocytes are frequently large with spindle and/or epithelioid appearance (Fig. 7.17), arranged mainly in bundles or nests or alveolar structures. The cytoplasm is often abundant, dense, and large; is finely vacuolated and even wispy, eosinophilic, or amphophilic; and, in some cases diffusely pigmented with sparse and small melanin granules. The nucleus of melanocytes presents a dispersed chromatin and a well- demarcated nuclear membrane. The nucleolus is often centrally located, prominent, basophilic, or eosinophilic. There are sometimes multiple (two or three) nucleoli, sometimes with cytoplasmic invaginations (intranuclear pseudoinclusion). The spindle and epithelioid cell nevus can be junctional, compound, or dermal. The spindle and epithelioid cell compound variant is the most common. Each type of the cell population may consist primarily of fusiform melanocytes, epithelioid cells, or both cell types. However, the pure epithelioid variant is most common in infancy. The presence of numerous epithelioid cells in an adult should prompt careful review and even levels if necessary to rule out a melanoma. It is not uncommon to find plurinucleate giant cells, also with four to five large nuclei. A useful rule of thumb is that the multiple nuclei are similar in a benign lesion. The malignant giant cell is associated with pleomorphic nuclei and nucleoli. The junctional component shows aggregate and nests of variable-size melanocytes unevenly distributed along the dermoepidermal junction (Fig. 7.18), often with major axis perpendicular to the skin surface. A halo, optically empty due to a fixation artifact phenomenon, separates the nests of the melanocytes from the epidermis. The junctional proliferation stops abruptly on the side margins and, if the nevus is compound, its lateral extension does not exceed the dermal component. The absence of lateral extension and dissemination supra-basal intraepidermal-type “pagetoid” cells represents an important criterion for differentiating epithelioid and spindle cell nevus from melanoma, especially in adults. In the child, in fact, you can also see frequently involved even up to the stratum corneum of the epidermis with trans-epidermal elimination of individual melanocytes or nests. In the superficial dermal layer, typical mitoses can be observed but also very rare atypical mitoses can sometimes be present even in the deep dermal portion; however, this character must be evaluated with caution because in the deep dermal portion, and along the proliferation margin, mitoses are more frequently present in melanoma. Marginal mitoses, namely, those within 250 μm of the peripheral border of the lesion, are of most concern and usually are associated with a metastatic potential of the lesion. In the dermal component, the melanocytes are arranged mainly in nests, bundles, or cords. The nests become smaller as they extend deeply and break up into single cells. These cells do not cause disruption of the dermal architecture so that the fibers appear to be falling apart. Rather, the cells appear as fibroblasts without destroying the architecture. Dr. Elston Helwig used to teach that the cells of the Spitz nevus look “at home in the dermis”, much like the space between the collagen fibers of the reticular dermis separated without destroying a feature often available in single file. The size of the cells decreases in the reticular dermis, becoming more rounded with little cytoplasm (maturation). This kind of transformation is a morphological appearance characteristic and important for the diagnosis of epithelioid and spindle cell nevus. Two other histological features are important for the histological diagnosis: the uniformity of the cell population morphology from side to side of the lesion and the presence of isolated single cells in a pseudo-infiltrative pattern at the base of the nevus, which reaches sometimes into the subcutaneous fat. The melanin pigment, when present, is in small quantities, most often in the superficial lesions, especially just below the epidermis. The spindle and epithelioid cell nevus is frequently accompanied by epidermal and stromal changes. Acanthosis, hyperkeratosis, pseudoepitheliomatous hyperplasia, and, less frequently, parakeratosis are frequently observed, especially in the epithelioid and spindle cell nevi of junctional or compound type, more rarely in the epithelioid and spindle cell dermal variant. The stromal alterations include frequently the presence of telangiectasia and edema of the papillary dermis, sometimes so marked as to create a clear zone of separation (“grenz zone”) between the epidermis and the dermal component of nevus. Near the dermoepidermal junction, the “bodies of Kamino” can be found; these are rounded eosinophilic bodies present singly or in small clusters; they were interpreted as “apoptotic bodies,” but they are now known to be portions of epithelial cell as well as nevus cell membranes admixed with cytosol. Lymphohistiocytic infiltrates are occasionally present and are scattered usually in the depth of the lesion. Dense infiltrates raise the question of a halo nevus or possibly an atypical lesion. Evidence of focal regression superficially with inflammation and fibrosis is usually a sign of a melanoma. Halo Spitz nevi, as any other halo nevus, have a prominent infiltrate that affects the entire lesion, not a focal portion. Numerous histopathological variants of the spindle and epithelioid cell nevus can be recognized. A desmoplastic variant [20] in which there is a striking pericellular fibrosis that encompasses the entire lesion. The brightly eosinophilic fibers highlight the often quite densely stained nevus cells. Clear epithelioid cell forms help to differentiate the lesion from a desmoplastic melanoma or sclerosing blue nevus. According to Hilliard [21], the staining pattern for p16 in desmoplastic melanomas and Spitz nevi in conjunction with the histopathologic features, S100 staining, Ki67proliferation index, and clinical scenario may aid in the difficult differential diagnosis between these two entities. There is also a balloon cell variant [22], a lichenoid variant, and as noted a halo variant [23]. Rarely a granulomatous response has been described. The myxoid variant [24], often with the presence of mast cells, is reported. The spindle and epithelioid cell nevus often poses problems of differential diagnosis with melanoma. In fact, some important characters for the histopathological diagnosis of malignancy, such as cellular atypia, intraepidermal spread, and the presence of mitosis, are not as conclusive if detected in epithelioid and spindle cell nevi. Knowledge of clinical features (age, location, mode of onset, duration, macroscopic and dermatoscopic appearance) is very important and sometimes essential for a correct interpretation of the lesion. One of the most important patterns is the cytology monomorphism present in Spitz nevus and absent in polyclonal proliferation of the melanoma. The junctional/superficial spindle and epithelioid cell nevus must be distinguished from dysplastic nevus and melanoma in situ. In favor of the dysplastic nevus are the proliferation of basal melanocytes with freckled “atypical” and polymorphic pattern and the presence of fused nests with major axis parallel to the skin surface, the elongation of the rete ridges, and the fibrosis of the superficial dermis. The presence of a continuous proliferation of atypical melanocytes with a tendency to invade aggressively the more superficial layers of the epidermis, atypical mitoses, and marked dermal inflammatory infiltrate, referring to the diagnosis of melanoma in situ or invasive horizontal growth phase melanoma. The compound spindle and epithelioid cell nevus can pose problems of differential diagnosis with invasive superficial spreading melanoma. In particular the epithelioid and spindle cell nevi that have marked trans-epidermal migration in nests or single cells epidermal infiltration can simulate a “pagetoid” spread and those with numerous mitoses especially in the deepest portion of the lesion. The dermal epithelioid cell and dermal spindle nevus can be misinterpreted as nodular melanoma; in these cases the clonal proliferation without evidence of deep dermal maturation, the presence of atypical mitoses, and the cellular necrosis and apoptosis can be important characters to distinguish Spitz nevus from melanoma. The use of additional diagnostic methods such as immunohistochemical stains with monoclonal antibodies and/or polyclonal (anti-S100, NSE, HMB45), the determination of DNA content (ploidy) by means of flow cytometry or image analyzer, and the evaluation of cell proliferation (PCNA) still do not allow to differentiate with certainty nevus epithelioid and spindle cell melanoma. In our experience and in recent report [25], p16 expression in nodular spitzoid melanomas and Spitz nevi, in conjunction with clinical and histopathological evaluation, may be a useful tool in differentiating between these two entities. Melanoma cases are associated with loss of p16 immunoreactivity without any correlation with their Breslow thickness, whereas the Spitz nevi have a strong positive nuclear and cytoplasmic expression of p16 staining (Fig. 7.19). More recently, techniques of in situ hybridization of DNA in paraffin slides have demonstrated some chromosomal abnormalities suggestive of melanoma [26]. Furthermore, the technique of comparative genomic hybridization (CGH) has revealed that in Spitz one can find an increased copy of the short arm of chromosome 11 [27]. Less often changes in chromosome 6 have been described [28]. These very limited changes are strikingly different than the myriad changes found in malignant melanoma.
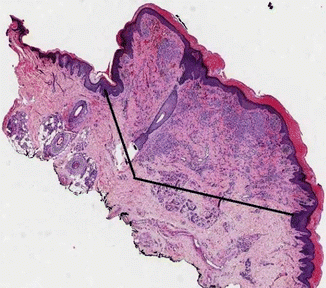
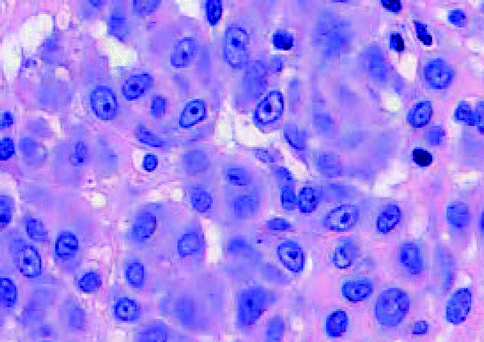
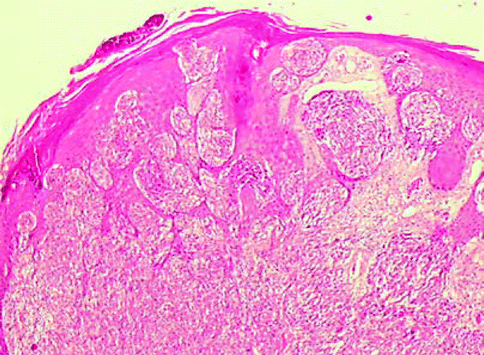
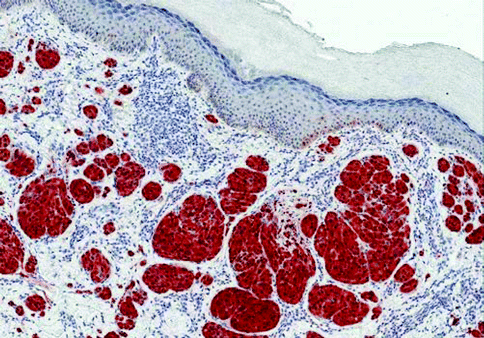

Fig. 7.16
Spitz nevus: reverse triangle

Fig. 7.17
Spitz nevus, epithelioid cells

Fig. 7.18
Spitz nevus, junctional and dermal proliferation

Fig. 7.19
Spitz nevus: nuclear and cytoplasm p16-positive stain
Dermal Melanocytosis
The blue nevus and its variants (cellular, atypical, and combined) belong together with desmoplastic blue nevus, deep-penetrating nevus, Mongolian spot, and the Ota and Ito nevi, are a group of lesions defined as the “dermal melanocytoses” [29]. These lesions are characterized by the proliferation of pigmented dendritic or epithelioid melanocytes located in the papillary and reticular dermis and by a prevalent immunohistochemical positivity with HMB45 antibody stain.
The Common Blue Nevus
The blue nevus is a benign melanocytic tumor in which the depth of melanin pigment present in nevus cells in the deep dermis is responsible for the characteristic color of the lesion which takes its name (Fig. 7.20). The blue nevus occurs predominantly in young people, with equal frequency in both sexes. The sites most affected are the skin on the backs of hands and feet and buttocks. Lesions also may be found on the trunk and on the scalp. Cases have been reported in extra-cutaneous site: oral cavity [30], uterus [31, 32], vagina [33], spermatic cord [34], and prostate (Fig. 7.21) [35, 36]; one case was described in a teratoma involving a mature cystic ovary [37] and in the bronchial tree [38]. Clinically, the lesions exhibit a blue, deep blue, blue-gray, or royal blue color. The blue nevus is characterized by three fundamental morphological aspects: (1) proliferation of dendritic melanocytes in the reticular dermis, (2) numerous melanophages, and (3) fibrogenetic stromal reaction. The dendritic melanocytes show typical elongate fusiform shape, with thin cytoplasmic extensions and prominent melanin pigment granules that sometimes obscure the nucleus. However, the pigment is always a brown finely granular one that differentiates the blue nevus cell from a melanophage in which the pigment is dense and coarse and often obscures the nucleus. In the blue nevus, the latter is oval to egg-shaped and displays finely dispersed chromatin, thin nuclear membrane, and inconspicuous nucleolus. Only rarely pleomorphism is present. The mode of cellular growth, individually or in small bundles, creeping between the collagen fibers of the dermis in a sometimes serpiginous pattern, without collagen fragmentation, is responsible for the apparent lack of cellularity of the lesion, which in low magnification can simulate the benign fibrous histiocytoma. Often one can observe a tendency of the nevus cells to arrange themselves around the skin appendages or along vessels or nerves. The fibrogenesis shows varying degrees, from mild to intense, until the formation of dense bundles that give the appearance of a desmoplastic or sometimes neuroid lesion. Commingled with the melanocytes melanophages with cytoplasm packed with large granules of melanin pigment are still present. In the typical form mitoses are usually not observed. The presence of a melanocytic proliferation-free zone immediately below the epidermis (grenz zone) is a characteristic feature. The epidermis may occasionally exhibit hyperplasia and hyperpigmentation of basal melanocytes very similar to the findings of a benign dermal fibrous histiocytoma. Frequently it is possible to observe multifocal areas of proliferation of the blue nevus. Among the variants of the blue nevus, one of the most common is the combined blue nevus where the lesion is presented in association with usually an acquired nevus. The ordinary nevus component may be a junctional, compound, or dermal nevus type. Less commonly, it may be a spindle and epithelioid cell Spitz nevus. Certainly one may find a combined congenital/blue nevus. The change may be present at birth. When it is acquired in the congenital setting, it appears as a change in the lesion. Because pigmented melanomas can occur in the deep portion of a congenital nevus and have a blue discoloration, we recommend always a biopsy of any acquired blue discolored area in one of these congenital lesions. The differential diagnosis of blue nevus is usually not a problem especially for the typical form. The differential diagnosis with other forms of dermal melanocytic proliferation must be considered. The nevus of Ota and Ito that, however, present a typical clinical pattern and location. The ectopic Mongolian spot is usually a congenital lesion that has similar coloration to the Mongolian spot in the lower posterior trunk. It is also a flat lesion with no consistency compared to blue nevi that are usually at least firm and slightly raised. The benign dermal fibrous histiocytoma, especially when there are marked hemosiderin deposits, and the tattoo should be also considered in differential diagnosis [39].
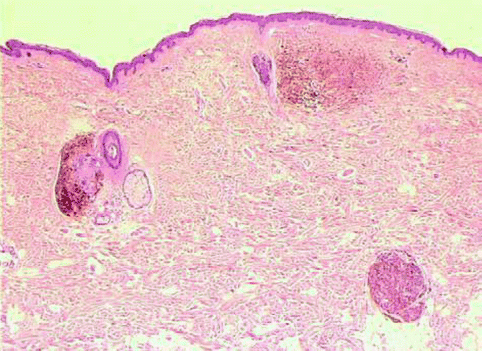
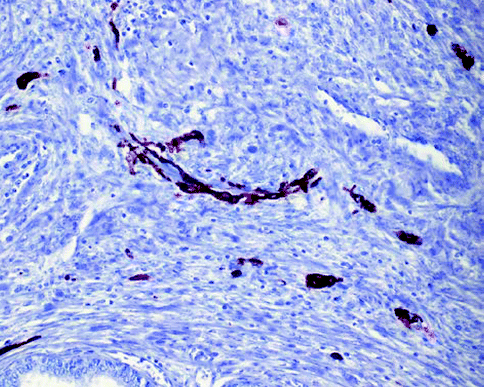

Fig. 7.20
Blue nevus

Fig. 7.21
Blue nevus prostate, HMB45 immunostain
Cellular Blue Nevus
Cellular blue nevus is a characteristic melanocytic tumor frequently localized in sacral coccygeal and gluteal area with a marked cellularity involving not only the reticular layer but also the hypodermis often with a characteristic dumbbell-shaped pattern” (Fig. 7.22). The tumor frequently exhibits a multinodular growth, and three different patterns can be identified: (1) mixed biphasic, (2) alveolar, and (3) neuro-nevoid. The mixed biphasic is the most frequent and is composed of large nodules of spindle cells intermingled with nests of epithelioid cells resulting from cross section of spindle cell fascicles. These nodules are separated from one another by zones of fibrosis. These fibrous zones contain melanophages but also exhibit characteristic blue nevus cells. Rare mitoses can be observed in these nodules as can some pleomorphism. The alveolar type presents rounded nests, with sharp edges; polyhedral and spindle cells, often pigmented with vesicular nuclei; inconspicuous nucleoli; and typical clear cytoplasm. The nests are surrounded by pigmented dendritic melanocytes and melanophages commingled with little stromal collagen which can occasionally occur edematous. In the neuro–nevoid type, the cells present schwannian differentiation and tend to aggregate in bundles that simulate the appearance of peripheral nerves; collagen and macrophages can be abundant. At times the cellular blue nevi can be so strikingly proliferative with ulceration that they raise the question with a nodular melanoma. However, the presence of dendritic cells with melanophages in fibrous stroma between the nodular islands of cellular blue nevi helps to differentiate this tumor from nodular melanoma. Likewise, the islands of cells are filled with cells containing clear cytoplasm with coarse melanin granules scattered amidst the clear cytoplasm. Overall the nuclei are repetitively similar and mitotic figures may be observed but the mitoses are not atypical and are not frequent in number. In nodular melanoma which resembles cellular blue nevi, there is very high grade nuclear atypia associated with mitotic activity and necrosis.
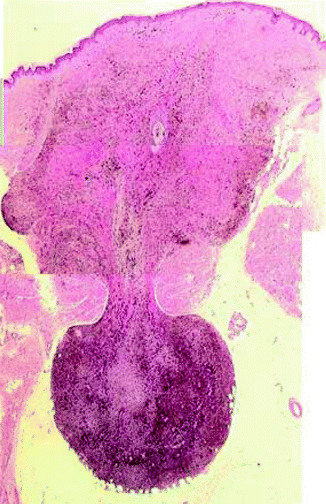

Fig. 7.22
Cellular blue nevus
Desmoplastic Nevus
Some nevi present or may become fibrous nodules for the presence of desmoplasia. The recognition of the desmoplastic nevus is important to distinguish from other benign skin lesions such as fibrous histiocytoma and dermal neurofibroma but also from desmoplastic melanoma [40]. The presence of desmoplasia in nevus may be related to regressive or reactive phenomena. The proliferation is usually centered in the papillary dermis but can also be extended to the reticular dermis and junctional activity often is minimal or absent. The epidermis overlying the tumor may present pseudoepitheliomatous hyperplasia, irregular acanthosis, and hyperkeratosis. The nevus cells are arranged singly or in small clusters, nests, or cords, and spindle cells predominating type C cells and epithelioid types A and B are less frequent or rare but always readily apparent. Sometimes there are bizarre giant cells with or ganglion-like aspects. Not uncommon is the finding of intranuclear inclusions caused by invaginations of the cytoplasm to the nucleus. Mitoses are rare and atypical mitoses are absent. The melanin pigment is irregularly distributed granules of different sizes and quantitatively variable. Rare are the melanophages. The stroma consists of eosinophilic collagen bundles usually much thicker than those normally recognized in the papillary dermis. The stroma often surrounds and isolates individual cells. Characteristic is a convex lens patter with sharp margins (Fig. 7.23). In Table 7.2 the main characteristics between desmoplastic nevus and desmoplastic melanoma are compared.


Fig. 7.23
Desmoplastic nevus, convex lens pattern
Table 7.2
Differential diagnosis between desmoplastic nevus and desmoplastic melanoma
Desmoplastic nevus | Desmoplastic melanoma | |
|---|---|---|
Junctional activity | Poor or absent | Nests of melanocytes with acral or lentiginous pattern |
Epidermis | Hyperkeratosis or irregular acanthosis
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|