Fig. 32.1
Intraepidermal melanocytic proliferations. a Atypical lentiginous melanocytic hyperplasia in lentigo maligna, b nested intraepidermal melanocytic proliferation in a compound dysplastic nevus with slight cytological atypia, c nested and pagetoid intraepidermal growth pattern in melanoma in situ
32.4.2 Histopathology of Benign Nevi
The histopathology of benign melanocytic tumors is quite variable. These nested proliferations of melanocytes may be confined to the epidermis (junctional nevus), present in both the epidermis and dermis (compound nevus) or limited to the dermis (dermal nevus). The most common nevi are composed of nests of benign appearing melanocytes with smooth nuclear contours, inconspicuous nucleoli, and variable amounts of amphophilic to eosinophilic cytoplasm. The pattern of melanocytes in the epidermis of benign nevi may be lentiginous (a proliferation of individual cells along the dermoepidermal junction) or nested (aggregates of three or more nevomelanocytes). In benign junctional and compound nevi the intraepidermal melanocytic proliferation is usually localized to the base of the epidermis (Fig. 32.1b). However, in some cases the melanocytes may be present in the upper half of the epidermis; this growth pattern is termed “pagetoid” when individual melanocytes are seen in the upper levels of the epidermis because it mimics Paget’s disease of the breast. While a pagetoid growth pattern maybe seen in specific rare subsets of benign nevi, it is a characteristic feature of melanoma in situ (Fig. 32.1c). In compound and dermal nevi, the melanocytes in the superficial dermis have round to oval nuclei with small nucleoli and occasionally delicately pigmented cytoplasm, these are termed as type A nevomelanocytes. With increasing depth in the dermis the tumor cells have more round nuclei, less cytoplasm, and inconspicuous nucleoli, termed as type B nevomelanocytes. At the deepest aspect of the nevus, the type C nevomelanocytes have small round nuclei with minimal cytoplasm and may mimic lymphocytes or fibroblasts. This transition of cytological appearances from superficial cells with larger nuclei, open chromatin, more cytoplasm, to smaller cells with minimal cytoplasm, is termed as “maturation” and is a feature of benign nevi that is helpful in distinguishing benign melanocytic tumors from melanoma (Fig. 32.2a).
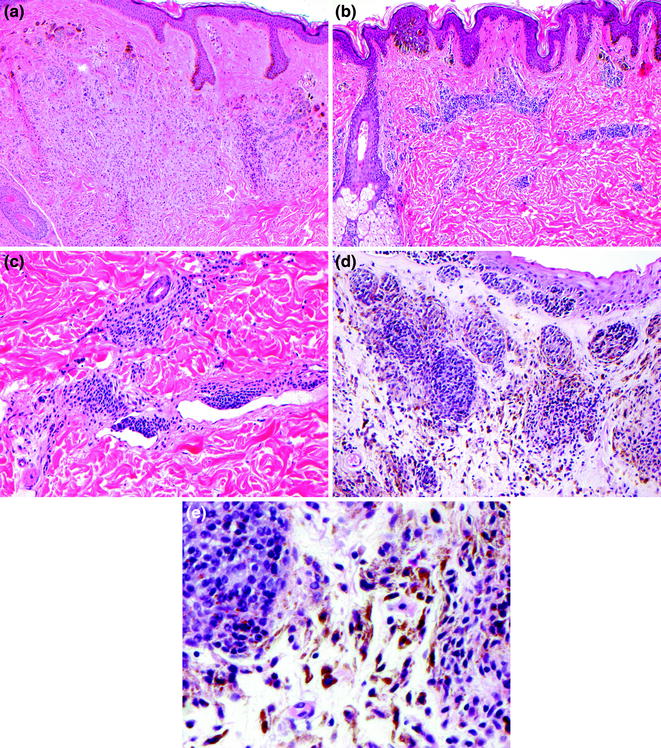

Fig. 32.2
Benign intradermal melanocytic proliferations. a Melanocytic maturation in the dermal component of congenital nevus, b perivascular growth pattern in congenital nevus, c subendothelial growth pattern in congenital nevus, d combined dermal and blue nevus of the conjunctiva, e epithelioid dermal nevus cells admixed with pigmented dendritic blue nevus cells in combined conjunctival nevus
In addition to the common benign nevi, there are several histologically distinct types of benign melanocytic nevi; these include nevi with features of congenital onset, dermal dendritic melanocytic proliferations (blue nevi and variants), nevi with cytological and architectural disorder (dysplastic nevi), and spindled and epithelioid cell nevi (Spitz nevi). While in most cases these melanocytic nevus variants may be readily identified as benign, in some cases these tumors may display histopathological features that mimic findings characteristic of melanoma.
Melanocytic nevi with features of congenital onset have variable dermal patterns of growth that are distinctive. Congenital nevi are usually compound or dermal and characteristically involve the reticular dermis. The dermal growth patterns of congenital melanocytic nevi may be strikingly perivascular (Fig. 32.2b), diffusely infiltrate throughout the reticular dermis to form a plaque, extend around adnexal structures, infiltrate into the arrector pili smooth muscle, and occasionally display a subendothelial growth pattern mimicking vascular invasion (Fig. 32.2c).
Most benign melanocytic nevi are composed of melanocytes with round or oval nuclei and variable amounts of cytoplasm, termed “epithelioid” because of the resemblance to epithelial cells. However, there also is a distinctive category of nevi that are composed predominantly of melanocytes with small round to oval nuclei and delicately elongated dendritic cytoplasm. The cytoplasm of these melanocytes is most apparent when pigmented (Fig. 32.2d). The presence of cells with pigmented cytoplasm in the dermis gives a blue hue to the skin due to the Tyndall effect of light scatter. These tumors are termed “blue nevi” because of this distinctive clinical appearance. Variants of blue nevi include the nevus of Ito which typically occurs on the shoulder or upper arm, the nevus of Ota which typically occurs on skin innervated by the ophthalmic and maxillary branches of the trigeminal nerve and the mongolian spot which typically occurs in lumbosacral region. Blue nevi may also occur in combination with other types of benign nevi, these combined blue nevi are most commonly found in the skin of the eyelid [4] (Fig. 32.2e).
32.4.3 Histology of Dysplastic Nevi
Dysplastic melanocytic nevi were first identified as a distinctive type of atypical nevus in the setting of familial melanoma [5, 6]. The description of dysplastic nevi as sporadic nevi in patients without familial melanoma has stimulated considerable controversy. A significant body of science has established dysplastic nevi as an intermediate between common benign nevi and malignant melanoma. These tumors with cytological and architectural disorder are clinically and histologically indistinguishable from dysplastic nevi that occur in patients with familial melanoma. While controversy regarding the criteria for diagnosis continues, the most straightforward approach to the histopathological diagnosis of dysplastic melanocytic nevi resulted from a consensus conference on this topic more than two decades ago [7]. Using these guidelines, the histological diagnosis of a dysplastic nevus is based upon cytological features of the melanocytes, architectural growth patterns, and the host response to the tumor. Adherence to these criteria allows for consistent diagnosis of dysplastic nevi. Both major criteria are required and at least two of the minor criteria are required. The two major criteria are: a lentiginous and nested cytologically atypical intraepidermal melanocytic proliferation, and a nested intraepidermal component that extends three rete ridges beyond the dermal component (termed a “shoulder” with the body representing the dermal melanocytic tumor). This second criterion is not required in purely epidermal (junctional) dysplastic nevi. The minor criteria include:
a prominent superficial vascular plexus,
superficial papillary dermal fibrosis in either an eosinophilic concentric or lamellar pattern,
a lymphocytic inflammatory infiltrate about the superficial vascular plexus, and
nest expansion and fusion at the dermoepidermal junction, termed “bridging.”
Cytological atypia is required for the diagnosis of a dysplastic nevus; however, there is a range of degrees of cytological atypia. While some will use architectural disorder, including limited pagetoid spread, to contribute to grading; most dysplastic nevi are graded based on the degree of melanocytic atypia. These tumors show nuclear variability along a continuum with gradual nuclear enlargement, increased chromatin density or clumping, nucleolar enlargement, and pleomorphism. With increasing degrees of atypia, the size of the nuclei increase; a good reference for size is the nucleus of the mid-layer keratinocyte. Because melanocytic atypia represents a spectrum, precise criteria are not possible. In general, mildly atypical cells have cytoplasmic retraction with a perinuclear halo and inconspicuous cytoplasm, the nuclei are oval, sickle shaped, angulated or rhomboidal, often with dense nuclear chromatin and do not exceed the size of a mid-layer keratinocyte nucleus. Moderately atypical nevomelanocytes have amphophilic or delicately pigmented cytoplasm, with enlarged rhomboidal, angulated, or oval nuclei, usually larger than a mid-layer keratinocyte nucleus, there is nuclear pleomorphism and nucleoli may be visible (Fig. 32.3). Severely atypical nevomelanocytes have features of melanoma cells, the cytoplasm may be amphophilic, eosinophilic or inconspicuous, nuclei are larger than mid-layer keratinocyte nuclei and have irregular folding, undulating nuclear contours, chromatin clumping or dense chromatin, nucleoli maybe be prominent and eosinophilic, and there is marked nuclear pleomorphism [8]. A limited pagetoid growth pattern with rare individual cells in the lower two-thirds of the epidermis may be seen in dysplastic nevi. Extensive pagetoid involvement of the upper layers of the epidermis is considered by many to be diagnostic of melanoma in situ, while others allow for a certain degree of pagetoid spread in severely atypical dysplastic nevi [9].
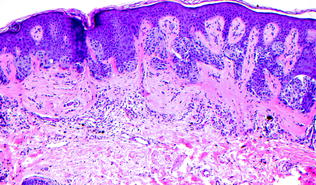

Fig. 32.3
Compound dysplastic nevus with moderate cytological atypia. There is bridging of intrapepidermal nests, subepithelial fibrosis, lymphocytic infiltrate and moderate cytological atypia of the intraepidermal melanocytes
32.4.4 Histology of Spindled and Epithelioid Cell (Spitz) Nevi
The diagnosis, predicted prognosis, and optimal therapy continue to be controversial for atypical melanocytic tumors with features of the spindled and epithelioid cell melanocytic tumors described by Spitz [10]. While most cases may be identified histopathologically as either a benign Spitz nevus or a melanoma, a subset of melanocytic tumors are histologically ambiguous sharing features of Spitz nevus and melanoma. These cases are important to identify as either atypical Spitz tumors (with a high risk of local metastasis but minimal risk of spread beyond the sentinel lymph node basin) or spitzoid melanoma (with prognosis likely similar to conventional melanoma). There has been debate regarding the terminology of these atypical melanocytic tumors [11, 12], nevertheless, the histopathological criteria for distinguishing atypical Spitz tumor, spitzoid melanoma, and conventional melanoma are more well-defined now than in past decade [13]. Extensive research on this topic has led to evolution of the standards of care for patients with atypical Spitz tumor and spitzoid melanoma [14–16]. Nevertheless, the histological criteria for the distinction of atypical Spitz tumor from melanoma are not consistently defined [17–19]. Some will use the term “atypical Spitz tumor” for all Spitz nevi with any atypical histological feature, and “melanoma” for melanomas with spitzoid features (including spitzoid melanomas) [13]. Consensus meetings have led to some unifying concepts [17, 18]:
histologically conventional benign Spitz nevi are not associated with metastasis (Fig. 32.4) [20, 21],
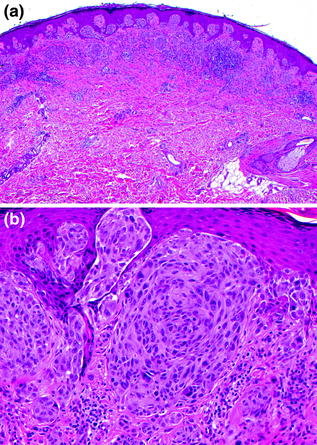
Fig. 32.4
Compound spindled and epithelioid cell nevus (Spitz). a A wedge-shaped intraepidermal and dermal melanocytic proliferation extends into the reticular dermis, b Kamino bodies (intraepidermal eosinophilic deposits) are present among the epithelioid melanocytes
atypical spindled and epithelioid melanocytic proliferations resembling Spitz nevi but with cytological and/or architectural atypia (e.g., atypical Spitz tumors) are frequently associated with sentinel lymph node metastases [12, 18, 22–33], and
Clear distinctions between these categories remain difficult and controversial because atypical Spitz tumor and spitzoid melanoma may exist on a continuum with Spitz nevi on one end and melanoma at the other. Histopathologically, atypical Spitz tumor has an overall resemblance to spindled and epithelioid cell nevus (Spitz nevus) but also displays a few atypical features. Spitzoid melanoma differs by showing more atypical features, with atypical findings sufficient for a diagnosis of melanoma (Fig. 32.5). The atypical histopathological findings include abnormalities of cellular organization, proliferation, and cytological atypia. Disorders of organization are manifested as: diameter > 10 mm, disordered intraepidermal growth pattern without supra-nest clefts, prominent pagetoid growth, ulceration, absence of Kamino bodies, confluent growth pattern, high cellular density, asymmetry at scanning magnification, poor circumscription, lack of maturation, lack of zonation (horizontal cytological consistency), and extension into the subcutaneous fat [13, 36]. Proliferation criteria include: more than two mitoses in the tumor or mitoses at the deep advancing margin of the tumor [37] and Ki-67 staining of more than 20 % of tumor cells [38]. Cytological atypia is manifested as: high nuclear-to-cytoplasmic ratio, “dusty” granular cytoplasmic pigmentation, nuclear chromatin clumping, nuclear membrane thickening, and irregularly shaped or enlarged nucleoli. In additional to the histopathological features, clinical characteristics including the age of the patient, and the color, size, and overall clinical appearance of the tumor also influence the final diagnosis.
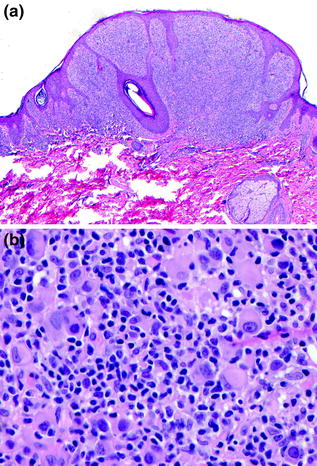

Fig. 32.5
Spitzoid melanoma. a A predominantly epithelioid intraepidermal and dermal melanocytic proliferation, b cytologically pleomorphic epithelioid melanocytes, lymphocytes, and mitosis
32.4.5 Histology of Other Benign Melanoma Mimics
As noted above in the description for dysplastic nevi and spitzoid neoplasms, the diagnosis of melanoma can be challenging in a subset of melanocytic tumors that are benign but share histopathological features with melanoma. One of the histopathological features that may be observed in both benign and malignant melanocytic tumors is pagetoid intraepidermal spread, a proliferation of individual melanocytes present in the upper levels of the epidermis. There are several types of benign melanocytic proliferations that also display pagetoid spread and can be distinguished from melanoma. Pagetoid growth pattern is seen in the following benign melanocytic tumors: congenital nevus in children less than 5 years of age, spindled and epithelioid cell (Spitz) nevus, pigmented spindled cell nevus, recurrent nevus, excoriated nevus, acral nevus (also known as MANIAC = melanocytic acral nevus with intraepidermal ascent of cells [39]), and occasionally dysplastic nevi. In most of these cases the histopathological attributes including the pattern of melanocytic nesting and absence of marked cytological atypia will aid in arriving at the correct diagnosis.
Another category of benign melanoma mimics includes combined nevi. These complex melanocytic tumors share features of two or more types of melanocytic nevi. The most diagnostically challenging of these are the combined nevi with deep pigmented melanocytes including the deep penetrating/plexiform spindled cell nevi (Fig. 32.6) and the clonal/inverted type A nevi [4, 40, 41]. Despite some histopathological similarities to melanoma these nevi are entirely benign and can be identified based on histopathological and IHC analysis.
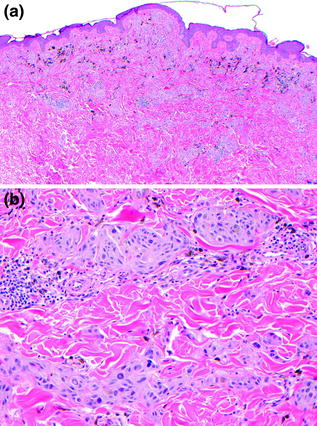

Fig. 32.6
Deep penetrating/plexiform spindled cell nevus. a A wedge-shaped dermal proliferation of lightly pigmented melanocytes, extending along neurovascular bundles with admixed pigment-laden macrophages. b The delicately pigmented tumor cells are admixed with coarsely pigmented macrophages and surround vascular and neural structures, extending deep into the dermis with plexiform growth pattern
32.5 Histopathology of Melanoma
32.5.1 Melanoma Subtypes
The initial classification of primary cutaneous melanoma occurred nearly 50 years ago based upon detailed observations and descriptions of the clinical and histopathological findings [42, 43]. There have been some additions to the classification scheme, however, the original descriptions form the foundation for the currently recognized subtypes of cutaneous melanoma [42, 44–47]. The original descriptions of cutaneous melanoma included the clinical appearance, age, gender, anatomic sites, and degree of sun exposure. The authors also described in detail the histological features including patterns of tumor cell growth in the epidermis and dermis, cytologic features, epidermal changes including atrophy and ulceration, the presence of solar elastosis, the anatomic level of invasion, the maximal tumor thickness as measured perpendicular to the epidermal surface from the top of the granular cell layer, vascular invasion, mitotic activity, and the pattern and density of lymphocytic host response. These clinical and pathological variables formed the framework for cutaneous melanoma classification into three major subtypes: invasive melanoma with adjacent intraepidermal component of superficial spreading type (superficial spreading melanoma, SSM), invasive melanoma with adjacent intraepidermal component of Hutchinson’s melanotic freckle type (lentigo maligna melanoma, LMM), and invasive melanoma without adjacent intraepidermal component (nodular melanoma, NMM). The adjacent intraepidermal component was defined as extending at least three rete ridges within the epidermis beyond the edge of the dermal component. The extension of melanoma in the epidermis and superficial dermis is termed “radial growth phase,” when the dermal invasive component become more prominent or mitotically active it is termed “vertical growth phase” (Table 32.1). In the years since this original description, additional cutaneous melanoma subtypes, including acral lentiginous, mucosal lentiginous, nevoid, desmoplastic, spindled, and others have been described. Each has distinctive clinical and histopathological features (Table 32.2).
Table 32.1
Definitions of radial and vertical growth phase in primary cutaneous melanoma
Characteristics of radial growth phase (RGP) melanoma |
• Single cell dermal invasion • Small invasive nests (dermal nests smaller than intraepidermal nests) • No dermal tumor cell mitoses • Inflammatory infiltrate present • Papillary dermis involved (Clark level II) • No expansile nodule (s) |
Characteristics of vertical growth phase (VGP) melanoma |
• Expansile nodule, nests in dermis larger than epidermis • Dermal mitoses • Stromal changes (desmoplastic) • Present in papillary dermis, +/– reticular dermis, +/– fat (Clark level III, IV or V) |
Table 32.2
Distinctive histopathological findings, frequency, and growth patterns in primary cutaneous melanoma
% of cases | Distinctive findings | RGP | VGP | |
|---|---|---|---|---|
Superficial Spreading | >70 | Pagetoid RGP, epithelioid VGP | + | −/+ |
Lentigo Maligna | 3 | Sun-damaged skin, lentiginous RGP extension down hair follicles, epithelioid or spindled VGP | + | −/+ |
Acral Lentiginous | 2 | Acral sites, lentiginous RGP extension down eccrine units, epithelioid VGP | + | −/+ |
Nodular | 20 | No radial growth phase, epithelioid VGP | − | + |
Desmoplastic | <1 | Often associated with lentigo maligna, scar-like VGP | +/- | + |
Nevoid | <1 | Symmetric nevic like dermal component, epithelioid VGP | −/+ | + |
Spindled | <1 | Often associated with lentigo maligna, spindled VGP | +/- | + |
Mucosal lentiginous | <1 | Oral, genitourinary, and gastrointestinal mucosa | + | −/+ |
32.5.1.1 Superficial Spreading Melanoma
The clinical appearance of superficial spreading melanoma is variable and includes a broad range of colors including tan, brown, gray, black, violaceous, pink, and occasionally blue or white. The edge of the tumor with adjacent normal skin is usually sharply marginated with a few irregular peninsula-like protrusions. The surface may be slightly elevated or have a palpable papule or a nodule that extends several millimeters above the skin surface.
The histopathological features of superficial spreading melanoma include an intraepidermal component with a pagetoid and nested growth patterns at all levels of the epidermis (Fig. 32.7). The intraepidermal tumor cells have a prominent epithelioid cytology with abundant cytoplasm that is eosinophilic, amphophilic or has a “dusty” distribution of fine cytoplasmic melanin granules. The nuclei may be large, have irregular nuclear contours, and contain one or more prominent nucleoli. These intraepidermal tumor cells are relatively uniform and appear cytologically similar to one another. In invasive dermal melanoma, the tumor cells have cytological features similar to the intraepidermal tumor cells. The dermal melanoma component may also be composed of numerous variably sized nests that may be associated with expansile nodule formation. In cases with abundant dermal tumor the cytological heterogeneity becomes more apparent, often with striking variation in cell morphology from one tumor nest to the next.
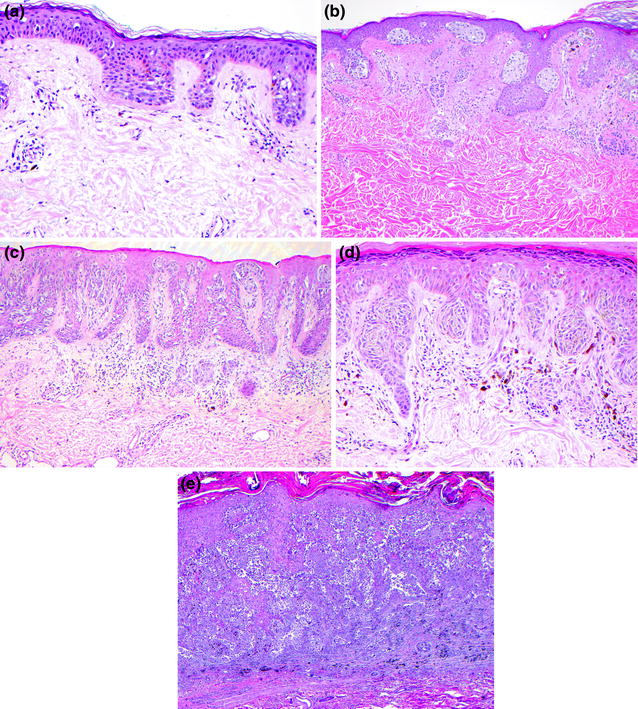

Fig. 32.7
Superficial spreading melanoma. a In situ melanoma with pagetoid intraepidermal growth pattern, b in situ nested and pagetoid intraepidermal proliferation with superficial dermal nevus, c pagetoid and nested intraepidermal melanoma with invasion of the superficial dermis, d the cytology of the intradermal tumor cells is similar to that of the intraepidermal component, e intraepidermal and dermal melanoma with multifocal dermal invasion
32.5.1.2 Nodular Melanoma
The clinical appearance of nodular melanoma is usually a relatively uniform brown, black or blue-black elevated lesion. Nodular melanoma may be a smoothly surfaced cutaneous nodule, an elevated plaque with irregular outlines, or a polypoidal ulcerated exophytic tumor. In contrast to superficial spreading and lentigo maligna types of melanoma, there is no surrounding flat pigmented lesion associated with the tumor.
The histopathology of nodular melanoma is that of a predominantly dermal tumor. When an intraepidermal component is present it directly overlies the invasive melanoma (Fig. 32.8). Occasionally, the epidermal component is so minimal as to suggest the possibility that the tumor represents a dermal metastasis. The vertical growth phase of nodular melanoma is composed of small nests and aggregates of tumor cells that together form the overall tumor nodule.
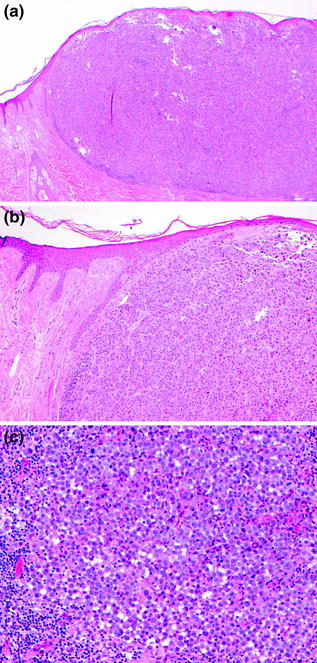

Fig. 32.8
Nodular melanoma. a Epithelioid tumor cells form an expansile dermal nodule with only focal involvement of the overlying epidermis, this is pure vertical growth phase tumor, b there is no radial growth phase melanocytic proliferation, c the epithelioid tumor cells have pleomorphic nuclei with prominent nucleoli and a lymphocytic infiltrate
32.5.1.3 Lentigo Maligna Melanoma
The clinical appearance of lentigo maligna melanoma is that of a relatively large, mostly flat, pigmented lesion with a variegated coloration that includes tan, brown, and black and may have flecks of black or brown. The outline of the tumor is irregular and may merge with the surrounding skin tones. Wood’s light examination may be helpful in identifying the edge of the melanocytic proliferation. Although lentigo maligna melanoma is predominantly flat, foci of invasion may be detected as a slightly raised papule that may be best detected by side lighting [48].
Lentigo maligna melanoma arises in the setting of lentigo maligna. There is a spectrum of atypical melanocytic proliferations that occur in sun-damaged skin of the elderly, usually on the face, scalp, and neck. These proliferations arise in a background of epidermal atrophy with marked solar elastosis. Histopathologically, the melanocytic proliferations range from subtle atypical melanocytic hyperplasia, to lentigo maligna, to lentigo maligna melanoma in situ, to invasive lentigo maligna melanoma (Fig. 32.9). The histopathology of lentigo maligna melanoma is characterized by an intraepidermal predominantly individual cell melanocytic proliferation localized to the basal layers of the epidermis. The cytology of the lentiginous proliferation is strikingly atypical; tumor cells have large densely chromatic nuclei and are occasionally multinucleated. When the proliferation is confluent the basal keratinocytes appear to be replaced by a continuous line of severely atypical melanocytes. The lentiginous proliferation extends down hair follicle epithelium, maintaining close approximation to the basal layer. Intraepidermal nests and pagetoid spread may be observed, however, these features are subtle when present, in contrast to superficial spreading melanoma, which is characterized by a nested and pagetoid intraepidermal component. While some observers include lentigo maligna and lentigo maligna melanoma in situ in one diagnostic category, others separate these two based on histopathological findings [49, 50]. Criteria for lentigo maligna melanoma in situ include a severely atypical lentiginous melanocytic proliferation with two or more of the following: (1) intraepidermal melanocytic nests, (2) pagetoid growth pattern, (3) confluence of melanocytes along the dermal epidermal junction [51]. When tumor cells extend into the dermis, the diagnosis is lentigo maligna melanoma. The dermal invasive component of lentigo maligna melanoma may display spindled cells and tumor cell pigmentation, have a scar-like desmoplastic appearance or may show features similar to those observed in the dermal component of superficial spreading and nodular melanoma. Desmoplasia and neurotropism are more commonly found in lentigo maligna melanoma than in other melanoma types.
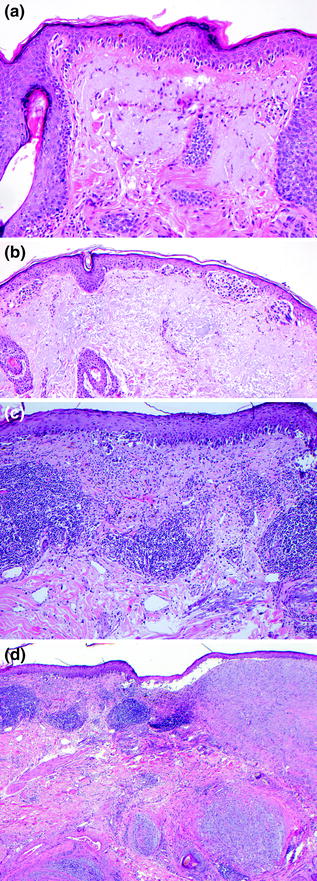

Fig. 32.9
Lentigo Maligna Melanoma. a In situ melanoma composed of a confluent lentiginous intraepidermal proliferation of cytologically atypical melanocytes associated with epidermal atrophy and solar elastosis, b in situ melanoma with a lentiginous and nested intraepidermal proliferation of cytologically atypical melanocytes, c intraepidermal and dermal melanoma with a confluent lentiginous and nested intraepidermal component and underlying dermal invasion with associated lymphocytic infiltrate, d invasive desmoplastic neurotropic melanoma arising the setting of lentigo maligna melanoma
32.5.1.4 Melanoma: Rare Subtypes
In addition to superficial spreading, lentigo maligna and nodular melanoma, there are several rare, clinically and histopathologically distinctive subtypes. These include acral lentiginous melanoma (Fig. 32.10), mucosal lentiginous melanoma, nevoid melanoma, desmoplastic melanoma (mixed and pure), and spindled melanoma. Acral lentiginous and mucosal lentiginous melanomas arise in anatomically distinctive sites: hands and feet in the case of acral melanoma, and oral, genital, and gastrointestinal mucosa in the case of mucosal melanoma. Some of these rare forms of melanoma may fit into an existing subtype, for example, nevoid melanoma is considered by some to be a variant of nodular melanoma [36, 52–54] (Fig. 32.11). There may be overlap between nevoid melanoma with Spitz-like tumors and tumors associated with BAP-1 loss; further research is needed to fully understand this subset of tumors [55, 56]. Desmoplastic melanomas are usually lentigo maligna type [57–62] (Fig. 32.12). It is now known that even further subcategorization is helpful when determining the treatment for desmoplastic melanoma. Patients with pure desmoplastic melanoma have a lower risk of metastases than those with mixed desmoplastic and conventional melanoma [59]. Other rare forms of melanoma include the pigmented epithelioid melanocytoma [63, 64] and Spitzoid melanoma [20, 21, 37, 65–69]. The treatment plan for these rare tumors is based on reports of similar tumors rather than the guidelines from the American Joint Commission on Cancer (AJCC) or other schemes.
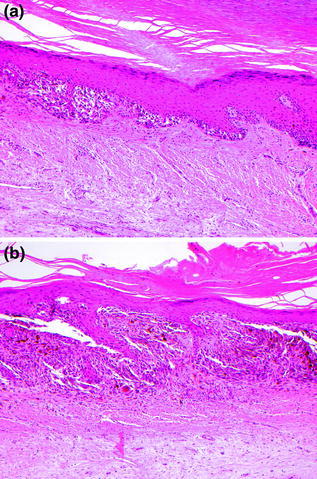
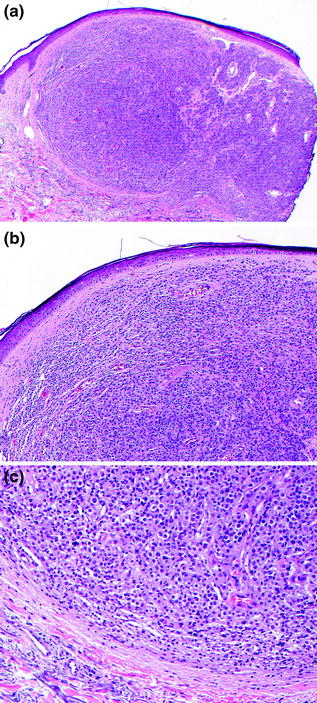
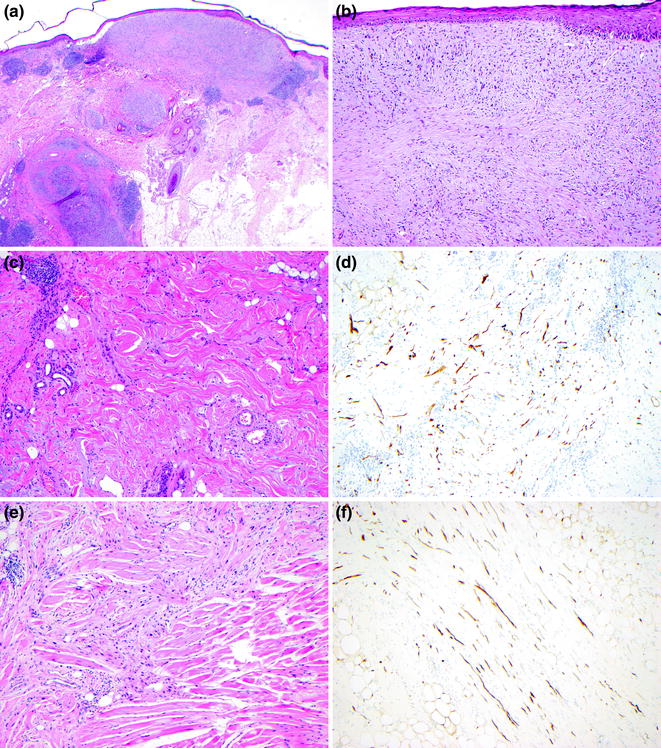

Fig. 32.10
Acral Lentiginous Melanoma. a In situ melanoma in a lentiginous and nested pattern, b the intraepidermal tumor cells are cytologically atypical with high nuclear-to-cytoplasmic ratios and nuclear enlargement

Fig. 32.11
Nevoid Melanoma. a This dermal proliferation of melanocytes is not associated with an overlying epidermal melanocytic proliferation, b the tumor cells have small to medium-sized nuclei without significant cytological atypia, resembling dermal nevus cells, c there is no maturation of tumor cells with increasing dermal depth, the tumor cells at the base have similar features to those at the superficial aspect of the tumor

Fig. 32.12
Desmoplastic melanoma. a There is an extensive infiltrative spindled and desmoplastic tumor arising in a background of lentigo maligna, the presence of lymphoid aggregates in the deep dermis and subcutis are a clue to the presence of neurotropism, b the superficial dermal tumor cells in this desmoplastic focus have spindled and dendritic cytology resembling scar, C. In some regions the tumor cell density is low, d an S100 stain in the region shown in panel C displays numerous desmoplastic melanoma cells, e the desmoplastic tumor invaded skeletal muscle, f an S100 stain highlights the dendritic melanoma cells infiltrating fat
32.5.2 Cutaneous Melanoma Histopathological Prognostic Factors
The histopathological features of cutaneous melanoma serve as the foundation for staging. Factors included in the AJCC staging system include primary tumor thickness, mitogenicity, and presence or absence of ulceration (Fig. 32.13). The histological identification of microscopic metastases as microscopic satellites, in-transit metastases, and sentinel lymph node metastases also factor into the AJCC stage. Other histopathological prognostic factors that may guide patient therapy include Clark level of invasion, tumor infiltrating lymphocytes, lymphovascular invasion, regression, and neurotropism (Fig. 32.13).
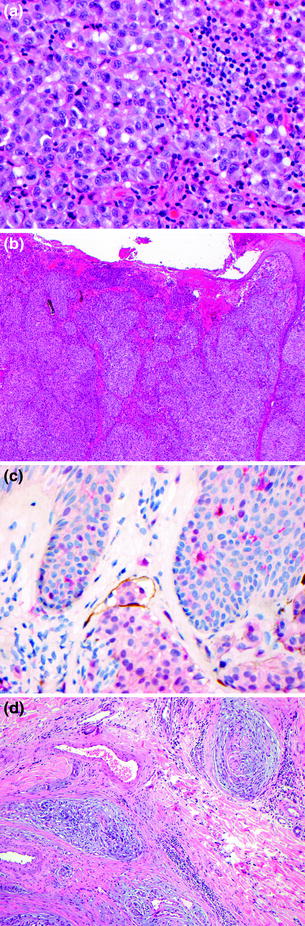

Fig. 32.13
Melanoma prognostic factors. a mitoses, b ulceration, c lymphovascular invasion (brown D240, pink S100 staining), d neurotropism
32.5.2.1 Primary Tumor Thickness (Breslow)
The maximal thickness of primary cutaneous melanoma, as measured under the microscope using an intraocular ruler, is one of the most powerful predictors of melanoma survival [47]. This measurement is a critical component of the pathology report and must be determined consistently. A calibration table is used to ensure accurate measurement across different brands and models of microscopes. This melanoma measurement is taken perpendicular to the epidermis from the top of the epidermal granular cell layer overlying the thickest part of the tumor to the deepest invasive melanoma cell. When ulceration is present, the measurement is taken from the topmost viable tumor cell at the base of the ulcer to the deepest point of tumor cell invasion. The deepest measured melanoma cell must be free and clear of adnexal structures: tumor cells that extend along perineural and periadnexal (adventitial) dermis and perivascular or intravascular extension are not included in the primary tumor thickness measurement. When the primary tumor has a polypoidal architecture, the Breslow thickness is obtained by measuring across the largest diameter of the lesion perpendicular to the skin surface [70].
32.5.2.2 Primary Tumor Mitogenicity
It has long been known that increased proliferative activity of invasive melanoma is associated with poor prognosis [71, 72]. Mitogenicity has been found to be of greatest prognostic power in patients with thin melanomas less than 1 mm thick [73–75]. Additionally, mitogenicity may be a powerful prognostic factor in patients with negative sentinel lymph nodes [76]. The presence of one mitosis in the dermal component of melanoma with thickness < 1 mm leads to upstaging from T1a to T1b. This upstaging is associated with more therapeutic intervention, usually sentinel lymph node removal and adjuvant therapy. Because the studies that revealed mitoses as clinically significant were retrospective studies that employed the “hot spot” technique of evaluating tumor mitogenicity, the AJCC Melanoma Staging Committee recommends that mitotic count be determined by this approach and reported as the number of mitoses per square millimeter of the primary tumor [77]. Determining mitogenicity is accomplished by examination of routine hematoxylin and eosin (H&E)-stained tissue sections. It is not necessary to do exhaustive tissue sectioning. After review of the invasive tumor the area with the most mitotic figures (“hot spot”) is identified, and the count starts using a high power 40× objective (Fig. 32.13a). After determining the number of mitoses in the first high power field the count is extended to adjacent fields until an area of 1 mm2 is assessed. To ensure accuracy across observers, individual microscopes are calibrated to determine the number of high power fields corresponding to 1 mm2. Mitogenicity is reported as n/mm2. If only one mitosis is found in the entire invasive component, the report states 1/mm2. If no dermal mitoses are identified the count is reported as 0/mm2. When the invasive component of the tumor measures less than 1 mm2 the count is performed on the entire dermal tumor and reported as n/mm2. The AJCC Melanoma Staging Committee strongly discourages the use of “<1/mm2” in reporting melanoma. It is important to distinguish between the reporting function, which should always be in a whole number/mm2 and the staging language which is described as a range, e.g., greater than or equal to 1/mm2.
32.5.2.3 Primary Tumor Ulceration
In the past decade ulceration has been identified as an important adverse prognostic factor in primary cutaneous melanoma. Unlike mitogenicity which is most powerful in thin tumors, ulceration serves as more powerful discriminator in tumors >1 mm thickness, perhaps in part because ulceration is very rare in tumors <1 mm. It may be difficult to distinguish thick-scale crust from an ulcer clinically, thus, in primary cutaneous melanoma staging ulceration is defined histopathologically. Tumor ulceration is defined as full-thickness interruption of the epidermis by tumor without prior history of mechanical trauma or surgery at the site. The epidermal disruption is associated with fibrin, inflammatory cells, and granulation tissue (Fig. 32.13b).
32.5.2.4 Tumor Infiltrating Lymphocytes in Primary Cutaneous Melanoma
The presence of lymphocytes infiltrating the vertical growth phase of malignant melanoma has been associated with a better prognosis [72, 78, 79]. The pattern of tumor infiltrating lymphocytes is graded as “brisk” when the lymphocytes diffusely infiltrate throughout the vertical growth phase tumor or form a continuous inflammatory front along the entire advancing tumor front, “non-brisk” when there is focal or multifocal infiltration of the vertical growth phase, and “absent” when there are no lymphocytes infiltrating the tumoral compartment. Notably, if a dense inflammatory infiltrate is present in the specimen adjacent to, but not infiltrating the melanoma, this is also termed “absent.” The presence of “brisk” tumor infiltrating lymphocytes is associated with a better prognosis, however, some of these patients may still develop metastasis and disease progression. Several additional studies have further characterized these as T cells [80]. Although tumor infiltrating lymphocyte grade is not currently part of cutaneous melanoma staging, advances in immunotherapy and further understanding of the role of lymphocytic subsets may lead to changes in future staging algorithms [81, 82].
32.5.2.5 Lymphovascular Invasion in Primary Cutaneous Melanoma
The identification of lymphovascular invasion in a primary melanoma correlates with an increased risk of metastasis [83–86] (Fig. 32.13c). Lymphovascular invasion is observed as the presence of tumor cells within the lumen of a lymphatic vessel. In some cases tumor cells may be observed surrounding vascular structures, a phenomenon known as “extravascular migratory metastasis” or “angiotropism” [87, 88]. Lymphovascular invasion has been demonstrated as prognostic factor in early stage melanoma [89].
32.5.2.6 Microscopic Satellites in Cutaneous Melanoma
The identification of microscopic melanoma metastases may occur in the tissue section with the primary tumor (microscopic satellite), in the skin within 5 cm of the primary tumor (satellite metastasis) and in the skin or soft tissue in a region between 5 cm from the cutaneous site and the regional lymph node basin (in-transit metastasis). Microscopic satellites identified in the primary tumor tissue section are defined as a discontinuous group or nest of melanoma cells, greater than 0.05 mm in diameter, that are located at least 0.3 mm from the dermal invasive tumor mass and separated from it by normal dermis or panniculus not affected by fibrosis or inflammation [90]. Described as a primary tumor prognostic factor, microscopic satellites are associated with poor prognosis [91, 92]. The AJCC staging system includes microscopic satellites, satellite metastases, and in-transit metastases along with intralymphatic metastases [93]. Primary tumor microsatellites are an independent predictor of reduced disease-free survival in patients with positive sentinel lymph nodes [94]. The terms “intralymphatic regional metastases” and “in-transit metastases/satellites” are now used to describe these patterns of local spread [77].
32.5.2.7 Neural Involvement in Primary Cutaneous Melanoma
Perineural invasion and neurotropism may be observed in melanoma. Neurotropism is most commonly associated with spindled or desmoplastic melanoma as an extension of tumor cells around and within cutaneous nerves (Fig. 32.13d). This neurotropic pattern of growth is often accompanied by a lymphocytic infiltrate and is most common on melanomas of the head and neck.
32.5.2.8 Anatomic Level of Invasion (Clark)
The level of invasion in relationship to the anatomical boundaries of the papillary and reticular dermis and subcutaneous fat were originally defined by Clark in 1969 [42]. Increasing levels of invasion correlate with poor outcome. Clark level I tumors are limited to the epidermis, level II tumors display individual cell and small melanoma cell nests in the papillary dermis, level III tumors have expansile nodules in the papillary dermis that push upon but do not invade the reticular dermis, level IV tumors have tumor cells in the reticular dermis, and level V tumors invade the subcutaneous fat. While this metric has largely been replaced by mitogenicity in the AJCC staging schema, Clark level remains important in cases where mitoses are difficult to assess due to poor processing or when tumor thickness cannot be accurately measured due to poor tissue orientation. In these rare cases, the identification of melanoma in the reticular dermis (Clark level IV) or subcutaneous fat (Clark level V) can assist in tumor staging.
32.5.2.9 Regression in Primary Cutaneous Melanoma
The phenomenon of regression was described by Clark as an important prognostic indicator and it has been associated with increased likelihood of metastasis, even in thin melanomas [72, 95]. Regression in primary cutaneous melanoma has the most prognostic significance when it is consistently defined. Regression occurs in the radial growth phase of the tumor and is characterized by complete absence of melanoma cells in the epidermis and dermis, flanked by viable melanoma cells. The regressed focus often displays epidermal atrophy with underlying fibroplasia, vascular prominence, chronic inflammation, and melanophages. The use of less stringent criteria for regression may contribute to the poor concordance between studies of tumor regression’s prognostic significance.
32.5.3 Melanoma Metastases and Sentinel Lymph Nodes
Sentinel lymph node mapping was described over two decades ago as a minimally invasive means of identifying microscopic metastases in regional lymph nodes [96]. This technique allows for more precise staging of patients with clinically localized cutaneous melanoma. The identification of even a single melanoma cell in a sentinel lymph node leads to upstaging from AJCC stage II to stage III. The anatomy of cutaneous lymphatic drainage allows for identification of the first or sentinel lymph node to drain a specific cutaneous site. The use of isosulfan blue dye injected at the primary cutaneous tumor site allows for identification of the draining blue lymph node in the regional lymph node basin. This sentinel node is the most likely lymph node to contain metastatic melanoma. The addition of lymphoscintigraphy with technetium-99 m–(99 mTc) labeled sulfur colloid, followed by intraoperative identification of sentinel lymph nodes using a handheld scanner with a gamma-sensor probe to detect 99 mTc has refined the technique for identifying early regional lymph node metastases. After the hottest lymph node is identified and the 99 mTc counts quantified, additional lymph nodes with >10 % of the counts of this hottest lymph node are removed and also considered sentinel [97]. On average, two to three sentinel lymph nodes are removed [76, 98], if melanoma is not detected in the sentinel lymph nodes, the remaining lymph nodes in the basin are unlikely to contain melanoma [99]. Patients diagnosed with sentinel lymph node metastases are usually offered complete lymphadenectomy and adjuvant therapy, although recently there has been a move away from offering completion lymphadenectomy to select patients with small sentinel lymph node deposits.
The detection of melanoma metastases in sentinel lymph nodes is best performed by careful examination of H&E-stained tissue sections, along with levels into the lymph nodes and IHC (Fig. 32.14), as occasionally tumor is most apparent on IHC sections. The diagnostic criteria for lymph node metastasis rely upon the identification of cytological features as well as the location and pattern of intranodal tumor spread: (1) the presence of individual cells or nests of epithelioid or spindled cells foreign to the lymph node, (2) cytological atypia including large pleomorphic nuclei with prominent nucleoli, variable cytoplasm occasionally with dusty cytoplasmic melanin granules, and (3) positive staining for one or more melanocytic markers (e.g., S–100, MART-1, Melan-A, HMB-45, MITF). Sentinel lymph node metastases are identified in 15–20 % of patients who undergo this procedure [100]. The differential diagnosis of metastasis in this setting includes benign melanocytic rests (nodal nevus) usually observed in the lymph node capsule or fibrous trabeculae. The reported frequency of these benign melanocytic deposits ranges from a few percent to more than 20 % [101]. The diagnostic criteria for benign melanocytic nevi in lymph nodes are: (1) individual cells in a linear array or nests of epithelioid or spindle cells foreign to the lymph node, (2) round or oval uniform nuclei without cytological atypia, (3) positive staining for one or more melanocytic marker (S-100, MART-1, MELAN-A, MITF; nodal nevi are usually negative for HMB-45), (4) identification of the cells on H&E-stained sections, and (5) cells are usually present in the fibrous capsule or trabeculae (Fig. 32.15). Overall these criteria allow for distinction of nodal melanocytic nevi from melanoma in most cases.
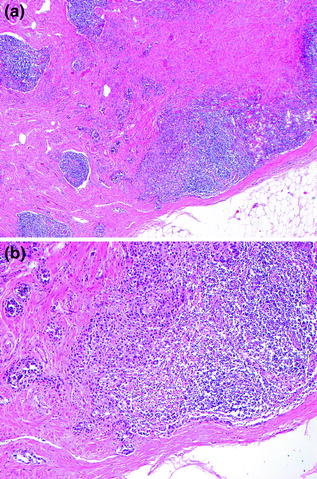
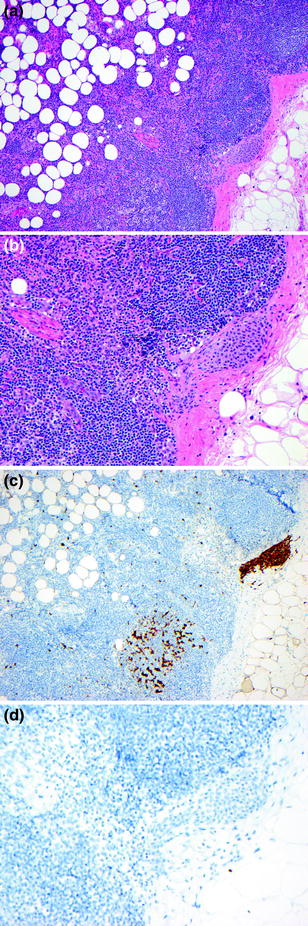

Fig. 32.14
Metastatic melanoma in lymph node parenchyma. a The tumor is present as nests embedded within fibrous tissue and associated with lymph node parenchyma, b The tumor cell nuclei are large and pleomorphic in comparison with the lymphocytes

Fig. 32.15
Benign nevus rests in lymph node capsule. b Cytologically banal nest of melanocytes are present in the fibrous capsule surrounding the lymphnode, b the nevic nuclei are small round to oval and uniform with little pleomorphism, c the nodal nevus and dendritic cells stain positively for S100, D. The nodal nevus does not stain for HMB-45 (same field as panel c)
32.5.4 Melanoma at Other Sites
32.5.4.1 Ocular Melanoma
Ocular melanoma is a rare form of melanoma representing approximately 3 % of melanomas, compared to >90 % of melanomas arising in the skin. Primary ocular melanoma usually arises in the uveal tract (85 %), but may also arise in the conjunctiva (5 %) or other ocular sites (10 %) [102]. Uveal melanoma is distinct from cutaneous melanoma in that treatment is largely based on clinical diagnosis and cytogenetic analysis. The most common form of ocular melanoma is that of the uveal tract, an intraocular structure composed of the iris, ciliary body, and choroid. The iris and ciliary body are located anterior to the retina, and contiguous with the choroid. The choroid, responsible for nourishing the retina and found between the epithelium and sclera, is composed of blood vessels, nerve fibers, and pigmented cells within a connective tissue matrix. Although uveal melanomas usually arise in the choroid (90 %), they can arise in any part of the uveal tract including the iris (4 %) and the ciliary body (6 %) [102, 103].
Clinical diagnosis of ocular melanoma based on ophthalmic examination, slit lamp examination, indirect ophthalmoscopy, and other ancillary testing has an accuracy of more than 99 % [104]. Ancillary diagnostic tests include ultrasonography, fluorescein angiography, indocyanine green angiography, optical coherence tomography, fundus autofluorescence, and fine needle aspiration (FNA). FNA allows for cytological evaluation and provides tumor cells for genetic analysis.
Histopathologic analysis of uveal melanoma is often based on cytological features observed on FNA and includes assessment of cell size/shape, cytoplasmic characteristics, nuclear and nucleolar features, degree of loss of cohesion, and proportions of cell types. In the past orbital enucleation allowed for more detailed histopathological analysis of ocular melanomas. Three histopathological subtypes of uveal melanoma have been described: spindle, mixed, and epithelioid (in order of worsening prognosis) [105]. Tumors composed predominantly of spindled cells comprise approximately 9 % of uveal melanomas, are slow growing, tightly cohesive and associated with a good prognosis. Approximately 5 % of uveal melanomas are composed of >50 % epithelioid cells with prominent eosinophilic nucleoli and ample cytoplasm are usually mitotically active, with dyscohesion and are associated with a poor prognosis. Mixed tumors, with 10–50 % epithelioid cells comprise the majority of uveal melanomas. Vasculogenic mimicry including the presence of closed vascular loops back to back on Periodic Acid–Schiff (PAS) stain is associated with increased mortality, and is often found in association with other negative prognostic indicators including epithelioid cell type and increased numbers of mitotic figures [106, 107].
Overall, important histopathologic features of ocular melanoma include mitotic count per 40 high power fields, mean diameter of the largest 10 nucleoli, presence of vasculogenic mimicry patterns including loops or other complex patterns, tumor infiltrating lymphocytes (more than 100 lymphocytes per 20 high power fields), and tumor infiltrating macrophages. In contrast to cutaneous melanoma, the presence of tumor infiltrating lymphocytes is a poor prognostic indicator in uveal melanoma. Basal tumor diameter, tumor height, presence of scleral invasion, and ciliary body involvement are important factors. Commonly seen characteristics include rupture of Bruch’s membrane (87.7 %), invasion of the retina (49.1 %), tumor cells in the vitreous (25.2 %), vortex vein invasion (8.9 %), invasion of tumor vessels by tumor cells (13.8 %), invasion into emissary canals (55.0 %), scleral invasion (55.7 %), and extrascleral extension 8.2 % [105]. Current AJCC staging guidelines are based primarily on tumor size and degree of extraocular spread.
32.5.4.2 Mucosal Melanoma
Melanoma rarely arises in mucosal sites, in particular the genitourinary, oral, and sinonasal mucosa and gastrointestinal mucosa [108–114]. These tumors have a very poor prognosis. The in situ phase of mucosal melanomas is usually similar to that seen in acral lentiginous melanoma: an often subtle lentiginous intraepithelial melanocytic proliferation. Some mucosal melanomas have more extensive intraepidermal tumor with pagetoid spread and nesting similar to superficial spreading melanoma. Adequate biopsy and local control are challenges in mucosal sites. IHC stains may be helpful in assessing the specimen margins [108]. The invasive component of mucosal melanoma is usually composed of nests and expansile nodules of pleomorphic tumor cells. The tumor cells may have epithelioid or spindled morphology, and occasionally plasmacytoid, rhabdoid, or neural differentiation is seen. Numerous pigment-laden macrophages may be present, and the tumor cells may have variable degrees of pigmentation. There are numerous mitotic figures and extensive tumor necrosis may be seen.
32.5.5 The Use of Immunohistochemistry in Melanoma and Melanocytic Neoplasia
32.5.5.1 Overview
IHC is a useful tool in the approach to melanocytic tumor diagnosis and staging. It is important to note that there are no reliable markers that distinguish benign from malignant melanocytic tumors. Most of the antibodies considered to detect “melanoma markers” actually detect pigment-related proteins that are present in normal melanocytes, benign nevi, and melanomas. These include Melan-A, MART-1, HMB-45, tyrosinase, and MITF (Fig. 32.16). S100 is the most sensitive IHC marker, present in 100 % of nevi and >99 % of melanomas whether primary or metastatic. The other markers show heterogeneous staining of 75–90 % of melanocytic tumors. These markers may be helpful in detection of small metastases in sentinel lymph nodes and also in discriminating metastatic melanoma from metastases of other tumor types. It is important to use a panel of markers when evaluating melanocytic proliferations because none of these markers routinely stain 100 % of the tumor cells. IHC stains to detect proliferation such as Ki-67 may identify a brisk proliferative activity in melanoma, an unusual finding in benign nevi. Markers that highlight the vasculature may aid in the detection of lymphovascular invasion. Finally, a few rare markers may be helpful in determining the presence or absence of specific mutations including BRAF V600E and BAP-1 or potential deletions such as with p16.
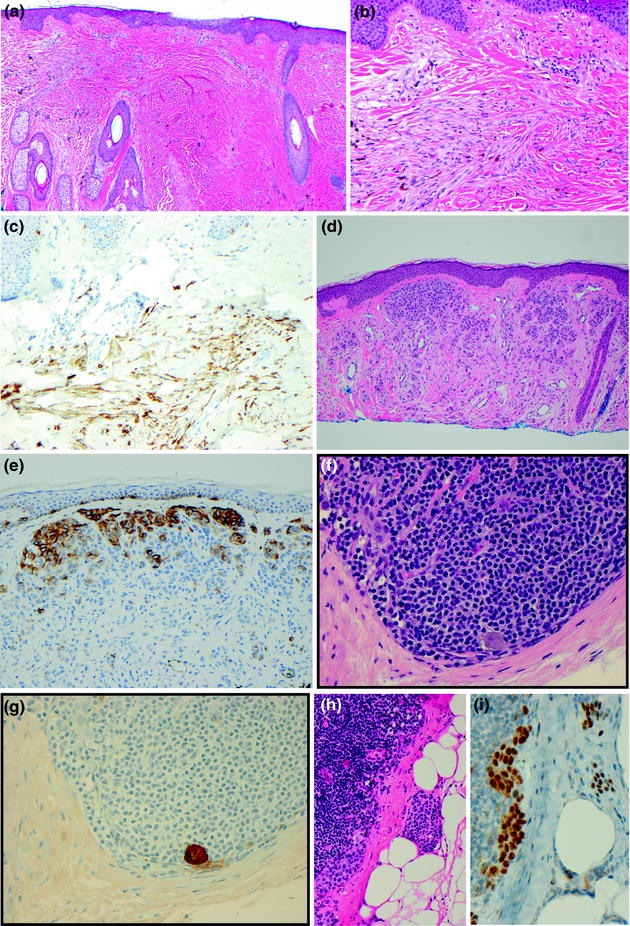

Fig. 32.16
Immunohistochemical staining in melanocytic tumors. a Blue nevus, a pigmented dendritic proliferation is present in the dermis, b blue nevus, the dermal tumor also has spindled cells, the differential diagnosis includes scar and desmoplastic melanoma, c blue nevus, the tumor stains positively for HMB-45 in a pattern supporting the diagnosis of blue nevus, d dermal nevus, e dermal nevus, HMB-45 stains only the superficial dermal tumor cells, f metastatic melanoma in a sentinel lymph node, g a single metastatic melanoma cell is identified on S100 stain, h nodal nevus and metastatic melanoma, i an MITF stain highlights the banal nuclear cytology of the intracapsular nodal nevus, in contrast to the pleomorphic large nuclei of the melanoma present in the subcapsular sinus
32.5.5.2 Cutaneous Melanocytic Tumors
There are several settings wherein the use of immunohistochemistry (IHC) in primary cutaneous melanocytic tumors is a helpful addition to routine H&E staining: (1) evaluation of intraepidermal melanocytic proliferations in sun-damaged skin, (2) discrimination of spindled and desmoplastic dermal melanocytic proliferations, (3) evaluation of proliferation in dermal melanocytic proliferations, (4) detection of lymphovascular invasion, (5) evaluation of dermal epithelioid cell proliferations, and (6) detection of therapeutic targets.
In sun-damaged skin solar-induced cytological atypia of keratinocytes and melanocytes may obscure an intraepidermal melanocytic proliferation. In this setting, HMB-45 and MITF are useful markers for highlighting the extent of the intraepidermal melanocytic proliferation. A cytokeratin stain may also be helpful in confirming the presence of atypical keratinocytes and allow for the detection of nonstaining nests corresponding to the MITF or HMB-45 positive melanocytes. Notably, MART-1 and Melan-A stains are sensitive cytoplasmic stains that highlight melanocytic dendrites and in some cases may detect keratinocytic pigmentation. These stains may over estimate the density of an intraepidermal melanocytic proliferation in sun-damaged skin.
The differential diagnosis of dermal spindled and dendritic cell proliferations may include blue nevus, desmoplastic melanoma, and scar. IHC can be very helpful in this setting, the melanocytes of blue nevus will stain positively for S100 and HMB-45, whereas desmoplastic melanoma is usually positive for S100 but without staining for HMB-45, scar will not have large atypical S100 positive cells, but may have scattered S100 positive dermal dendrocytes, scar does not stain for HMB-45. Some authors have reported SOX-10 as a useful stain in discriminating desmoplastic melanoma from scar, however recent studies have shown SOX-10 staining in scar. The distinction of desmoplastic melanoma from neurofibroma may also be challenging, both have an S100+, HMB-45-immunophenotype. In the mixed form of desmoplastic melanoma, other melanocytic markers including MART-1 and Melan-A may help to highlight the nondesmoplastic portion of the tumor.
The presence of lymphovascular invasion in primary cutaneous melanoma is associated with a poor prognosis [89]. It is often difficult to distinguish vascular invasion from a peritumoral stromal retraction. Dual IHC for D240 and S100 allows for the identification of melanoma cells within lymphovascular spaces [115] (Fig. 32.13C). CD31 may also be helpful in the evaluation of tumor in close apposition to vascular spaces as described in extravascular migratory metastases [87].
Occasionally cutaneous melanocytic proliferations are composed of dermal proliferations of pleomorphic epithelioid cells that may raise a broad differential diagnosis. In these cases there may be histopathological overlap between Spitz nevus, atypical Spitz tumor, nevoid melanoma, spitzoid melanoma, and nodular melanoma. There is no reliable IHC stain to routinely distinguish these cases, however in some cases the immunophenotype may contribute to the histopathological evaluation. HMB-45 shows a distinctive staining pattern in benign melanocytic nevi characterized by staining of the superficial dermal melanocytic proliferation and diminished staining with increasing tumor cell depth in the dermis. No tumor cell staining for HMB-45 is seen at the deep dermal aspect of the nevus. Melanoma on the other hand displays a patchy positive staining pattern for HMB-45 rather than the gradual gradient of staining observed in nevi. A subset of epithelioid cell melanocytic tumors that resemble Spitz nevi or nevoid melanomas have been described in patients with cutaneous/ocular melanoma, atypical melanocytic proliferations, and other internal neoplasms (COMMON syndrome), these tumors display loss of staining for BAP-1 [55]. Loss of p16 has also been described as a prognostic factor in melanoma, and some observers use the absence of p16 staining to triage cases for fluorescence in situ hybridization (FISH) analysis [116–118]. This is based on the result that in FISH analysis homozygous deletion of chromosome 9p21 is associated with a poor prognosis. In these cases p16 loss is seen immunohistochemically [119, 120]. Loss of p16, however, is not diagnostic of homozygous 9p21 deletion; in situ hybridization is helpful to confirm the copy number status of 9p21.
An evaluation of tumor cell proliferation may also be helpful in discriminating benign from malignant dermal melanocytic proliferations. Staining for Ki-67 when present in more than 20 % of the tumor cells supports a diagnosis of melanoma. This stain is also useful in identifying hot spots of proliferation in dermal melanocytic tumors. In cases with a prominent inflammatory infiltrate, it may be difficult to determine if Ki-67 is highlighting melanocytes or lymphocytes. Dual staining for a melanocytic marker, such as MART-1 with Ki-67 may be particularly helpful in this setting. Importantly, melanoma may have a low proliferation rate, therefore, the absence of significant Ki-67 positivity does not exclude the diagnosis of melanoma. Another stain that holds promise is anti-phosphohistone H3 (PHH3). By highlighting mitotic figures at any stage of mitosis this is a highly sensitive detection method. Additional studies that identify appropriate quantitative thresholds for reporting the results of anti-PHH3 are needed before it will be used in routine reporting of primary melanoma.
Finally, IHC staining may aid in identifying therapeutic targets. The detection of BRAFV600E in cutaneous melanoma or melanoma metastases may help to guide therapy [121]. Future studies may lead to evaluation of immune markers such as PD-1 and PD-L1, however, the clinical utility of these stains is yet to be demonstrated in a robust clinical study. Staining for c-Kit does not predict mutational status or sensitivity to tyrosine kinase inhibitors.
32.5.5.3 Ocular Melanoma
Similar to cutaneous melanomas, melanocytic markers are the most sensitive IHC markers for uveal melanoma. These tumors stain uniformly positively for S100 and HMB-45, most uveal melanomas also stain for MART-1, MITF, melan-A, and tyrosinase [122]. Of note, melan-A and tyrosinase also stain normal uveal melanocytes in a variable manner, but with less intensity [122]. Given the importance of identifying mitotic figures in assessing ocular melanoma prognosis, staining for PHH3 may help assess mitotic count [123]. Some markers have been ascribed prognostic significance, including cyclin D1, which is associated with more aggressive course and histologically unfavorable disease. Cyclin D1 expression is present in 1–30 % of cases and is an independent risk factor for metastasis [124]. It is also possible to assess loss of expression of the BAP1 gene using IHC probes for its protein product; loss of BAP1 expression is correlated with poorer survival in uveal melanoma [56, 125].
32.5.5.4 Melanoma Metastases and Sentinel Lymph Nodes
IHC is most helpful in the detection of microscopic metastases in sentinel lymph nodes and in the differentiation of metastatic melanoma from nonmelanoma metastases. S100 is the most sensitive melanoma marker, present in more than 99 % of melanomas; however, S100 is also present in some carcinomas and sarcomas and thus lacks specificity. Other markers of melanocytic differentiation, including HMB-45, MART-1, Melan-A, and MITF, are less sensitive (staining 75–90 % of melanomas) but are more specific than S100. A panel of stains is the most effective way of using these tests to assist in arriving at the correct diagnosis.
The histopathological analysis of sentinel lymph nodes includes the evaluation of H&E-stained tissue sections and IHC from multiple levels of each lymph node [101, 126, 127]. While there is marked variability in analytical platforms for the detection of melanoma in sentinel lymph nodes, some common practices exist: (1) submit the lymph node tissue entirely, (2) perform level sections deep into the block (beyond the initial set of tissue sections), and (3) use IHC. S100 and either MART-1 or Melan-A or most commonly employed; other less frequently used markers include HMB-45 and MITF. Additionally, some laboratories apply a cocktail of reagents including Melan-A/MART1 and HMB-45/Melan-A/Tyrosinase. Protocols that do not include levels into the block or IHC are associated with a false negative rate of approximately 15 % [101, 128–130]. Intraoperative frozen sections analysis is not a sensitive means of detecting melanoma in lymph nodes and is not recommended [131].
In some cases the presence of intranodal melanocytic rests may present a diagnostic challenge. To date most reports indicate that HMB-45 does not stain nodal nevi, similar to the absence of staining for HMB-45 deep dermal nevomelanocytes of a cutaneous nevus. Others have reported an absence of Ki-67 staining in nodal nevi, leading to the adoption of a double MART1/Ki-67 stain by some laboratories [132]. MITF and SOX10 are also helpful in the evaluation of nodal melanocytic tumors, these nuclear stains allow for the evaluation of nuclear size and pleomorphism.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree