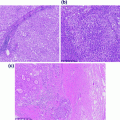
Fig. 26.1
Normal pancreas
26.2.5 Pancreatic Cancer and Precursor Lesions
Despite representing only a small portion of the epithelial component, the vast majority (greater than 90 %) of pancreatic neoplasms have a ductal origin. PDA comprise 80–90 % of this group [29]. There are several similar entities that can develop into or are at least associated with PDA. These entities are PanIN, intraductal papillary mucinous neoplasia (IPMN), and mucinous cystic neoplasia (MCN). One of these entities, PanIN, was initially identified as a series of increasingly atypical proliferative changes in the epithelium of the pancreatic ducts. These lesions have long been recognized, but have only been reported previously with descriptive terminology [30–32].
26.2.5.1 Pancreatic Intraepithelial Neoplasia
PanIN is a relatively common finding in resected specimens and is seen in association with PDA and other tumor types [33]. It represents a spectrum of proliferative lesions in the ductal system, all of which contain cytoplasmic mucin. The lesions are separated into three grades (PanIN-1, PanIN-2, and PanIN-3), depending on the extent of cytological atypia and architectural complexity (see Fig. 26.2). PanIN-1 lesions (previously called mucinous metaplasia or mucous cell hypertrophy) are characterized by tall columnar mucinous cells with nuclei lacking any atypia or loss of polarity. These lesions are further divided into PanIN-1A and PanIN-1B, based on the presence or absence of papillary and micropapillary formations and nuclear stratification. PanIN-1A lacks both the architectural and cytological features. PanIN-2 lesions (previously called atypical hyperplasia or moderate dysplasia) are characterized by prominent nuclear stratification, loss of polarity, and mild nuclear atypia. Finally, PanIN-3 lesions (previously called carcinoma in situ or severe dysplasia) show marked nuclear atypia, significant loss of polarity, frequent mitotic figures, and papillary tufts “floating” in the lumen [34]. Recently, a two-tier classification was recommended by an international consensus meeting to replace the current three-tier classification used for PanIN as well as IPMN and MCN. In the revision, PanIN-1 and PanIN-2 are categorized as low grade. PanIN-3 is categorized as high grade PanIN. This change in classification brings the terminology in line with the clinical management of these lesions [35].


Fig. 26.2
Pancreatic intraepithelial neoplasia (PanIN). a PanIN-1A; b PanIN-1B; c PanIN-2; d PanIN-3
Lower grade lesions, i.e., PanIN-1, are often incidental findings in neoplastic and nonneoplastic pancreas resections. In one study, PanIN-1A lesions were identified in 43 % of older adults with nonneoplastic resections [36]. PainIN-2 and PanIN-3 are significantly more common in pancreases with PDA [33, 34]. Recent retrospective data suggest that there is a very low risk of cancer progression in PanIN-1 and PanIN-2 lesions [37]. However, Brat et al. [31] have reported rare cases where PanIN-2 lesions were identified before the development of PDA. The natural history of these lesions is exceedingly difficult to observe given the lack of reproducible radiographic findings and serologic markers.
26.2.5.2 Intraductal Papillary Mucinous Neoplasms
IPMNs are intraductal papillary proliferations of mucin-producing cells. Intraluminal mucin is often evident, and accumulation of the mucin often leads to cystic dilation of the ducts, which may be localized to the immediate vicinity or may involve the entire ductal system. They may manifest as multilocular cystic masses or abundant papillary nodules in either the main duct or the branch duct and are classified accordingly [38–42].
IPMNs share many cytological features with PanIN and are characterized by mucinous cells with varying degrees of atypia. However, most IPMNs are larger and involve cystically dilated ducts that are at least 1 cm in diameter [34, 43, 44]. The atypia is stratified into three grades (i.e., low, intermediate, and high) on the basis of the most severely dysplastic area and parallel the spectrum of changes seen in PanIN-1 through PanIN-3. IPMNs with low grade dysplasia (also called intraductal papillary mucinous adenomas) are characterized by mucinous cells that lack any nuclear atypia or loss of polarity (see Fig. 26.3). Intermediate grade dysplasia is characterized by more prominent nuclear stratification, loss of polarity, and mild nuclear atypia. IPMNs with high grade dysplasia (also called intraductal papillary mucinous carcinoma in situ) have significant loss of polarity and marked nuclear atypia (see Fig. 26.4). Similar to the changes in PanIN terminology, both IPMNs with low grade dysplasia and intermediate grade dysplasia are now categorized as IPMN, low grade, in the revised classification and IPMN with high grade dysplasia as IPMN, high grade [35]. This two-tier classification scheme more accurately parallels the 2012 consensus guidelines of the International Association of Pancreatology for the management of IPMNs and MCNs, which recommend low and intermediate grade dysplasia as being amenable to observation and high grade dysplasia as requiring clinical attention and intervention [45]. In addition to the grading of dysplasia, three papillary patterns have been described [38, 46–48]. The most common pattern is the gastric type (50 %), which is characterized by cells that resemble gastric foveolar epithelium with basally oriented nuclei and abundant mucinous cytoplasm. The papillae in these lesions are often less exuberant and can be flat [49–52]. The second most frequently recognized pattern is the intestinal type (35 %), characterized by cells that resemble adenomas of the colorectum [53] with villiform papillae; like the adenomas in the colorectum, there is often nuclear pseudostratification and intracellular mucin. The least common pattern is the pancreaticobiliary type (15 %) that is characterized by cuboidal cells often with more complex papillae formation [54], including numerous branched papillae, micropapillae, and cribriform areas. The cells are typically not pseudostratified, but show marked variation in size, irregular contours, prominent nucleoli, and loss of nuclear polarity. While an overlap of these patterns can be seen, the intestinal and pancreaticobiliary patterns are not commonly seen in a single tumor.
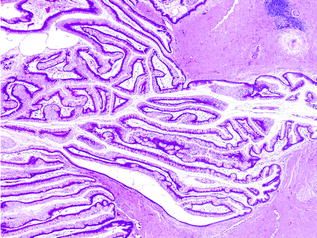
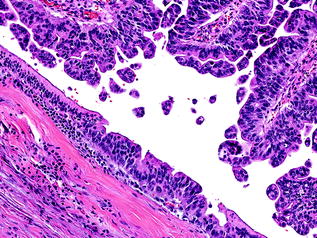

Fig. 26.3
Intraductal papillary mucinous neoplasm (IPMN) with low grade dysplasia

Fig. 26.4
IPMN with high grade dysplasia
IPMNs express keratins, such as CK7, CK8, CK18, and CK19. The degree of CK20 immunoreactivity depends on the histologic subtype [42, 55]. Expression of glycoproteins, MUC1 and MUC2, also varies with the histologic subtype [46, 47, 53, 56]. Expression of MUC1 is more common in pancreaticobiliary-type IPMNs, whereas MUC2 is more commonly expressed in intestinal-type IPMNs. Gastric-type IPMNs do not express MUC1 or MUC2. Moreover, MUC2 expression in intestinal-type IPMNs is paralleled by other markers of intestinal differentiation, such as CK20 and CDX-2. MUC6 is preferentially expressed in pancreaticobiliary-type IPMNs suggesting pyloric differentiation [57]. Almost all IPMNs, regardless of their histologic subtype, express CEA, CA19–9, and MUC5AC [58].
26.2.5.3 Mucinous Cystic Neoplasms
MCNs are typically single, multilocular cystic masses with a thick, fibrotic capsule. The cysts are often large, greater than 10 cm. Unlike PDAs and IPMNs, these tumors do not communicate with the ductal system. The septa between cysts are often thin, but some can appear trabeculated and thickened. Papillations are not uncommon. Similar to IPMNs, the cysts contain mucinous material. Degenerative changes with hemorrhage may occur and may resemble a pseudocyst [59–62].
MCNs are characterized by a distinctive, subepithelial stroma that resembles ovarian stroma (see Fig. 26.5), composed of densely packed spindle cells with uniform, wavy nuclei, sparse cytoplasm, and scattered clusters of plump, epithelioid cells suggestive of luteinization [63, 64]. In addition, the spindle cells demonstrate immunoreactivity for estrogen and progesterone receptors, inhibin, and melan-A [62, 63, 65]. The epithelium, when not denuded, is characterized by cuboidal to columnar cells with abundant apical mucin and basally located nuclei. These cells lie flat or form papillae, and occasionally, goblet cells, neuroendocrine cells, and Paneth cells can be found interspersed [66].
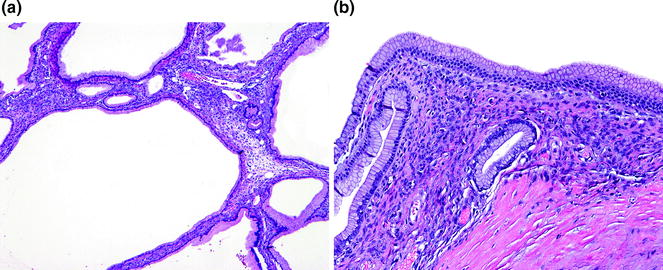

Fig. 26.5
Mucinous cystic neoplasm (MCN) with low grade dysplasia. a Low-power. b High power of ovarian-type stroma
Similar to IPMNs, the degree of dysplasia in the epithelial component is graded as low, intermediate, or high based on the area with the most severe dysplasia. MCN with low grade dysplasia (also called mucinous cystadenoma) is characterized by minimal cytological atypia and minimal architectural complexity (see Fig. 26.5). MCN with intermediate grade dysplasia has moderate cytological atypia, including loss of nuclear polarity, and mild architectural complexity. MCN with high grade dysplasia (also called mucinous cystadenocarcinoma in situ) is characterized by marked cytological atypia, including irregular, hyperchromatic nuclei, mitotic figures and significant architectural complexity [61, 62] (see Fig. 26.6). The proposed change in classification for PanINs and IPMNs is also recommended for MCNs. MCNs with low grade dysplasia and intermediate grade dysplasia are categorized as MCN, low grade; MCN with high grade dysplasia is categorized as MCN, high grade, in the 2014, revised classification system [35].
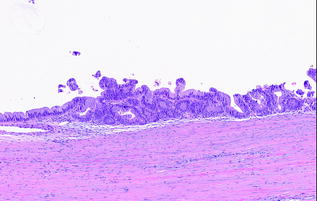

Fig. 26.6
MCN with high grade dysplasia
Invasive adenocarcinoma, resembling conventional PDA, can develop in association with MCN, especially those with high grade dysplasia (see Fig. 26.7). Other histological variants, e.g., undifferentiated carcinoma with osteoclast-like giant cells, have also been reported [67]. MCN with an associated invasive carcinoma are also called invasive mucinous cystadenocarcinoma. Similar to IPMNs, the invasive component in MCNs can also be focal and often necessitates in toto submission of the tumor. Overall, the rate of malignancy in MCNs ranges from 10 to 28 % [68], and it has been suggested that they may be less aggressive than their conventional counterparts, especially, when there is only minimal invasion [69].
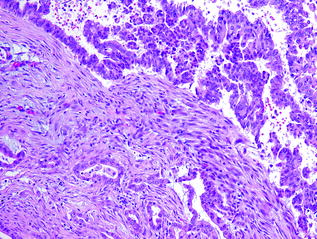

Fig. 26.7
MCN associated with invasive carcinoma
26.2.5.4 Conventional Ductal Adenocarcinoma
Most PDAs are firm, discrete tan-white masses with ill-defined, infiltrative borders. Frank necrosis and hemorrhage is infrequent. In an uninjured pancreas, the appearance of the tumor is easily distinguished from the normal pancreas, however, it can be difficult to visualize the mass-forming tumor in a background of fibrosis, secondary to either chronic pancreatitis or neoadjuvant therapy [26].
Conventional PDA represents one of two general groups of pancreatic cancer, the second group being histological variants, which are most likely of ductal origin. They are characterized by a proliferation of variably sized glands haphazardly set in a desmoplastic stroma [72]. The neoplastic glands are composed of cuboidal to columnar cells with variable amounts of cytoplasm and mucin. The nuclei vary in size and shape, and there is frequently a loss of polarity. Rarely, the nuclei may retain their normal basal orientation (see Fig. 26.8). In addition to the conventional (or tubular) pattern, PDAs can also demonstrate other morphological patterns, including a foamy gland pattern resembling low grade PanIN [73]; a large duct (or microcystic) pattern commonly seen with duodenal infiltration [74]; a vacuolated pattern resembling adipocytes or signet ring cells [75]; a solid, nested pattern resembling squamous cell carcinoma or hepatoid carcinoma [76]; a micropapillary pattern; and a lobular carcinoma-like pattern with neoplastic cells in cords or single files (see Fig. 26.9). With rare exceptions, these patterns are not considered to have clinical significance. PDAs with a micropapillary pattern are considered to be particularly aggressive. Regardless of the morphological patterns, the neoplastic glands destroy the normal lobular architecture. Lymphovascular and perineural invasion are almost invariably seen and infiltration into peripancreatic fat is also common [77]. Interestingly, colonization of the normal epithelium and the basement membrane can be seen with invasion into the common bile duct, duodenum, and native pancreatic ducts, mimicking the appearance of a precursor lesion.
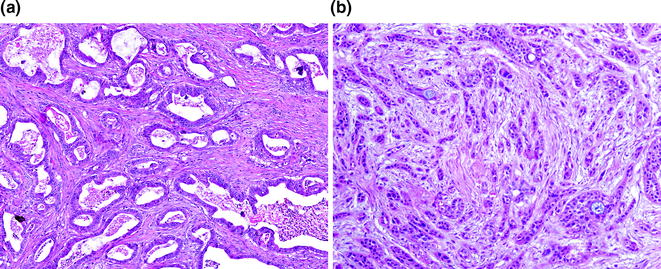
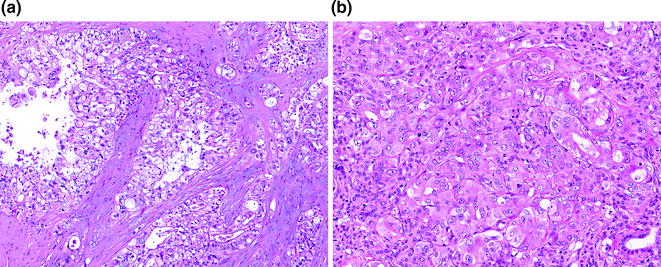

Fig. 26.8
Conventional pancreatic ductal adenocarcinoma (PDA). a Well differentiated; b poorly differentiated

Fig. 26.9
Other morphologic patterns of PDA. a Vacuolated pattern. b Solid, nested pattern
The TNM grading system is based on the extent of gland formation and is advocated by the College of American Pathologists. Well-differentiated (grade 1) adenocarcinomas are characterized by greater than 95 % gland formation. Adenocarcinomas with 50–95 % gland formation are considered moderately differentiated (grade 2). Poorly differentiated (grade 3) adenocarcinomas have less than 50 % gland formation. Undifferentiated carcinomas, which are discussed below, are considered grade 4. The Klöppel grading system, which is endorsed by the World Health Organization (WHO), assesses mucin production, mitoses, and nuclear atypia in addition to gland formation [78], however, it is cumbersome in practice. Adsay and colleagues [79] have proposed a third system based on the predominant and secondary patterns of infiltration, but it is not widely used. In a comparison between the TNM and Klöppel grading systems, no significant differences in predictive value were found [80]. Regardless of the grading system used, the histologic grade has been shown to be prognostically significant, with higher grades, i.e., grades 3 and 4, being more unfavorable [79, 80].
PDAs stain for CK7, CK8, CK18, CK19, and epithelial membrane antigen (EMA) [81]. CK20 is positive in 25 % of cases with typical immunoreactivity being focal and weak [82] except in mucinous noncystic carcinoma in which immunoreactivity is diffuse and strong [56]. In addition, conventional PDAs usually express the cell surface-associated mucin proteins, MUC1, MUC3, MUC4, and MUC5AC. However, MUC2 expression is only seen in those with intestinal differentiation and MUC6 is expressed in those with pyloric gland differentiation [56, 83, 84]. Similar to IPMNs and MCNs, immunohistochemical stains to glycoproteins, e.g., CA19–9, CEA, TAG-72, and CA-125, are often positive in conventional PDAs. Of these, the latter three proteins are expressed only to a limited degree in low grade PanIN lesions and are not typically expressed in the normal pancreas [85].
Histological Variants of Adenocarcinoma
There are several histological variants that are most likely of ductal origin, as evident by an associated component of conventional PDA. These variants include mucinous noncystic carcinomas, squamous cell, and adenosquamous carcinomas, undifferentiated carcinomas, medullary carcinomas, and hepatoid carcinomas. With a few exceptions, the natural history and molecular alterations of these variants are not well understood due to their infrequency.
Mucinous Noncystic Carcinoma
Mucinous noncystic carcinoma (also called colloid carcinoma) is a unique variant, both clinically and histologically. These lesions are characterized by pools of mucin, within which are flat strips of small clusters of neoplastic cells that are either attached to the edge of the mucin pool or floating within it (see Fig. 26.10). Signet ring cells are not infrequently seen in these pools [86]. They are typically associated with IPMNs or MCNs [87]. Relative to conventional PDA, mucinous noncystic carcinomas have a more favorable, protracted clinical course [86].
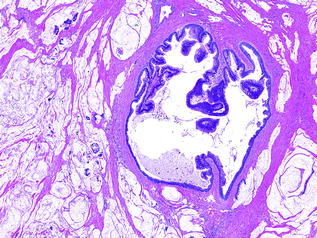

Fig. 26.10
IPMN with mucinous noncystic carcinoma
Squamous Cell Carcinoma and Adenosquamous Carcinoma
Squamous cell and adenosquamous cell carcinomas represent a small minority of pancreatic cancers [88, 89]. They resemble squamous cell carcinomas from other sites, with variable keratinization. The diagnosis of adenosquamous carcinoma only requires an arbitrary 30 % squamous component [90] (see Fig. 26.11). As such, pure squamous cell carcinomas are exceedingly uncommon (see Fig. 26.12). Both squamous cell and adenosquamous cell carcinomas have a similar clinical course to conventional PDA.
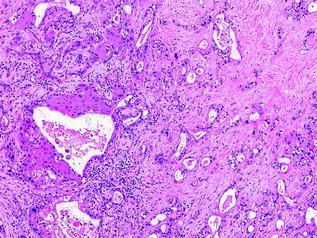
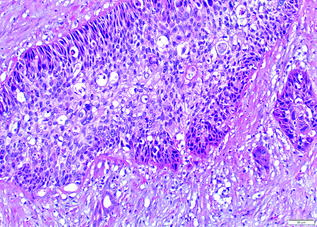

Fig. 26.11
Adenosquamous carcinoma of the pancreas

Fig. 26.12
Squamous cell carcinoma of the pancreas
Undifferentiated Carcinoma
Undifferentiated carcinomas encompass a wide range of histological appearances, and include sarcomatoid (or spindle cell) carcinomas, anaplastic carcinomas, carcinosarcomas, and undifferentiated carcinomas with osteoclast-like giant cells [91]. Undifferentiated carcinomas are thought to be ductal in origin as a conventional PDA component or preinvasive component, e.g., PanIN or MCN [92–94], can be identified. The epithelioid component is frequently markedly atypical and dyshesive. The sarcomatoid component may consist of only spindle cells or large anaplastic giant cells (see Fig. 26.13). In addition, there may also be heterologous differentiation, e.g., skeletal muscle, cartilage, or bone formation [95]. Undifferentiated carcinomas with osteoclast-like giant cells, as the name indicates, contain a variable number of osteoclast-like giant cells scattered throughout the neoplasm [96]. The giant cells are immunoreactive for CD68, supporting a histiocytic origin [97] (see Fig. 26.14). Undifferentiated carcinomas are aggressive with most patients dying of disease within 2 years of initial diagnosis [98].
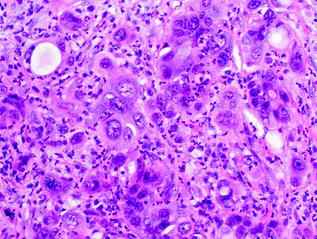
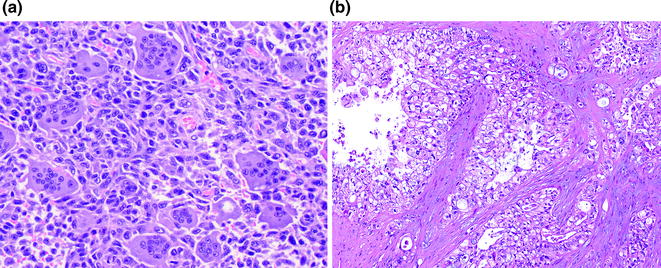

Fig. 26.13
Undifferentiated carcinoma of the pancreas

Fig. 26.14
Undifferentiated carcinoma with osteoclast-like giant cells. a H&E; b CD68 immunohistochemistry highlighting giant cells
Medullary Carcinoma
Medullary carcinomas are uncommon tumors that are characterized by a syncytial growth pattern composed of poorly differentiated neoplastic cells. These neoplasms are typically associated with a prominent inflammatory infiltrate containing neutrophils and lymphocytes. Similar to ones at other sites, medullary carcinomas can be associated with microsatellite instability [99–101] and are associated with a good prognosis [101, 102].
Hepatoid Carcinoma
Hepatoid carcinomas are exceedingly rare and are characterized by polygonal cells with granular and eosinophilic cytoplasm, centrally located nuclei, and prominent nucleoli. The neoplastic cells, which have hepatocellular differentiation, are arranged in solid sheets, nests, or trabeculae [103–105].
26.2.6 Molecular Pathogenesis
Cancer is fundamentally caused by accumulation of mutations in oncogenes and tumor suppressor genes. These mutations can be acquired somatically, or patients can inherit them. PDA is among the best tumors characterized molecularly. Numerous mutations in oncogenes and tumor suppressor genes, both inherited and acquired, have been identified and provide an unprecedented insight into the molecular pathogenesis of pancreatic cancer.
26.2.6.1 Somatic Alterations
The molecular alterations in sporadic pancreatic cancer have been extensively investigated in the last few decades and have given us insights into PDA tumorigenesis. Whole exome sequencing has revealed an average of 48 somatic mutations with four genes being altered in greater than 50 % of cases [106]. These four genes are KRAS, CDKN2A (also called CDKN, p16, and INK4A), TP53 and SMAD4 (also called DPC4) [107–109] (see Table 26.1).
Table 26.1
Most common sporadic alterations in pancreatic ductal adenocarcinoma
Chromosome | Gene | Prevalence (%) | Mechanism |
|---|---|---|---|
12 | KRAS | 95 | Missense mutation |
9 | CDK2NA | 95 | LOH, homozygous deletion, promoter methylation |
17 | TP53 | 75 | LOH |
18 | SMAD4 | 55 | LOH, homozygous deletion |
KRAS is the most frequently altered oncogene in PDAs. The gene, which is located on chromosome 12, encodes for a small GTPase that is vital to several cell signaling pathways, including MAPK, ERK, and AKT pathways. The point mutations involve three target codons almost exclusively (12, 13, and 61). Mutations involving codon 12 are detected in greater than 90 % of PDAs [110]. KRAS mutations are also identified in histological variants, including undifferentiated carcinomas with osteoclast-like giant cells [111] and mucinous noncystic carcinomas [86], as well as both IPMNs and MCNs. In IPMNs, the frequency of KRAS mutations ranges from 30 to 80 % with increasing frequency in those with high grade dysplasia and invasive carcinoma [112–119]. A similar trend in frequency of KRAS mutation is observed in MCNs [115, 120, 121]. Notably, KRAS mutations have been identified in more than 90 % of the earliest PanIN lesions (PanIN-1), implicating KRAS mutations as a key initiating event in pancreatic neoplasia [122].
CDKN2A is the most frequently altered tumor suppressor gene in PDAs. The gene, which is located on chromosome 9p, encodes for the protein, p16, which is involved in cell cycle regulation. Inactivation of the gene is observed in 95 % of pancreatic cancers [106] and is thought to promote unrestricted growth. In addition to DNA alteration, CDKN2A inactivation can be accomplished through epigenetic silencing via aberrant methylation [123]. Similar to KRAS mutations, CDKN2A mutations occur early in the progression from PanIN to pancreatic cancer.
TP53 is another frequently altered tumor suppressor gene and is inactivated in 75 % of PDA [106]. The gene, which is located on chromosome 17p, encodes the protein p53, which plays an important role in cellular stress responses by activating DNA repair, inducing growth arrest, and triggering apoptosis. TP53 mutations are also found in MCNs and IPMNs [124]. In contrast to KRAS and CDKN2A, TP53 mutations occur later in the PanIN-to-invasive carcinoma sequence, being observed in high grade PanIN lesions (i.e., PanIN-3) [125].
SMAD4 is a tumor suppressor gene on chromosome 18q [126]. The protein, SMAD4, is involved in the transforming growth factor beta (TGF-β[beta]) signaling pathway. SMAD4 mutations are associated with a poor prognosis and widely metastatic disease [17, 127] and can be detected using immunohistochemistry. Loss of SMAD4 expression by immunohistochemistry is found in 55 % of PDAs [128] (see Fig. 26.15). Similar to TP53, mutations in SMAD4 are observed in PanIN-3 lesions, implicating its late role in the genetic progression to cancer [125].
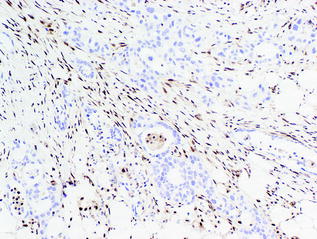

Fig. 26.15
Loss of SMAD4 by immunohistochemistry in conventional PDA
In addition to somatic mutations, large chromosomal gains and losses, and complex karyotypic abnormalities occur frequently in PDA. Some changes target known driver genes, whereas others identify regions with specific loci that have unknown roles [129]. The chromosomal abnormalities may be related to early telomere shortening, which is reported to occur in approximately 90 % of low grade PanIN lesions [130]. Together, progression from PanIN to PDA and the sequential acquired alterations, telomere shortening, and chromosomal instability can be mapped (see Fig. 26.16).
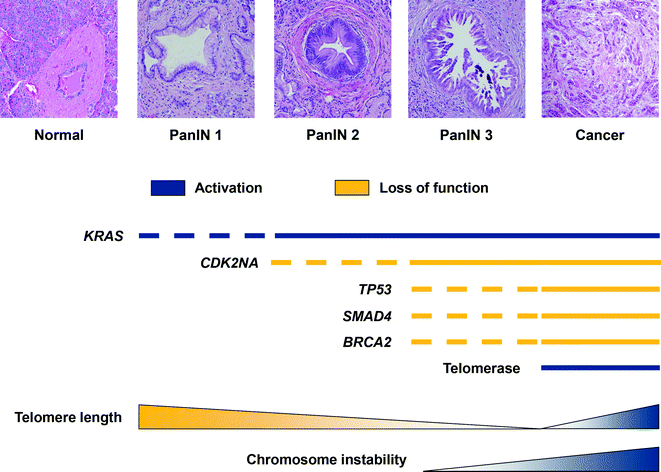

Fig. 26.16
Sequential molecular alterations and increasing chromosomal instability
Despite a common progression to pancreatic cancer, two somatic mutations unique to MCNs and IPMNs have been identified. The affected genes include GNAS and RNF43 [119, 124]. The latter, RNF43, encodes a protein with intrinsic E3 ubiquitin ligase activity. Mutations in RNF43 are found in both MCNs and IPMNs [124]. GNAS, which encodes for a stimulatory G-protein subunit, is unique in that GNAS mutations are only seen in IPMNs [119].
26.2.6.2 Germline Alterations and Mutations
Approximately 10 % of PDAs have a familial basis, which is defined as at least a pair of first-degree relatives diagnosed with pancreatic cancer. Case-control and cohort studies have shown that those with a family history of pancreatic cancer have a 1.9 to 13-fold increased risk [131–134]. The genetic basis for the majority of familial pancreatic cancer is unknown [135]. Several germline genetic syndromes also lead to an increased risk for PDA (see Table 26.2).
Table 26.2
Germline alterations in pancreatic cancer and cancer risk
Gene | Syndrome | Estimated lifetime cancer risk |
|---|---|---|
BRCA1, BRCA2 | Familial breast cancer | BRCA1a, 2.16 % (by age 80) BRCA2, 3.36 % (by age 80) |
PALB2 | Familial breast cancer | Elevated |
CDK2NA | Familial atypical multiple mole melanoma (FAMMM) syndrome | 17 % (by age 75) |
STK11 | Peutz-Jeghers syndrome (PJS) | 11–32 % |
PRSS1 | Hereditary pancreatitis | 30–40 % (by age 70) |
ATM | Ataxia-telangiectasia | Unknown |
MSH2, MSH6, MLH1, PMS2 | Lynch syndrome | 1.45–5.88 % (by age 70) |
Germline BRCA2 mutations are associated with a significantly elevated lifetime risk of breast, ovarian, prostate, and pancreatic cancer [136–139]. PALB2 (also called FANCN), which encodes for a protein that partners with the BRCA2 protein, is also implicated in familial PDA. Germline mutations in this gene account for 1–3 % of familial PDA [140–142]. Both BRCA2 and PALB2 encode proteins crucial to the Fanconi anemia pathway, and germline mutations in other genes involved in the pathway, including FANCC and FANCG, have been reported in young patients diagnosed with PDA [143–145]. However, FANCA mutations have not been implicated [146]. In addition to conventional PDA, IPMNs have also been reported in patients with inherited BRCA2 mutations and a family history of PDA [147].
Unlike germline mutations in BRCA2 and PALB2, it is unclear if inherited mutations in BRCA1 are also at higher risk. Studies show conflicting results, with one large study conducted by the Breast Cancer Linkage Consortium observing a 2.26-fold increased risk [148]. Others have not shown an increased prevalence of BRCA1 mutations [149, 150].
Germline mutations in CDKN2A causes familial atypical multiple mole melanoma (FAMMM) syndrome, which results in a 38-fold increased risk of PDA and melanoma [151–156].
Peutz-Jeghers syndrome (PJS) results in the development of gastrointestinal hamartomas and pigmented macules on the lips, and buccal mucosa. It is associated with an increased risk of PDA. Germline mutations in STK11 (also called LKB1) explain 80 % of PJS cases [157]. PDAs in these patients also show somatic loss of the wild-type STK11 allele [158]. In addition, rare cases of IPMNs have been reported in patients with this syndrome [159].
Germline mutations in PRSS1 and SPINK1 cause hereditary pancreatitis [160, 161], which is characterized by repeated episodes of acute pancreatitis in young patients. As a result of the continuous and relapsing inflammation and repair, these patients have a markedly increased risk (58-fold) for PDA [162].
Germline mutations in ATM, which encodes a protein in cell cycle regulation and DNA damage response, have been identified in approximately 2 % of familial pancreatic cancer [163]. The germline mutation is heterozygous; biallelic germline mutations in the gene result in ataxia-telangiectasia, which is characterized by cerebellar ataxia and sensitivity to ionizing radiation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree