eliminate bronchioloalveolar carcinoma,
define the term adenocarcinoma in situ (AIS),
define the term minimally invasive adenocarcinoma (MIA),
revive the term “lepidic,”
promote comprehensive histologic subtyping,
emphasize and introduce the term micropapillary carcinoma,
detach the term mucinous adenocarcinoma, and
discourage use of the term NSCLC and subclassify the tumors in as much detail as possible.
The histological features of each are described separately below.
20.5.1 Non-small Cell Carcinoma
20.5.1.1 Adenocarcinoma
Clinical Features
Adenocarcinoma is the most frequent cell type of lung cancer, accounting for over 50 % of cancers in most recent series. To date, most validated and investigational predictive biomarkers have been identified in adenocarcinoma as compared to other cell types and a new subtype classification of adenocarcinoma has been proposed by the International Association for the Study of Lung Cancer, American Thoracic Society and the European Respiratory Society that takes into account the molecular pathology of these tumors [9]. The current classification of lung adenocarcinoma by the World Health Organization recognizes several distinct morphologic subtypes of adenocarcinoma: papillary (Fig. 20.1), micropapillary (Fig. 20.2), acinar (Fig. 20.3), solid (Fig. 20.4), and lepidic (Fig. 20.5) [11]. The majority of lung adenocarcinomas exhibit combinations of morphologic patterns [12–14]. While the biologic basis for the histologic subtypes remains an area of active investigation [14], there is evidence that some subtypes may be associated with specific molecular alterations [14–19] or a better outcome [20–22].
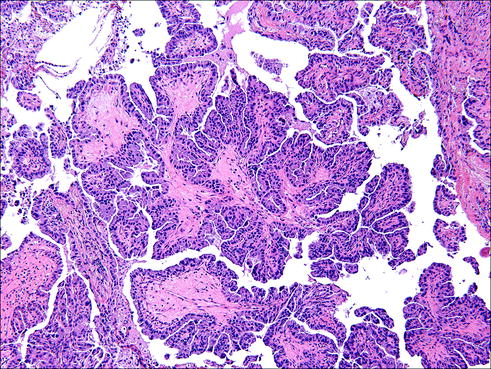
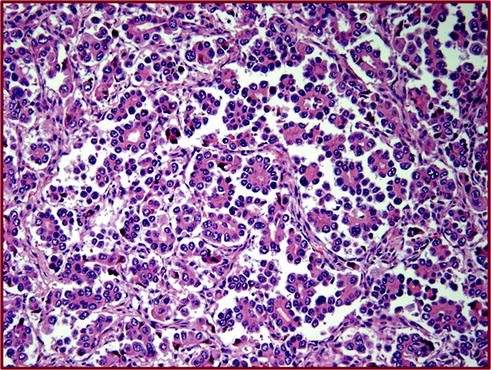
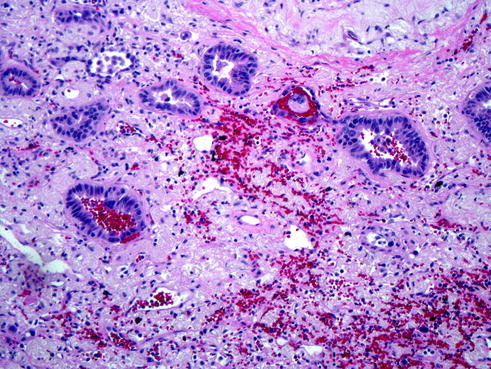
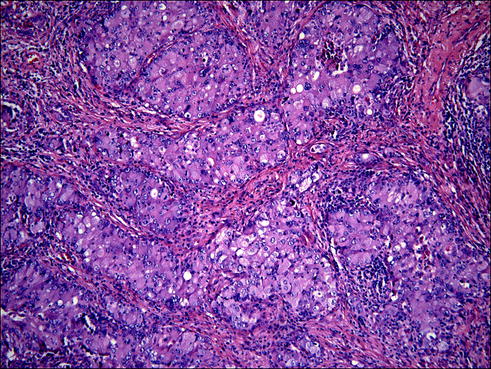
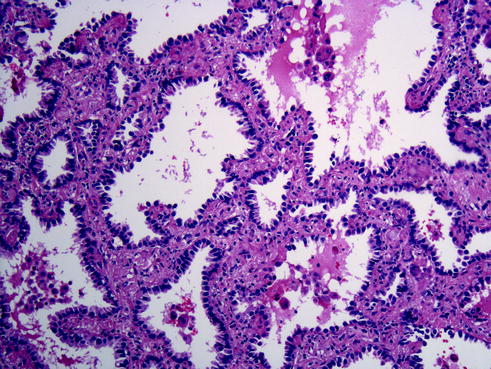

Fig. 20.1
Papillary adenocarcinoma is characterized by finger-like projections of tumor cells connected by a stromal core abundant in vascular structures, with tumor cells protruding to the outside of these core structures. Due to the spatial 3D arrangements, some of these papillae give the false impression of floating into the tumor spaces

Fig. 20.2
Micropapillary adenocarcinoma is characterized by a piling up and clustering of tumor cells in the alveoli. These clusters miss the vascular cores, in contrast to papillary adenocarcinoma

Fig. 20.3
Acinar adenocarcinoma is the classical pattern of adenocarcinoma with tumor cells arranged in tubular architectures also called acinar

Fig. 20.4
Solid lung adenocarcinoma characterized by irregularly shaped islands of tumor cells sometimes separated by stroma (desmoplastic reaction). This architectural pattern has no particular shapes, and is considered one of the worst behaving and aggressive type of lung cancer

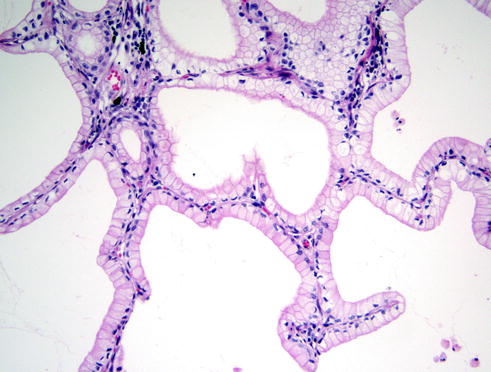
Fig. 20.5
The lepidic pattern characterized by the tumor cells missing any invasion into the stroma. In this architectural pattern, the tumor cells spread along the alveolar walls, replacing the normal pneumocytes type II. This pattern is most commonly associated with some of the other previously mentioned patterns
Pathologic Features
Gross Findings
Grossly, adenocarcinoma typically has an irregularly lobulated configuration, with a gray-white cut appearance. As they are predominantly peripheral parenchymal masses, adenocarcinomas, in contrast to squamous cell carcinoma of the lung, are rarely associated with large airways. Anthracotic pigment is commonly entrapped in the tumor mass. Gross necrosis is uncommon except in larger masses. They may be found in association with fibrosis and pleural puckering. The penetration of the pleura may require additional studies such as elastic stains are important in tumor staging (see below).
Microscopic Findings
Adenocarcinoma in situ (AIS): AIS, formerly BAC, is an important subtype of pulmonary adenocarcinoma (Fig. 20.6). This cancer has received increasing attention in recent years owing to its increasing incidence and rate of sensitivity to epidermal growth factor–tyrosine kinase inhibitors [24]. AIS is a primary lung tumor with a peripheral location, well-differentiated cytology, lepidic growth pattern, and a tendency for both aerogenous and lymphatic spread. The key feature is preservation of the underlying architecture of the lung with no invasion.
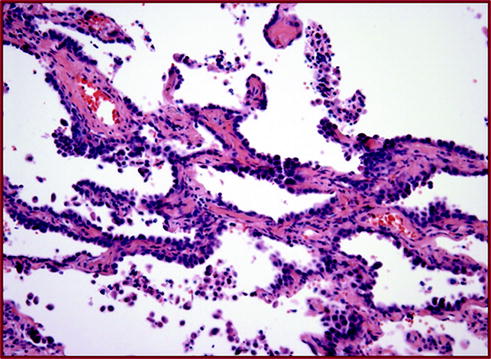

Fig. 20.6
Adenoma in situ (AIS) This tumor is composed only by the noninvasive lepidic pattern. No invasion is identified into stroma, lymphatics, or pleura. Patients with this type of in situ carcinoma are considered to be cured by simple surgical excision
Minimally invasive adenocarcinoma (MIA): MIA was introduced to define patients with a near 100 % 5-year disease-free survival. It is defined as a lepidic predominant tumor measuring 3 cm or less that has an invasive component of 5 mm or less [25]. MIA is characterized by a combination of ground glass opacity (GGO) and a central solid opacity, with the solid component measuring 5 mm or less. Nonmucinous MIA is more common than mucinous MIA and most often appears as a GGO. Mucinous MIA appears radiologically as a solid or part-solid nodule.
Invasive adenocarcinoma: Changes were inserted in the classification of invasive adenocarcinomas. The current classification of lung adenocarcinoma by the World Health Organization recognizes several distinct morphologic subtypes of adenocarcinoma: papillary, micropapillary, acinar, solid, and lepidic [11]. The majority of lung adenocarcinomas exhibit combinations of morphologic patterns [12–14]. While the biologic basis for the histologic subtypes remains an area of active investigation [14], there is evidence that some subtypes may be associated with specific molecular alterations [14–18] or a better outcome [20–23]. Overtly invasive adenocarcinomas are classified according to the predominant subtype after the use of comprehensive histologic subtyping to estimate the percentages of the various components in a semiquantitative fashion in 5–10 % increments. The adenocarcinoma patterns are: lepidic, acinar, papillary, micropapillary, and solid. The invasive adenocarcinoma variants are mucinous adenocarcinoma (Fig. 20.7), colloid, fetal, and enteric morphologies. The term lepidic predominant adenocarcinoma consists of mixed subtype tumors containing a predominant lepidic growth pattern of type II pneumocytes and/or Clara cells (formerly known as nonmucinous BAC) that have an invasive component >5 mm. A micropapillary predominant subtype is added because it has been recognized as a poor prognostic category. Signet ring (Fig. 20.8) and clear cell carcinoma (Fig. 20.9) subtypes are now recorded as cytologic features whenever present with a comment about the percentage identified.
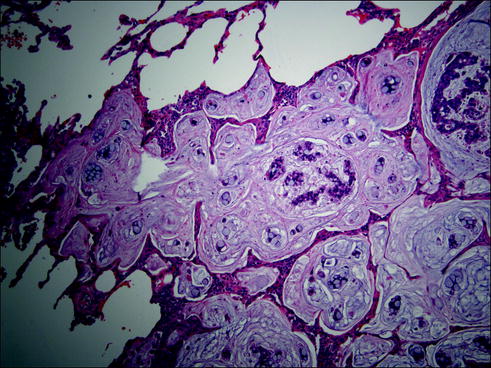
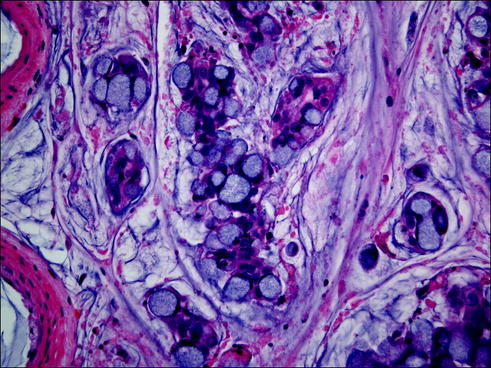
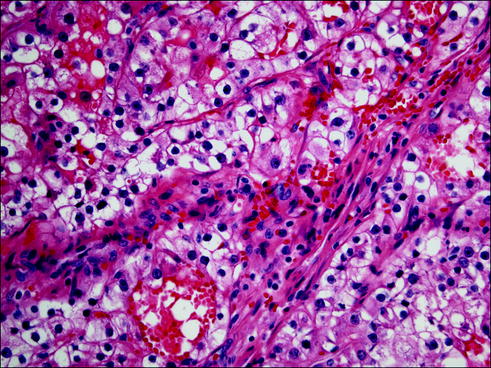

Fig. 20.7
Mucinous adenocarcinoma composed of tumor cells secreting large amounts of mucus in the alveolar spaces and stroma

Fig. 20.8
Signet ring cell carcinoma are cells with a peculiar cytology: the mucin is collected in the cytoplasm, pushing the nucleus to the periphery of the cell, giving the cell a peculiar appearance resembling an archaic ring with an attached seal structure

Fig. 20.9
Some of the carcinomas have a glycogenized (clear) cytoplasm. This particular type of cytology (clear cell cytology) has no particular prognostic or biological significance
High-power examination reveals that the tumor cells are typically polygonal with large vesicular nuclei, prominent nucleoli, and moderately abundant cytoplasm. Unlike SCC, the cytoplasmic borders are often poorly defined or indistinct. In addition, a variety of cell types have been described, such as clear cell, mucinous, fetal, sarcomatoid, and signet-ring cell. Moreover, it is not uncommon for adenocarcinoma to be present in association with other types of lung carcinoma (e.g., combined with SCLC, or large-cell neuroendocrine carcinoma, or SCC, or sarcomatoid carcinoma).
20.5.1.2 Squamous Cell Carcinoma
Clinical Features
SCCs represent approximately 30 % of all NSCLC. Their incidence has been decreasing compared to adenocarcinomas, possibly due to changes in smoking habits. It is strongly linked to a history of cigarette smoking.
Pathologic Features
Gross Findings
Most SCCs arise centrally from the segmental or subsegmental bronchi. However, the incidence of SCC of the peripheral lung is increasing. Grossly, tumor masses are usually gray white to yellow tan. SCC is the most common type to give rise to a thick-walled irregular cavity with central necrosis. The texture can be firm or gritty and may be surrounded by areas of obstructive consolidation. Some of the proximal tumors have an exophytic, papillary, and endobronchial growth pattern. Because of their frequent central location (in the mainstem, lobar, or segmental bronchi), diagnosis by cytological examination of sputum, bronchoalveolar lavage (BAL), bronchial brushing and washing, or endoscopic biopsies can be performed with generally satisfactory results. Also, because of their central location, direct extension of the primary tumor mass into the adjacent hilar lymph nodes is common.
Microscopic Findings
SCC is a malignant epithelial tumor with keratinization and/or intercellular bridges (Fig. 20.10). SCC is graded as well differentiated if prominent keratinization, intercellular bridges, or pearl formation is present. They are moderately differentiated if these features are easily seen but not extensive. Poorly differentiated SCCs have only focal morphologic features of squamous differentiation. Keratinization may take the form of squamous pearls or individual cells with markedly eosinophilic dense cytoplasm. The presence of intracellular mucin in a few cells does not exclude tumors from this category. In situ SCC may be seen in the adjacent airway mucosa.
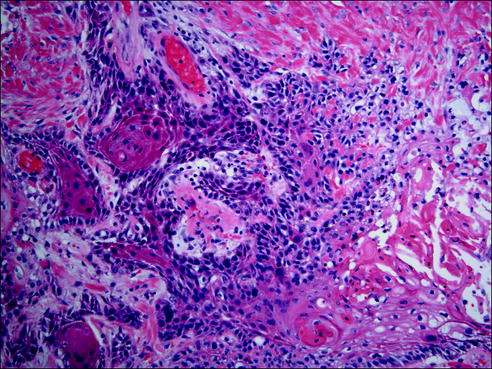

Fig. 20.10
Squamous cell carcinoma is a malignant epithelial tumor with keratinization and/or intercellular bridges
SCCs can present as histological variants which include: papillary, clear cell, small cell, and basaloid patterns. Rarely, these patterns are seen throughout the tumor, but more commonly, they are focal. Even though invasive growth is not identified, papillary SCC can be diagnosed if there is sufficient cytological atypia. However, small biopsy specimens that show a very well-differentiated papillary squamous epithelium should be interpreted with caution since separation of a papillary squamous carcinoma from a papilloma can be difficult. Furthermore, the squamous epithelium of a squamous papilloma may extend into the bronchial glands and should not be confused with invasion.
20.5.1.3 Large-Cell Carcinoma
Large-cell carcinoma (LCC) is an undifferentiated malignant epithelial tumor accounting for approximately 10 % of all lung cancers in many series. It is strongly associated with cigarette smoking. The lesion tends to occur in the periphery and grows rapidly. It usually presents at a late stage, resulting in a poor outcome.
Pathologic Features
Gross Findings
About half of the cases display a relationship to a large airway. Grossly, LCCs are frequently greater than 5 cm in size and have a white to gray or fish-flesh cut appearance. Gross tumor necrosis is commonly appreciable.
Microscopic Findings
LCC is a diagnosis of exclusion made after ruling out the presence of a component of SCC, adenocarcinoma, or SCLC. Because of this, the diagnosis is best made on resection specimens, and not on small biopsy specimens. In general, the cells typically have large nuclei, prominent nucleoli, and a moderate amount of cytoplasm. They are typically arranged in sheets or large nests, frequently revealing foci of necrosis.
Morphologically, they have lobular, trabecular, or palisading growth patterns surrounding typically centrally located comedo-type necrosis. These tumors consist of relatively small monomorphic cuboidal to fusiform cells with moderately hyperchromatic nuclei, finely granular chromatin, absent or only focal nucleoli, scant cytoplasm, and a high mitotic rate. Neither intercellular bridges nor individual cell keratinization are present. A high percentage of cases have associated carcinoma in situ. Immunohistochemical stains for adenocarcinoma, SCC, and neuroendocrine markers are negative. About half of the tumors with this histological pattern are pure basaloid carcinomas. Immunohistochemistry (IHC) reveals that these neoplasms commonly express cytokeratin, but do not express TTF-1 or p63. Ultrastructural analysis of LCCs usually shows evidence of squamous, glandular, or neuroendocrine differentiation, suggesting that these are, in fact, very poorly differentiated NSCLCs.
20.5.2 Small Cell Carcinoma
SCLC is defined as a neuroendocrine tumor with more than 10 mitoses per 2 mm2 and small cell cytologic features. Cells have an oval or vaguely spindled shape and have scant cytoplasm. Nuclei are hyperchromatic and have absent or very small nucleoli (Fig. 20.11). Crush artifact may be prominent on small biopsies, but this is not pathognomonic for the diagnosis of SCLC. In larger core biopsies or resected specimens, the cells may appear slightly larger than in a transbronchial biopsy and may have distinct cytoplasm. Numerous prominent nucleoli and large cells should not be seen.
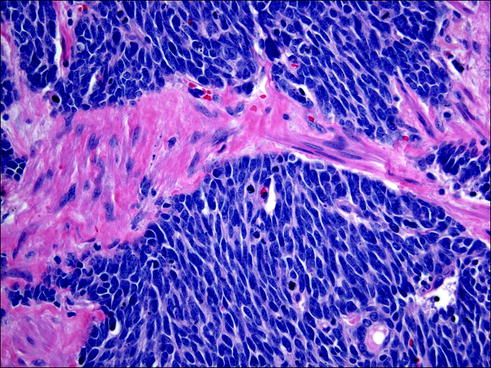

Fig. 20.11
Small cell carcinomas are extremely aggressive tumors with a neuroendocrine differentiation. Cells have an oval or vaguely spindle shape with very scant cytoplasm, nuclear molding with absent or very small nucleoli
20.6 Lung Cancer Grading
Histological grading (well, moderately, and poorly differentiated adenocarcinomas) has prognostic significance and recently published analyses have been validated in clinical practice using histological and cytologic criteria [26].
20.7 Lung Cancer Staging
Stage is the most important prognostic and predictive factor for patients with lung neoplasms, and pathologists are expected to provide accurate staging information in lung-resection specimens. Separate staging systems have been proposed for patients with NSCLC and SCLC. The seventh edition of the published guidelines of the American Joint Commission on Cancer (AJCC) compiles the most recent clinical and pathologic staging of patients with lung cancer and other neoplasms (Table 20.1). The designation “T” refers to a primary tumor that has not been previously treated. The symbol “p” refers to the pathologic classification of the tumor/node/metastasis (TNM), as opposed to the clinical classification, and is based on gross and microscopic examination. “pT” entails a resection of the primary tumor or biopsy adequate to evaluate the highest pT category, pN entails removal of nodes adequate to validate lymph node metastasis, and pM implies microscopic examination of distant lesions. Overall survival for lung cancer is 16 %; however, survival is stage-dependent. Overall survival rates for patients with stages I–IV NSCLC are 60–80, 25–50, 10–40, and 4:5 %, respectively.
Patients with Stage I usually undergo surgical resection with lobectomy, segmentectomy, or wedge resection. Usually for patients with stage II disease (and for those with Stage III disease diagnosed upon final pathology following resection), treatment should include anatomic surgical resection followed by adjuvant chemotherapy. Patients with resectable disease (Stages I and II) who have medical contraindications for surgery are candidates for curative radiation therapy. Patients with stage IIIA NSCLC receive neoadjuvant chemotherapy followed by surgical resection. Patients with Stage IIIB disease are treated with radiation therapy in combination with chemotherapy; those with stage IV disease receive this regimen, predominantly as palliative therapy.
20.8 Molecular Alterations and Precision Oncology in Lung Cancer
NSCLC is the second most common cancer diagnosed in the United States and the leading cause of cancer-related mortality, with an estimated 221,200 new cases and 158,040 deaths anticipated in 2015 [27]. Lung cancer was the leading cause of cancer death among men in 2012 [28]. Among women, lung cancer was the leading cause of cancer death in more developed countries, and the second leading cause of cancer death in less developed countries [28]. Globally, the overall lifetime risk of lung cancer is about 1 in 13 for men and 1 in 16 for women. The risk is significantly higher for smokers and lower for nonsmokers [29–31]. However, lung cancer rates in Chinese women (20.4 cases per 100,000 women) were higher than rates among women in some European countries despite a lower prevalence of smoking. This is thought to reflect indoor air pollution from unventilated coal-fueled stoves and cooking fumes [32]. Other known risk factors for lung cancer include exposure to occupational and environmental carcinogens such as asbestos, arsenic, radon, and polycyclic aromatic hydrocarbons [33]. Recently, outdoor pollution has also been determined to cause lung cancer [34, 35]. More than one-half of the lung cancer deaths attributable to ambient fine particles were projected to have been in East Asian countries [36].
In the past years, given the development of new targeted therapies, tremendous efforts have been directed towards identifying potentially druggable molecular alterations, especially against known activating mutations. Although numerous mutations have been described in lung adenocarcinoma [37], the mutation status remains unknown in more than 50 % of cases [38]. So far, we can identify at the present moment therapeutic targets in only 20 % of lung cancers.
Molecular profiling has become the standard of care for advanced (metastatic) lung cancer. For nonsquamous NSCLC, which accounts for more than half of all lung cancer cases, routine testing for epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) rearrangements is recommended. In cases with identified EGFR (approximately 15 % of NSCLC) or ALK alterations (approximately 5 % of NSCLC), molecularly targeted therapy with EGFR-or ALK-targeting drugs is now the preferred initial approach to treatment [39].
20.8.1 Targeted Therapies in Lung Cancers with Epidermal Growth Factor Receptor (EGFR) Abnormalities
20.8.1.1 EGFR
Recognized mechanisms of EGFR gain of function in NSCLC include somatic activating mutations in the exons encoding the tyrosine kinase domain and EGFR gene amplification [40–42]. The EGFR mutation status is best determined by gene sequencing abnormalities of EGFR status may also be observed with gene copy number determined by fluorescence in situ hybridization (FISH) or chromogenic in situ hybridization (CISH), and protein expression determined by IHC with mutation-specific antibodies. Several mutations have been recently described in the tyrosine kinase domain of EGFR [40, 43]. EGFR is expressed in 50 % of NSCLCs, and its expression is correlated with poor prognosis [44]. These two factors make EGFR and its family members prime candidates for the development of targeted therapeutics [45]. EGFR kinase domain mutations target four exons [18–21], which encode part of the tyrosine kinase domain (the entire kinase domain is encoded by exons 18–24) and are clustered around the ATP-binding pocket of the enzyme [46–50].
EGFR gene amplification is detected in some EGFR-mutation-positive patients as well [51]. A subset of lung adenocarcinomas has activation of growth factor receptor (EGFR) by mutations and/or amplification but the interaction between them is complex and unclear. Some EGFR-amplified lung adenocarcinomas have distinct genetic alterations, unique clinicopathologic features, and worsened prognosis [51, 52]. Furthermore, EGFR amplification and EGFR mutations are heterogeneously distributed within any given tumor. These are novel and important findings with implications for the efficacy of treatment with tyrosine kinase inhibitors in patients with EGFR-mutant lung adenocarcinoma [51].
Recent discoveries described EGFR mutation-specific antibodies that could help in the rapid screening of lung cancers with EGFR mutations (Fig. 20.12) [52].
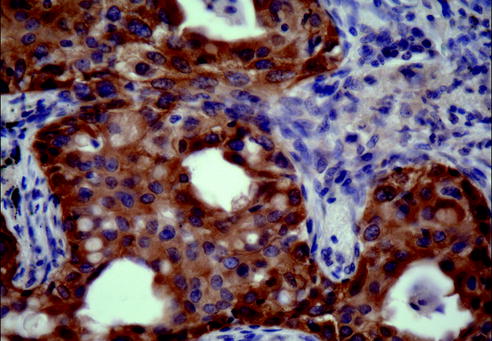

Fig. 20.12
Using exon 19 deletion-specific antibody recently described one is able to directly visualize the location of tumor cells with EGFR exon 19 deletion mutations and show heterogeneity in receptor overexpression among different tumor cells (Immunohistochemistry, exon 19 deletion-specific antibody, 200× magnification)
Mutations in the tyrosine kinase domain of the EGFR have prognostic significance since patients with EGFR-mutant NSCLC have prolonged disease-free survival compared with those with wild-type disease, regardless of the treatment received [2, 53, 54]. Although EGFR mutations are predictive of response to EGFR tyrosine kinase inhibitor (TKI) therapy, they do not appear to be predictive of a differential effect on survival [54].
20.8.1.2 Targeted Agents Against Lung Cancer with EGFR Mutations
EGFR-mutant NSCLC generally refers to cases with sensitizing mutations in the EGFR kinase domain (exon 19 deletions or exon 21 L858R substitutions). These activating mutations result in constitutive activity of the EGFR kinase domain, generating survival and proliferative signals through the PI3 K-Akt-mTOR and Ras-Raf-MEK pathways. In these cases, EGFR inhibitors such as erlotinib, gefitinib, and afatinib in the first-line setting yield response rates in excess of 75 %, and overall survival exceeding 2 years [39]. In contrast, in NSCLC without actionable molecular alterations treated with conventional chemotherapy, response rates are approximately 30 %, median overall survival is 12 months. The first two TKI agents approved for use in lung cancer that target lung cancer with EGFR mutations were gefitinib (2002) and erlotinib (2003). EGFR mutation is a specific target for therapy by TKIs and is a validated biomarker of treatment response [42]. The clinical utility of this biomarker is supported by prospective clinical trials that have demonstrated a progression-free survival benefit of TKI as first-line therapy in EGFR-mutant patients [55]. Based on current data, predictive biomarker tests for EGFR should involve mutational analysis. Molecular profiling has become the standard of care for advanced (metastatic) lung cancer and routine testing for EGFR is recommended [56, 57]. In cases with identified EGFR alterations, molecularly targeted therapy with EGFR-targeting drugs is now the preferred initial approach to treatment.
Resistance to TKI therapy is associated with KRAS mutation and specific acquired EGFR mutations such as T790 M [58, 59]. These molecular events, as well as other genetic alterations in cMet (amplification), ERBB3 (overexpression), and epiregulin (autocrine loop activation), account for approximately 50 % of cases of TKI-resistance [50, 60–62].
20.8.2 Genotype-Phenotype Correlations
In patients with lung adenocarcinoma treated with erlotinib and gefitinib, favorable responses were associated with adenocarcinoma with lepidic patterns [18]. This finding led to trials of gefitinib and erlotinib in patients that showed that 17–22 % of patients had a response to gefitinib [63, 64]. The weak correlation between EGFR mutation status and adenocarcinoma subtypes [65, 66] led to the adoption of reflex genetic testing of all lung cancers and investigation for treatable molecular targets [67]. Genetic abnormalities can be seen in different histology types although with various frequency. One characteristic correlation is that mucinous adenocarcinoma (Fig. 20.13) may be exclusively TTF1 negative, EGFR mutation negative but may have Ras mutation, and expresses CDX2 possibly because of their presumed derivation from bronchiolar mucinous goblet cells [15, 68]. However, more recently, molecular genetic analyses of lung adenocarcinoma have recently become the standard of care for treatment selection [57].