Fig. 10.1
Columnar cell lesions. a Benign columnar cell change. The mildly dilated acini are lined by bland cells with columnar-shaped nuclei bearing apical snouts and with focal intraluminal microcalcification in secretions. b Flat epithelial atypia. Dilated acini lined by small, regular, uniform epithelial cells with round nuclei and nuclear stratification. Apical snouts and luminal calcifications are also noted in this case. c Flat high-grade DCIS. Dilated acinus lined by a flattened layer but this is formed from large pleomorphic cells (some more than 3× the diameter of adjacent red blood cells) with necrosis in the lumen. This represents flat high-grade DCIS rather than FEA, which is low grade
Various terminologies have been used to define columnar cell lesions with atypia; FEA has also been called, amongst others, columnar cell change with atypia, atypical cystic lobules, atypical cystic duct, atypical lobule type A, hypersecretory hyperplasia with atypia, columnar cell metaplasia with atypia, ductal intraepithelial neoplasia (DIN)1a, clinging carcinoma monomorphic type and small ectatic ducts lined by atypical ductal cells with apocrine snouts (for review, see Schnitt 2003) [2].
10.2.1 Histology
FEA is seen as dilatation of terminal duct lobular units (TDLUs) by one or more layers of mildly cytologically atypical cells resembling those of low-grade ductal carcinoma in situ (DCIS) (◘ Fig. 10.1b). By definition, FEA does not comprise high-grade nuclei. Those very uncommon, high nuclear grade lesions that are believed to share the same pathway as the other columnar cell processes are classified as flat high-grade DCIS (◘ Fig. 10.1c). Multilayering of nuclei is allowed but not a complex architecture in FEA; if this is seen, the diagnosis is of ADH or low-grade DCIS depending on the size and extent of the abnormality.
The acini in FEA are often of rounded internal contour and typically lined by cuboidal epithelium showing apocrine snouts, indicative of the columnar phenotype. Mild nuclear hyperchromasia, a high nuclear/cytoplasmic ratio and inconspicuous nucleoli are features of the lesion. An adjacent stromal lymphocytic infiltrate may be identified. These lesions are commonly associated with luminal calcifications.
The diagnosis of FEA is morphological. Immunohistochemistry is unhelpful as the lesion shares the same immunohistochemical profile (oestrogen receptor (ER) positive and Ck5/6 and Ck14 negative) as the non-atypical columnar lesions and as ADH and low-grade DCIS. In some studies it has been shown that these lesions suffer from poor interobserver reproducibility of diagnosis amongst general pathologists [3], but excellent concordance has been reported amongst breast pathology specialists following training, for example, with teaching sets of images [4]. However, concordance is generally better for exclusion of the diagnosis of atypia than its diagnosis [4].
10.2.2 Significance of FEA
FEA is currently regarded as precursor of low-grade in situ and/or invasive carcinoma, albeit with a low risk of progression. It commonly coexists with other lesions of the low nuclear grade neoplasia family [5] and shares common genetic alterations with those lesions, namely, deletion of chromosome 16q and gain of 1p. The changes are similar to those of associated DCIS or invasive carcinoma, if present [6, 7].
Relatively few studies, however, have looked at the long-term follow-up of those lesions. Eusebi and colleagues [8] reported one recurrence of 25 low-grade clinging carcinomas (FEA) over a period of 17.5 years. The recurrence was of essentially similar disease with no invasive component identified. Similarly, within the EORTC 10853 DCIS trial, 4 of the 58 low-grade clinging lesions showed local recurrence (but no invasive element) after a median follow-up of 10.5 years [9]. Another study showed no subsequent cancer following the diagnosis of FEA (n = 101) over a 10-year follow-up [10]. A Dutch series of 259 patients with 8-year follow-up reported development of cancer in 3.5% (nine invasive cancers, three of which were contralateral) [1]. A matched case control study showed a higher relative risk (2.32) for breast cancer development in patients with FEA [11].
However, another, more recent, analysis of the 282 women (2.4%) with FEA (from 11,591 women with benign biopsy) found a similar standardised incidence rate, at median follow-up of 16.8 years, for women with atypical hyperplasia with and without FEA (4.74 and 4.23, respectively) and for those with usual epithelial hyperplasia with and without FEA (2.04 and 1.90, respectively), indicating that FEA has a much lower risk of progression than ADH or DCIS and is probably more equivalent to the minimal risk seen with florid usual epithelial hyperplasia [12].
Although the long-term risk of development of breast carcinoma is very low, FEA may coexist contemporaneously with other lesions in the low-grade neoplasia family group. The upgrade rates following the diagnosis of FEA are approximately 15% [13–15], but are higher if ADH is also present (average 29%) [14] (◘ Table 10.1). A recent systematic review reported a pooled DCIS underestimation rate of 9% for FEA (confidence intervals (CI) 5–14%) and 20% (CI 13–28%) when ADH was present, compared with only 1.5% (CI 0.6–4%) for non-atypical lesions [16].
Table 10.1
Relative risk for subsequent breast cancer development and upgrade rate following the diagnosis of high-risk lesions
Lesion | Relative risk of subsequent carcinoma | Upgrade risk of contemporaneous carcinoma |
|---|---|---|
Flat epithelial atypia (FEA) | ×2 | 13–21%, average 15% (in pure form); if coexistent with AIDEP, then higher risk (pooled 29%) |
Atypical intraductal epithelial proliferation (AIDEP)/atypical ductal hyperplasia | ×4.4–5 ×8 if associated family history | 18–87% with 14-gauge core biopsy; pooled value 21% after vacuum-assisted biopsy |
Atypical lobular hyperplasia (ALH) | ×4–5 | 0–43%; pooled data 19% |
Classical lobular carcinoma in situ (LCIS) | ×8–10 | 0–60%; pooled value 27% |
Pleomorphic lobular carcinoma in situ (PLCIS) | Not known | 30–60%; pooled value 40% |
Radial scar (RS)/complex sclerosing lesion (CSL) | ×2 | 4–9% (if no epithelial atypia), 28–44% (if epithelial atypia); pooled value 25% |
Intraductal papilloma | ×2 | 3–13% (if no epithelial atypia); 33–37% (if epithelial atypia) |
10.3 Atypical Ductal Hyperplasia (ADH)
The term refers to an intraductal proliferation with both architectural and cytological atypia. It shares some histological features with DCIS but does not fulfil the quantitative and qualitative criteria for a DCIS diagnosis. ADH is an uncommon lesion diagnosed mainly in association with screen-detected calcification and may be an incidental finding [17].
The diagnostic criteria of ADH are not perfect, and the diagnosis suffers from lack of consistency amongst pathologists in some series. Complete concordance amongst 6 pathologists was achieved in 58% of 24 cases [18]. Data from the UK Breast External Quality Assurance Scheme [19] show low consistency in reporting ADH, albeit a large number of histological sections cut from any one block for circulation are unlikely to show the same representative features of this lesion that is, by definition, small and microfocal.
10.3.1 Histology
ADH diagnosis is quantitative. It is diagnosed where DCIS has been considered, but the lesion has not fulfilled the quantitative criteria for diagnosing DCIS (i.e. two membrane-bound spaces fully involved by the low-grade atypical intraductal epithelial proliferation, or at least 2 mm in size) (◘ Fig. 10.2a). ADH is therefore only diagnosed when there ispartial involvement of ducts by a monomorphic population of atypical cells that also shows architectural atypia, such as rigid bars or bridges or a micropapillary pattern (◘ Fig. 10.2b). The nuclei are similar to those of low-grade DCIS. The low-grade nuclei of both low-grade DCIS and ADH show a uniform pattern of immunoreactivity with ER (◘ Fig. 10.2c), Ck5/6 and Ck14. Cases with high-grade nuclei are not designated as ADH, but rather as high-grade DCIS, regardless of the lesional size.
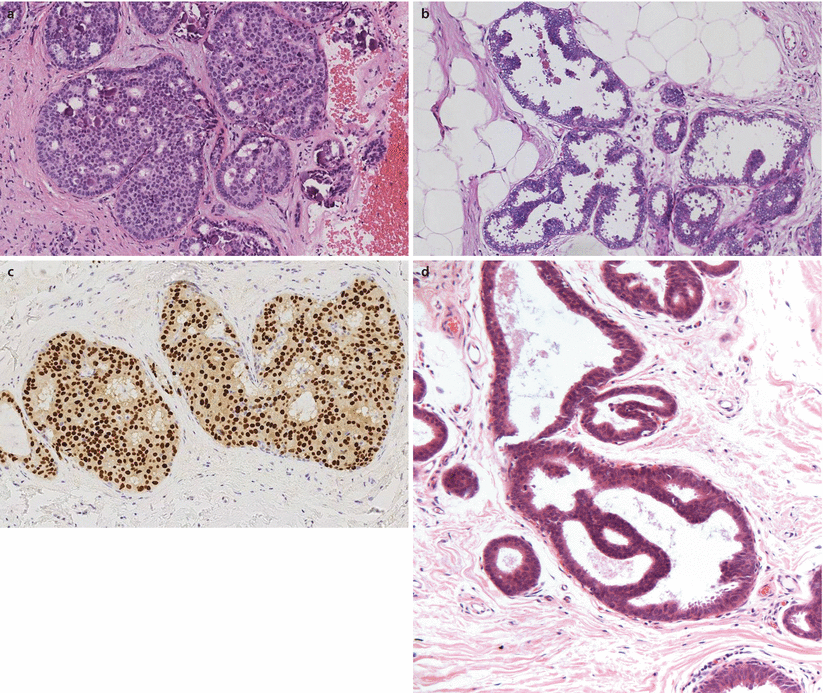

Fig. 10.2
Atypical ductal hyperplasia and DCIS. a Low-grade cribriform DCIS. Ducts expanded by a population of small, uniform evenly spaced epithelial cells with a cribriform architecture. There is microcalcification within secretions. As this involved more than two spaces, this was classified as DCIS rather than atypical ductal hyperplasia. b ADH. This small atypical epithelial proliferation has a focal micropapillary architecture with bridges. This does not involve two complete spaces and represents ADH rather than DCIS. c Oestrogen receptor immunoreactivity in ADH/DCIS. Uniform and strong nuclear reactivity is seen in all lesional cells, assisting in confirmation of clonality. d Atypical intraductal epithelial proliferation (AIDEP) in core biopsy. This biopsy shows a duct space with an architecturally atypical proliferation on a background of FEA. It is not possible to assess true extent and this is regarded as AIDEP
Given the quantitative/extent criteria for diagnosis of ADH, it is not possible to definitively diagnose this entity on core biopsy specimens, as it is impossible to ascertain if there is additional disease in the adjacent breast and therefore whether the focus seen is actually a small portion of a larger area of low-grade DCIS. Whilst this may seem like semantics, in order to maintain the specific implications of the diagnosis (i.e. as a lesion of increased risk of subsequent carcinoma), it is recommended that the term ADH is only used for lesions which have been adequately sampled and that the term atypical intraductal epithelial proliferation (AIDEP) is used for core biopsy samples [20] (◘ Fig. 10.2d).
10.3.2 Significance of ADH
Patients with atypical hyperplasia have a 4.3 times higher risk of subsequently developing breast cancer than patients without proliferative lesions. A family history of breast cancer (FH) increases breast cancer risk 2.4 times (95% CI, 1.4–4.3) [21, 22] in some, but not all, series. ADH is believed to be precursor for low-grade DCIS [23].
10.4 Lobular Neoplasia
This refers to a spectrum of lesions characterised by solid proliferation of small non-cohesive cells within the terminal duct lobular units. The term was originally coined by Haagensen in 1978 [25] to refer to lobular carcinoma in situ (LCIS) lesions and subsequently to encompass both atypical lobular hyperplasia (ALH) and LCIS. In addition to classical LCIS, other rare variants of lobular neoplasia include pleomorphic LCIS (PLCIS), pleomorphic apocrine LCIS (PALCIS) and LCIS with central comedo-type necrosis.
Lobular neoplasia (classical and variants) shows inactivation of the e-cadherin gene, hence the loss of cellular cohesion. Polymorphism and mutation of the e-cadherin gene have been identified in these lesions [26]. A useful diagnostic test is, therefore, e-cadherin immunohistochemical staining, with absent or weak e-cadherin immunohistochemical staining typical in lobular lesions.
LCIS is a disease of premenopausal women with an average age of 49 years [27]. Lesions are often identified incidentally, and the true incidence is difficult to ascertain. However, it is reported to be identified in 0.5–3.6% of all breast biopsy specimens. Lobular neoplasia can be multifocal/multicentric in up to 85% of cases and bilateral in approximately 30%. The earliest report suggested that LCIS was always multifocal and recommended simple mastectomy for treatment [28], but this was a lesion largely identified in association with invasive lobular carcinoma. More recently, it has been recognised that the invasive carcinoma developing following a diagnosis of LCIS is three times more likely to be ipsilateral than contralateral [29] indicating some precursor behaviour, at least in some cases. Importantly, clinically, however, the presence of LCIS at the margins of surgical specimens has been shown not to affect the excellent local control after breast conservation and radiotherapy for lobular cancer [30].
10.5 Histology
10.5.1 ALH and Classical LCIS
As the name suggests, this lesion typically involves the lobular units, rather than ducts (◘ Fig. 10.3a,b). The characteristic lobular cells are generally small and uniform and show loss of cellular cohesion (◘ Fig. 10.3c). Intracytoplasmic vacuoles (◘ Fig. 10.3d) are often conspicuous, and pagetoid involvement of terminal ducts can be prominent (◘ Fig. 10.3e). The latter is diagnosed when lobular carcinoma cells infiltrate under the normal residual duct epithelium. Lobular cells can be divided into type A: small uniform nuclei, indistinct nucleoli and minimal cytoplasm and type B: larger cells with more abundant cytoplasm and variation in nuclear size and morphology. Neither of these types has high-grade features.
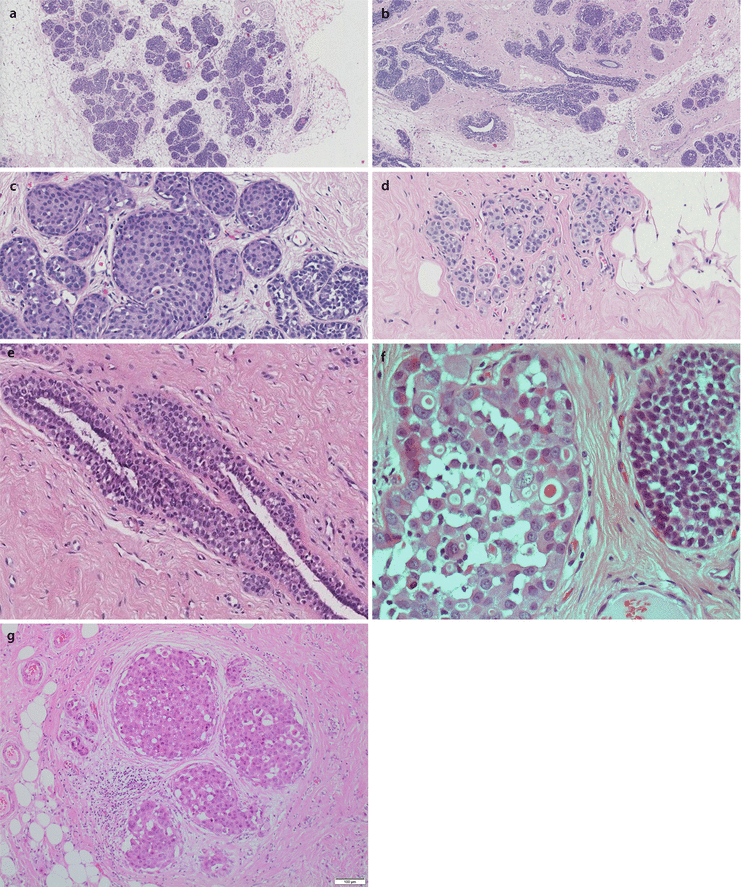

Fig. 10.3
Lobular carcinoma in situ and variants. a LCIS. The lobular geography of this epithelial proliferation can be seen at low power, as can the expansion of the acinar spaces. b Expanded acinar spaces are seen in this case of LCIS. Although there is some increased cellularity around ducts, these are not expanded by cells, and this represents pagetoid spread by the neoplastic cells (see also e). c LCIS. Lobular unit with expansion of acini by discohesive epithelial cells (see as spaces around cells) with moderately sized nuclei. d ALH. Acini are not expanded, but are filled by a population of cells with prominent intracytoplasmic vacuoles. Luminal spaces are not evident and there is discohesion of cells. e Lobular neoplasia pagetoid spread: Duct space with normal ductal epithelium undermined by a population of moderately sized, discohesive forms, present between the myoepithelial layer and innermost ductal cells. In such circumstances the degree of acinar expansion cannot be assessed, and thus the process cannot be accurately assigned to either ALH or LCIS, although this tends to be more conspicuous in the latter. f Pleomorphic and classical LCIS. The acinar space on the left is expanded by a population of large, pleomorphic epithelial cells with large nuclei (cytonuclear grade 3) along with prominent discohesion and intracytoplasmic vacuoles; this represents PLCIS. The acinar space on the right bears more regular, moderately sized but discohesive cells which are those of LCIS. g Pleomorphic apocrine LCIS (PALCIS). Several ducts contain a solid proliferation of large, pleomorphic and discohesive cells. The cells have abundant eosinophilic cytoplasm and prominent nucleoli. Mitotic figures are also noted
The distinction between ALH and LCIS is quantitative. LCIS (◘ Fig. 10.3c) is diagnosed when more than half the acini are filled and distended (more than 8 cells across) by the characteristic lobular cells, whilst lesser degrees are regarded as ALH (◘ Fig. 10.3d). Lobular neoplasia is often ER positive and HER2 negative and, as noted above, typically e-cadherin negative.
10.5.2 Pleomorphic LCIS
Pleomorphic LCIS is diagnosed when there is distension of the TDLUs by discohesive lobular cells showing large pleomorphic (cytonuclear grade 3) nuclei (◘ Fig. 10.3f). It is a relatively recently described variant of lobular neoplasia which is often associated with comedo necrosis and microcalcification and is therefore most commonly identified through mammographic screening. As with classical LCIS, PLCIS shows inactivation of the e-cadherin gene and is typified by an absence of staining by e-cadherin immunohistochemistry. It is more likely to be ER negative and HER2 positive than classical LCIS, but the two lesions often coexist [31] (◘ Fig. 10.3f).
Pleomorphic lobular carcinoma in situ can rarely comprise a proliferation of pleomorphic but overtly apocrine cells when it is classified as being pleomorphic apocrine type/pleomorphic apocrine LCIS (PALCIS) [32] (◘ Fig. 10.3g). The lesional cells have abundant eosinophilic, granular eosinophilic cytoplasm and conspicuous nucleoli but also lobular features, such as discohesion and a lack of e-cadherin expression. Whilst this entity shares the loss of 16p and gain of 1q seen in other forms of LCIS, it shows more genetic alterations compared to classical LCIS and more usual types of PLCIS. Historically PLCIS lesions are likely to have been diagnosed as high-grade DCIS. This, along with limited clinical evidence, supports the view that PLCIS should be categorised and treated as per high-grade DCIS and pure PLCIS lesions on core biopsy should be coded as B5a [33–36].
10.5.3 LCIS with Necrosis
This is a very rare variant characterised by prominent distension of lobules by classical LCIS but with conspicuous comedo necrosis. The lobular proliferation in those cases is usually florid, and mitoses may be seen, unlike more usual forms in which they are infrequent. Although data in the literature regarding clinical behaviour is very limited, this variant is thought to be more aggressive than classical LCIS [37]. Therefore, in its pure form, the lesion should best be categorised as B4. Subsequent management, i.e. whether this requires complete/wide local excision, is unclear.
10.6 In Situ Carcinoma with Mixed Ductal and Lobular Features
In occasional cases, despite thorough morphological and immunohistochemical assessment, it can be difficult to categorise an in situ lesion as being of pure ductal or lobular nature. Those lesions that genuinely show features of both phenotypes (typically supported by a mixed e-cadherin immunophenotype) should be classified as mixed, as per the WHO classification of tumours of the breast [27].
10.7 Significance of LCIS
Lobular neoplasia has been, for a long time, regarded as a marker of increased breast cancer risk. The relative risk for subsequently developing breast cancer was four- to fivefold for ALH and 8–10 for LCIS [38, 39]. This view was supported by observations that the disease can be multifocal and bilateral. More recent data, however, supports the concept that, in at least some cases, the lesion is likely to be a precursor of breast cancer [29]. It is commonly associated and shares the same genetic alterations, as invasive lobular carcinoma; both show loss of chromosome 16p and gain of 1q [40]. It represents one of the lesions encountered within the spectrum of the low nuclear grade neoplasia family [5].
Both classical ALH and LCIS should be coded as B3, of uncertain malignant potential, when identified on core biopsy [20]. The upgrade rate to DCIS or invasive carcinoma following the diagnosis of LCIS ranges from 2 to 25% in one systematic review [41], although another suggests an average upgrade of 27% [42], more equivalent to the data reported in UK practice where 30.3% of cases with lobular neoplasia were upgraded to DCIS or invasive carcinoma [43] (◘ Table 10.1). The upgrade rate for PLCIS is higher (30–60%, average 40%) [37], supporting the view that this is a more aggressive precursor lesion. A review of the studies published in the English literature that included 1229 lobular neoplasia patients [42] similarly reported an upgrade rate on excision of 42% for PLCIS. Of note, however, in many of the series, there is marked bias in the data, in that in many series by no means, all of the patients have undergone surgical excision. The upgrade rate in many series is, however, surprisingly high, even for cases of incidental lesions and for ALH, and some authors have therefore recommended surgical excision following a lobular neoplasia diagnosis [42].
Several issues have led to this wide variation in upgrade rate reported in the literature and to the differences in management that various authors have recommended when lobular neoplasia is diagnosed on core biopsy. First, many of the report series include a heterogeneous group of lesions and include screen-detected and symptomatic cases, women undergoing sporadic/population-based and family history/high-risk screening, isolated lesions and those presenting as mass lesions. Second, radiological-pathological correlation is not reported in many series. Finally, some studies have included both classical and PLCIS, which have different upgrade rates.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







