Fig. 18.1
Renal anatomy and histology. a Anatomically, the kidney is separated into an outer cortex and inner medulla. The glomeruli and convoluted tubules are located in the cortex. b The plasma ultrafiltrate is concentrated through a series of tubes that empties into the collecting ducts and eventually into the calices, renal pelvis, and ureter. c The glomerulus contains arterioles and capillaries surrounded by podocytes and mesangial cells, and filters plasma. Adjacent to the glomeruli in this cross-section are proximal convoluted tubules and distal convoluted tubules
18.4 Disease States and Benign Tumors
18.4.1 Autosomal Dominant Polycystic Kidney Disease (PKD1 or PKD2 Gene)
Autosomal dominant polycystic kidney disease (ADPKD) is an inherited developmental disorder of the kidney that is characterized by the development of cysts. The PKD1 gene is responsible for 85–90 % of cases, and the PKD2 gene is responsible for the remaining 15–20 %. These genes encode for polycystin-1 and polycystin-2, respectively, which are used in the assembly of cilia in the renal tubules. The resultant cysts develop anywhere along the nephron and involve both renal cortex and medulla, and can also develop in the liver and pancreas. Cystic destruction of the nephrons and collection of necrotic fluid within the cysts causes renal failure, massively enlarged kidneys, hypertension, hematuria, and flank pain.
The cysts are lined with an attenuated columnar to cuboidal epithelium and thickened basement membrane, and involved by a mixed inflammatory infiltrate. Some studies have suggested an increased risk of renal cell carcinoma in those with ADPKD, but a definite causation has not been found.
18.4.2 Benign Cysts
Benign cysts can develop as part of the global effect of diseases such as von Hippel–Lindau disease or tuberous sclerosis. The cysts develop in the renal cortex, and are lined by hyperplastic epithelium, although there is no known association of these cortical cysts with malignancy.
18.4.3 Oncocytoma
Oncocytomas are benign neoplasms that grossly are well-circumscribed, brown in appearance, and may have a central scar. The oncocytoma cells have a dense nested architecture and individual cells are eosinophilic (oncocytic) due to their high mitochondrial content. The cells are typically uniform in appearance and positive for KIT by immunohistochemistry. Unlike many RCCs, oncocytoma cells are negative for cytokeratin 7. The main differential diagnosis is the eosinophilic variant of chromophobe RCC (Ch-RCC, discussed in more detail in Sect. 18.7.3), in which cytokeratin 7 is usually positive.
The mitochondrial accumulation in oncocytoma tumor cells has been hypothesized to be a compensatory mechanism for insufficient oxidative phosphorylation [2]. A number of molecular abnormalities have been detected in sporadic oncocytomas, chiefly loss of chromosomes Y and 1, and alterations of chromosome 11q13. The alterations of chromosome 11q13 involve the CCDN1 gene, causing overexpression of cyclin D1 gene product. However, many oncocytomas have no detectable abnormalities by fluorescence in situ hybridization (FISH) or chromosomal karyotyping. When familial cases of oncocytoma have been reported, the patients have been found to have Birt–Hogg–Dubé syndrome (discussed in Sect. 18.7.3.2) [3, 4].
18.5 Renal Cell Carcinoma
Renal cell carcinoma (RCC) arises from the epithelium of the renal tubules, and represents more than 90 % of the renal malignancies in adults. Molecular and cytogenetic studies have elucidated specific genetic changes in the majority of histologic subtypes. Epithelial malignancies of the kidney are comprised of a number of histologically, immunophenotypically, and genetically distinct profiles, with resultant different clinical course and increasing numbers of targeted therapeutic agents.
Although the earliest models of kidney cancer utilized studies of inherited RCCs, hereditary cancer syndromes account for only an estimated 2–4 % of RCC cases. However, further comparison between RCCs in hereditary cancer syndromes and sporadic RCCs has shown common pathways and molecular alterations within each RCC type. Identification of the causal gene mutations has improved the diagnostic accuracy as well as underscored the importance of distinguishing between types of RCC.
18.5.1 Histologic Diagnosis and Immunohistochemistry
RCC variants have been categorized by morphology and histologic features, with immunohistochemical staining playing a somewhat limited role. Both benign renal tubules and RCCs in general show positive staining with Paired Box 8 (PAX8) antibodies. This feature is primarily useful in confirming the renal origin of metastatic RCC.
The histology and molecular alterations associated with the subtypes of clear cell RCC (CC-RCC), papillary RCC (P-RCC), and Ch-RCC will be discussed in particular in Sect. 18.7. However, it is important to note that a panel of cytokeratin 7, AMACR, carbonic anhydrase 9 (CA-9), and CD10 can be useful in delineating between CC-RCC, clear cell tubulopapillary RCC, and P-RCC. CC-RCCs are negative for cytokeratin 7, whereas P-RCC, tubulopapillary RCC, and Ch-RCC are positive for cytokeratin 7. CC-RCC is positive for CA-9, whereas most P-RCC and Ch-RCC are negative. While P-RCCs are positive for AMACR, 20 % of CC-RCCs are positive, and clear cell tubulopapillary RCCs are negative [5].
A separate panel of HNF1beta, CD10, and S100a1 is used to help differentiate between Ch-RCC and oncocytoma. Ch-RCC demonstrates a complete loss of HNF1beta protein expression, and is negative for S100a. Oncocytomas retain HNF1beta protein expression, S100a expression, and stain with Hale’s colloidal iron. These staining differences are utilized to differentiate between the eosinophilic variant of Ch-RCC and oncocytomas [5].
18.5.2 Fuhrman Nuclear Grading
Fuhrman nuclear grading was the first important independent predictive factor of disease-free survival, and is still used in the grading of most RCCs [6]. This grading system was first published in 1982 and is based on size of nuclei, presence of nucleoli, and nuclear membrane contours. Fuhrman grade I nuclei are small (<10 microns in diameter), round, and with absent nucleoli. Fuhrman nuclear grade II includes nuclei 15 microns in diameter with slight nuclear irregularities and small nucleoli visible under high power magnification (40x). Grade III nuclei are larger (20 microns), with very irregular outlines and prominent nucleoli visible at low power magnification (10x). Grade IV nuclei are similar to Grade III nuclei, but with the addition of pleomorphic, bizarre, or multilobated nuclei with macronucleoli. The most severe nuclear grade seen in one high-powered field is reported [7].
Recent modifications to the grading schema have limited Fuhrman nuclear grading to clear cell and papillary types, emphasized the importance of nucleolar features in grading, and reserved nuclear grade IV for those with extreme nuclear pleomorphism, sarcomatoid differentiation, or rhabdoid differentiation [8].
18.5.3 Staging
The American Joint Committee on Cancer (AJCC) TNM staging for RCCs, last updated in 2010, stages RCCs based on primary tumor size, involvement of adjacent structures (adrenal gland, renal vein, Gerota’s fascia, vena cava), involvement of lymph nodes, and distant metastasis for anatomic staging [9].
The pathologic stage has most consistently been shown to have prognostic utility in RCC. Pathologic staging after histologic typing has been shown to be even more effective in prognosticating outcomes. As discussed below, histologic types have been shown to behave differently. The size of tumor and extent of involvement leading to pathologic stage are powerful predictors of prognosis [10], with stage 1 RCC having a 5-year disease survival of 80–95 % [11].
18.6 The Warburg Effect and Molecular Alterations in Carcinoma
The Warburg effect is a theory of carcinomatosis first posited by Otto Warburg in the 1920s [12]. A fundamental aspect of cancer is the use of aerobic glycolysis for energy production. Tumors that switch to aerobic glycolysis instead of oxidative phosphorylation for energy production must fundamentally alter the expression of a number of genes involved in cell metabolism, oxygen sensing, and fatty acid synthesis [12]. As will be discussed in the remaining sections, a number of RCC types exemplify the Warburg effect of altering cell metabolism and respiration to suit tumor cell growth.
18.7 RCC Types and Associated Familial Cancer Syndromes
A large portion of the research into molecular underpinnings of renal tumors has been due to the study of familial cancer syndromes. While the syndromes represent a minority of the total renal carcinomas diagnosed, this initial research shed insight to the molecular drivers of sporadic renal cell carcinomas. It is estimated that 1–4 % of RCCs are due to hereditary cancer syndromes [13].
18.7.1 Clear Cell Carcinoma and Von Hippel–Lindau (VHL) Syndrome
18.7.1.1 Histology of CC-RCC
CC-RCC accounts for approximately 75 % of the cancers in the kidney [14, 15]. Sporadic CC-RCC is the most common and most aggressive subtype of RCCs. CC-RCC is grossly bright golden-yellow due to a high lipid content in the lesional cells. The tumor cells are arranged in nests, sheets, and tubules. The cells have clear cytoplasm and single round nuclei. Intralesional hemorrhage is common, due to the highly vascular septations coursing between tumor nests. Higher grade lesions have eosinophilic granular cytoplasm and higher grade nuclei, and can have focal sarcomatoid features (Fig. 18.2).
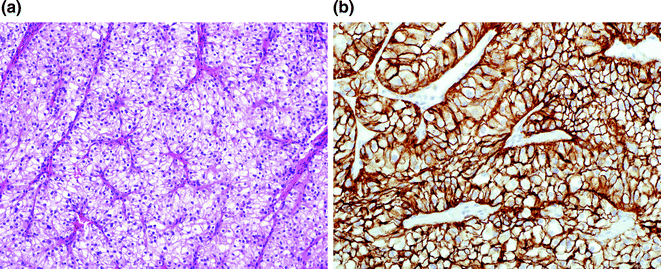

Fig. 18.2
Clear cell renal cell carcinoma (CC-RCC) and CA-9 staining. a CC-RCC is composed of clear cells with nested architecture with intervening small capillaries. Fuhrman nuclear grading is determined by nuclear size, nucleoli, and nuclear contours. b Cytoplasmic and membranous accrual of CA-9, visualized by immunohistochemistry is a feature of CC-RCC, but can be seen in peri-necrotic tumoral cells in similarly low oxygen conditions
18.7.1.2 von Hippel–Lindau Syndrome
Von Hippel–Lindau (VHL) syndrome is caused by the inherited autosomal dominant germline mutation of the VHL tumor suppressor gene on chromosome 3p25. The syndrome has a high degree of penetrance, and an estimated incidence of 1/36,000–1/45,500. The inherited germline mutation silences one allele of the VHL gene, and a point mutation, deletion, or promoter hypermethylation of the remaining allele results in clinical manifestations of the disease. As a result, the onset of malignancies is earlier in those with von Hippel–Lindau syndrome than with sporadic tumors of the same kind, with an average onset in the third to fourth decade versus seventh decade of life. Tumors include cerebellar hemangioblastoma, retinal blastoma, pheochromocytoma, pancreatic cysts, inner ear cysts, and multiple renal cysts and CC-RCC [16]. The VHL gene was detected in 1993, and a peripheral blood test for the germline mutation is now commonly used in detection within at-risk families.
The gene product pVHL is necessary for degradation of hypoxia-inducible factor HIF-1α[alpha], which itself controls downstream angiogenesis. When pVHL is faulty, HIF-1α[alpha] accumulates and leads to stabilization of its downstream targets such as vascular endothelial growth factor (VEGF), glucose transporter 1 (GLUT1), carbonic anhydrase 9 (CA-9), epidermal derived growth factor (EGFR), and platelet derived growth factors (PDGFR). These genes are involved in angiogenesis, cell migration, and cell metabolism in eventual tumor formation [17].
18.7.1.3 mTOR Complex
The mammalian target of rapamycin protein (mTOR) is a protein that exists as a dimer and functions, in part, in regulation of cell growth and metabolism. The mTORC1 complex has been activated in a cited 60–85 % of CC-RCCs, and through unknown mechanisms also drives the progression of CC-RCCs. The mTOR pathway has been more clearly discussed in tumors of tuberous sclerosis complex and angiomyolipomas [17].
18.7.1.4 Sporadic CC-RCC
The Cancer Genome Project recently sequenced 417 cases of CC-RCC. The underlying genetic changes included alterations in the genes controlling cellular oxygen sensing and maintenance of chromatin states. Whole-exome sequencing of the tumors from the Cancer Genome Project identified over 36,000 somatic mutations. There were 19 significantly mutated genes, most commonly VHL, PBRMI, BAP1, and SETD2. Loss of 14q, which leads to loss of HIF-1α[alpha], was associated with more aggressive disease. Most sporadic CC-RCCs contain alteration of the VHL gene, with up to 87 % of CC-RCCs showing VHL inactivation through sequence alterations or promoter methylation [18]. In addition to mutation in VHL, up to 41 % of sporadic CC-RCCs have mutation in PBRM1 leading to altered chromatin biology. Copy number variations (CNVs) are common with CC-RCC. Alterations in BAP1 and SETD2 are correlated with poor survival.
The Cancer Genome Project reports that 7 % of CC-RCC showed an epigenetic silencing of VHL that was unassociated with somatic VHL mutation. An additional 289 other genes showed evidence of epigenetic silencing, and increased promoter hypermethylation correlated with higher clinical stage and grade. RNA expression in this study identified four subsets of mRNA classes, the m1 subtype of which was associated with chromatin remodeling and higher frequency of PBRM1 mutations [19].
CC-RCCs that harbor no VHL mutations and have low CA-9 expression are clinically more aggressive [20], and may be a different disease altogether from its phenotypically similar CC-RCC with VHL loss.
18.7.1.5 VHL Targets HIF1α and EPAS1 (also Called HIF2α)
Renal cell carcinomas share similar pathways relating to oxygen utilization and nutrient sensing. In conjunction with derangements in the mTOR pathway, which also controls HIF synthesis, alterations in VHF and HIF-1α[alpha] pathways lead to significant increased accrual of CA-9. This increased CA-9 has been found in CC-RCC tumor cells and in the hypoxia-driven necrosis found in other RCCs. Nonclear cell RCCs share common metabolic pathways, and some relate back to the HIF1α[alpha] pathway. The genes VHL, MET, FLCN, FH, TSC1, TSC2, TFE3, and SDH are used to some extent by the cell to sense oxygen or energy. This commonality has led to the hypothesis that RCCs are fundamentally a metabolic disease, following the Warburg effect [12].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree