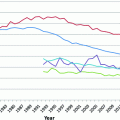
Fig. 12.1
Normal breast (a), and lactational changes (b)
Table 12.1
Markers of luminal epithelial cells and myoepithelial cells in the terminal ductular-lobular unit (TDLU)
Luminal cells | Myoepithelial cells |
|---|---|
CK18 | CK14 |
CK19 | CK5/6 |
CK8 | P63 |
CK7 | Calponin |
GFCD15 | Caveolin |
EMA | D2-40 |
KIT | Smooth muscle actin (SMA) |
The stromal counterpart recognizes the intervening stroma between lobules (interlobular stroma) and the stroma within lobules (intralobular stroma). The first is composed of highly collagenized paucicellular fibroadipose tissue that occasionally may be populated by multinucleated giant cells of unknown significance (that should not be mistaken for malignant or inflammatory cells); the latter is made of loose fibrovascular stroma populated also by lymphocytes, plasma cells, macrophages, and mast cells [7]. The intralobular stroma responds to hormonal changes and upon estradiol stimulation becomes more edematous.
Hormones elicit their action also on epithelial cells, where estrogen, progesterone, and androgen promote differentiation and proliferation of luminal cells and oxytocin is responsible for contraction of myoepithelial cells.
Hormone receptors (estrogen and progesterone receptors, ER and PR, respectively) are expressed in the normal breast but at low levels (and lower in the ducts than in the lobules): typically, single positive cells are scattered in the epithelial population, never reaching 100 %. In addition, there is heterogeneity of expression among different lobules [7].
The gland produces milk and is active only during pregnancy, when the full development of the breast occurs in humans. In this period of the adult life, epithelial cell proliferation resumes under the influence of ER, PR, prolactin, and growth hormones, leading to an increase in the number of lobules at the expenses of both intralobular and interlobular stroma [7]. In addition, the mammary gland undergoes secretory changes featuring luminal cells acquiring a foamy cytoplasm (they contain numerous lipid vacuoles) and a characteristic hobnail appearance with nuclei and prominent nucleoli. Secretions accumulate in the expanded lobules (Fig. 12.1).
So-called “lactational changes” can also occur outside of pregnancy and may be alarming due to features of cytologic atypia; therefore, a differential diagnosis including atypical lesions and carcinoma, in particular ductal carcinoma in situ (DCIS) of clinging pattern, must be considered. In the context of fine-needle aspirates, extreme caution is needed to avoid false-positive results.
Over time, with the decrease of ER and PR stimulation in the postmenopausal period, the gland undergoes regressive changes with fibroadipose involution. More specifically, there is atrophy of the TDLUs (reduction in size and complexity of the acini), loss of specialized intralobular stroma, and ducts may become ectatic [7].
The nipple and the areola are lined by keratinizing squamous epithelium with minimal extension into the terminal portion of the lactiferous ducts. Clear cells can occasionally be present in the epidermis of the nipple/areola complex. These are benign epithelial cells and should not be confused with Paget’s disease. Some are simply clear keratinocytes, others are known as “Toker cells” [9].
12.4 Benign Epithelial Proliferations
12.4.1 Adenosis
Adenosis is a lobulocentric proliferative lesion largely derived from the TDLU that occurs most often as part of a spectrum of proliferative abnormalities commonly referred to as fibrocystic changes [10]. Both epithelial and myoepithelial cells participate in adenosis [10]. When by itself it may either be isolated to single lobules and represent a microscopic lesion that comes to attention clinically only if it contains mammographically detected calcifications, or it can rarely form a palpable or a radiographically detectable mass. This is the case when a confluence or fusion of the affected lobules creates an “adenosis tumor” [11]. Presentation with a mass is more frequently found when adenosis is seen in fibrocystic disease [10].
Microscopically, adenosis tends to have a more prominent glandular pattern in premenopausal women, whereas sclerosis and diminished gland formation are conspicuous after menopause. The most cellular type of adenosis is florid adenosis, characterized by hyperplasia of epithelial and myoepithelial cells. Proliferation of ductules and lobular glands severely distorts the architecture of the underlying lobules. The hyperplastic structures appear to elongate, becoming tortuous and entwined in a fashion that results in many more ductular cross sections than are present in an anatomically normal lobule [10].
Epithelial cells lining the tubules and glands of adenosis are most often flattened, cuboidal, or slightly columnar, and are arranged in one or two orderly layers surrounded by myoepithelial cells. Mitoses are vanishingly rare, and are more numerous during pregnancy. Apocrine metaplasia is uncommon in florid adenosis. Luminal secretions may undergo calcification, but this is less common and less extensive in florid than in sclerosing adenosis [10].
12.4.1.1 Sclerosing Adenosis
In sclerosing adenosis there is preferential preservation of myoepithelial cells with variable atrophy of epithelial cells, accompanied by lobular fibrosis. The swirling lobulocentric pattern encountered in florid adenosis is retained, but epithelial cells are less conspicuous, the ductular structures and their lumens are largely attenuated, and the myoepithelial cells predominate [10]. The identification of sclerosing adenosis is important given that it may display an infiltrative pattern in the stroma and fat especially when sclerosing adenosis is not limited to a lobulocentric pattern [10]. The differentiation between invasive carcinoma must be made and such a scenario may be challenging especially in needle core biopsy samples, which lack the orientation provided by surrounding tissue of a surgical biopsy [10]. Low-power microscopic assessment reveals the remaining lobulocentric pattern and immunohistochemistry (IHC), including cytoplasmic markers of smooth muscle differentiation and the nuclear marker P63, both label myoepithelial cells (Table 12.1). Microcalcifications are more frequently formed in the sclerosing type of adenosis and become progressively more numerous with increasing sclerosis [10].
Several epidemiological studies have demonstrated that a diagnosis of benign entities, such as sclerosing adenosis, as well as fibrosis and cysts, confers a low relative risk of development of cancer [12–14]. These lesions, however, do not display genetic aberrations in common with those of true precursors and invasive breast cancer [14, 15].
12.4.2 Usual Ductal Hyperplasia
Usual ductal hyperplasia (UDH) is an intraductal proliferation of a mixed population of epithelial cells, leading to the formation of secondary lumens. The latter are often distributed at the periphery of the ducts and associated with streaming of the central proliferating cells [16, 17], producing the so-called “slit-like” spaces. This mixed population of cells displays features of both luminal and myoepithelial differentiation, with heterogeneous positivity for ER and PR, and consistent expression of high molecular weight cytokeratins, such as CK5/6. IHC for hormone receptors and CK5/6 can indeed be very helpful in the context of lesions with equivocal morphological features (Fig. 12.2; Table 12.2) to help differentiate UDH from atypical ductal hyperplasia (ADH)/low-grade DCIS. Another feature that helps in differentiating between these two entities is the presence of intranuclear pseudoinclusions, which are typically found in florid UDH and are rare in ADH/low-grade DCIS [18, 19].
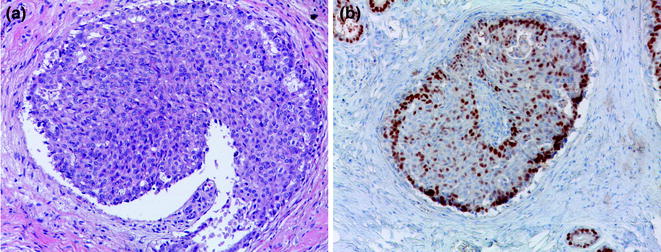

Fig. 12.2
Usual ductal hyperplasia (a H&E; b ER)
Table 12.2
Utility of most commonly used IHC antibodies in breast pathology, other than evaluation of prognostic and predictive factors for primary carcinomas
Marker | Diagnostic scenario |
|---|---|
High molecular weight cytokeratins | Heterogeneous in UDH, negative in ADH/low-grade DCIS |
ER | Heterogeneous in UDH, homogeneous (≅100 %) in ADH/low-grade DCIS |
P63 | Suspicion for invasion: sclerosing lesions versus invasive carcinoma, microinvasion in DCIS; Diagnosis of myoepithelial lesions and spindle cell MBC |
HER2 | Microinvasion in (HER2+) high-grade DCIS |
E-CAD | IC-NST versus ILC and LCIS versus DCIS (mind possible aberrant E-CAD expression in lobular lesions) |
The role of UDH in the evolution of breast cancer has largely been debated [20, 21]. UDH has been shown to be associated with a low risk of breast cancer development and was initially considered to be a nonobligate precursor of both ADH and DCIS [22, 23].
Genetic data suggest that the majority of UDH constitutes only a risk indicator rather than a true breast cancer precursor [24]; however, loss of heterozygosity (LOH) analyses of UDHs have revealed that at least a subgroup of these lesions are clonal and that the prevalence of LOH in UDHs (4.5–13 %) is lower than that found in ADH and DCIS [25]. Chromosomal comparative genomic hybridization (CGH) studies have produced conflicting results on the presence of numerical chromosomal aberrations in UDHs [22, 23]. While some have demonstrated the presence of unbalanced chromosomal aberrations in UDHs [22, 23], others have failed to identify recurrent genetic aberrations [26] or any aberration at all [27]. It should be noted, however, that the studies demonstrating unbalanced chromosomal changes in UDHs employed whole genome amplification methods [22, 23], which have been shown to introduce artifacts in CGH and microarray-based CGH (aCGH) [28], or failed to correct the results for some of the known artifacts of CGH analysis performed with DNA extracted from formalin-fixed paraffin-embedded (FFPE) samples subjected to whole genome amplification [14].
In a similar way to copy number analyses, phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha (PIK3CA) mutational studies on UDH have provided conflicting results. While, Li et al. [29] found no PIK3CA mutations in 16 cases analyzed by Sanger sequencing, Ang et al. [30] used a PCR-mass spectroscopy-based technique and found PIK3CA mutations in around 50 % of cases. Interestingly, PIK3CA mutations in UDH tended to be in exon 20 while in carcinomas the majority of mutations were in exon 9. Additionally, in the vast majority of cases, concurrent invasive carcinomas had discordant genotype, while concordance was high for matched in situ and invasive carcinomas. It is likely that differences from these two studies are related to the different techniques and their distinct sensitivities. In addition, due to the highly sensitive methodology used by Ang et al., it is unclear whether PIK3CA mutations found in UDH samples were clonal (i.e., present in all/nearly all lesional cells) or were present in only a small fraction of cells.
Taken together the available evidence suggests that the vast majority of UDHs lacks clonal genetic changes; when these are present, they are usually randomly distributed and do not affect regions usually involved in invasive breast cancers. Therefore, it is reasonable to conclude that the majority of UDHs are risk indicators rather than nonobligate precursors of breast cancer development; however, a small minority may be associated with the development of breast cancer [14].
12.4.3 Sclerosing Lesions
12.4.3.1 Radial Scar, Complex Sclerosing Lesion
A radial scar (RS) is defined as a benign lesion with a typical stellate profile, which is microscopically characterized by obliterated ducts and pseudoinfiltrative tubules, immersed in an elastotic stroma (Fig. 12.3). These tubules are lined by epithelial and myoepithelial cells, surrounded by contracted ducts and lobules exhibiting a variety of features including epithelial hyperplasia, duct ectasia, adenosis, papillomatosis, and apocrine cysts (Fig. 12.3; Table 12.2) [31].
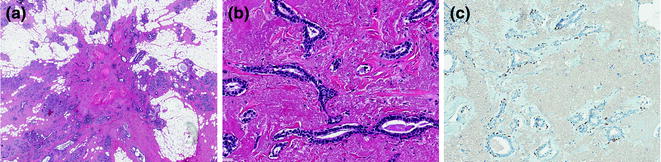

Fig. 12.3
Radial scar/complex sclerosing lesion (a, b H&E; c P63)
The term “complex sclerosing lesion” (CSL) is applied to those lesions larger in size and more complex in features. Most pathologists diagnose “complex sclerosing lesion” in lesions larger than 1 cm.
Contradictory results have been reported on the role of RS/CSL in the development of breast cancer, namely whether RSs/CSLs are risk indicators of breast cancer development or if they constitute true nonobligate precursors of DCIS and invasive breast cancer [32–34]. Long-term follow-up of women with RS/CSL indicates a 1.5–2 fold increase in subsequent breast cancer risk [35–37], which persists after adjusting for concurrent proliferative disease [38].
Some have observed clonal differences between hyperplasic lesions and other areas within RSs. 16q and 8p allelic imbalance were detected, as well as different genetic losses. There is also some evidence to suggest that RSs may be associated with the development of breast cancer. Manfrin et al. [39] reported on a high incidence (32 %) of carcinomas in a series of 117 asymptomatic patients with mammography detected RSs. Given the observation that RSs harbor 16q LOH, it is not surprising that the majority of invasive breast cancers developing in the context of RSs are of low histologic grade [39], such as tubular and classical lobular carcinomas. Nevertheless, a rare association between RSs/CSLs and low-grade metaplastic carcinomas has also been described [40, 41]. Overall, the presence of a coexisting carcinoma in RSs/CSLs ranges from 3.6–32 % [39, 42, 43].
From a genetic standpoint, RSs/CSLs have been shown to harbor PIK3CA-activating mutations in 63.6 % [44], and these were more prevalent when epithelial atypia was superimposed (56.3 % vs. 83.3 %) [44]. Interestingly, activating mutations affecting PIK3CA, the second most frequently mutated gene in breast cancer [45], have also been documented in papillomas [46], columnar cell lesions (CCLs) [47], and UDH [30], and it has been hypothesized that PIK3CA mutations may be more relevant for proliferation than for malignant transformation in breast epithelium [44].
In conclusion, despite some studies finding molecular changes in RSs/CSLs, it is not clear whether those lesions should be broadly categorized as clonal neoplastic lesions and considered true nonobligate precursors. It is possible that those detected molecular changes reflect the epithelial proliferations that may be or may not be present, rather than the lesion as a whole.
“Infiltrating epitheliosis” (IE), is sometimes perceived as synonym of RS and CSL, however, some authors have suggested that these terms should not be used interchangeably [48]. IE may be associated with a higher risk of carcinoma, and defined morphological criteria for this differentiation have been published [48]. Historically, the term IE was first used by John Azzopardi in 1979 [49].
The overall appearance of IE is more infiltrative than usual sclerosing lesions, as the involved ducts often have jagged or irregular edges and the proliferating epithelium often appears to “flow-out” into adjacent stroma [50]. As a matter of fact, invasive carcinoma is often considered in the differential diagnosis. In addition, florid UDH-like epithelial proliferation is an integral component of IE. As in UDH, IE displays a heterogeneous epithelial phenotype (admixture of CK5/6-positive basal/intermediate-type cells and both ER-positive and ER-negative/C-KIT-positive luminal-type cells) [50]. However, at a variance to UDH and most sclerosing lesions, myoepithelial cells, if not absent, are usually immunophenotypically altered and not detected by routinely used antibodies [50]. Despite these distinctive histologic features, most pathologists currently classify IE in the RS/CSL spectrum and therefore its true incidence and association with carcinoma are unknown [50].
A recent study has analyzed the mutational repertoire of eight IE cases by targeted capture massive parallel sequencing and consistently found mutations in components of the PI3K pathway: seven samples harbored PIK3CA hotspot mutations, while the remaining case displayed a PIK3R1 somatic mutation. Of note, analysis of one case composed of IE, DCIS, and low-grade adenosquamous carcinoma revealed the three components were clonally related [50]. In conclusion, these data supports the notion that IE is part of the RS/CSL spectrum, with a high prevalence of PIK3CA mutations, but a subset of them may constitute the substrate for carcinoma development.
12.4.4 Benign Tumors of the Nipple
12.4.4.1 Nipple Adenoma
A nipple adenoma is defined as a compact proliferation of small tubules showing both epithelial and myoepithelial layers arising around the collecting ducts of the nipple [16]. Epithelial hyperplasia may be florid within the tubules or in the collecting ducts. It should be noted that necrosis of comedo-type may occasionally be present in cases with florid UDH and should not be interpreted as a sign of malignancy. Furthermore, a nipple adenoma may mimick an invasive carcinoma as marked sclerosis may impart a pseudoinfiltrative pattern.
Clinically, it usually presents with nipple discharge and erosion of the nipple itself or with an underlying nodule. An association with carcinoma is on record but it represents a rare event [16]. Therefore, simple excision of those lesions, and whenever possible, conservation of the nipple, is the treatment of choice.
12.4.4.2 Syringomatous Adenoma
Syringomatous adenoma is a rare lesion, defined as a nonmetastasizing, locally recurrent, and locally invasive tumor of the nipple/areolar region displaying sweat duct differentiation [16]. Clinically it presents as a firm, discrete mass that on gross observation shows ill-defined margins. Microscopically, it is composed of nests and branching cords of cells, glandular structures, and small keratinous cysts that permeate the stroma of the nipple (bundles of the muscle and perineural spaces) [16]. This infiltrative pattern is coupled with bland cytology with regular nuclei and rare mitoses. Frequently, the glandular structures show an inner luminal layer and an outer layer of basal cells occasionally positive for smooth muscle actin.
The optimal treatment is excision with wide free margins. Of note, extension of the tumor can be appreciated also at a great distance from the main mass. Recurrence has been reported [16].
Syringomatous adenoma has to be differentiated from tubular carcinoma (which rarely involves the nipple) and low-grade adenosquamous carcinoma (which occurs in the breast parenchyma) [16]. The latter has a similar tendency for local recurrence and minimal metastatic potential, and may be morphologically indistinguishable from syringomatous adenoma, being therefore the anatomical site the main determinant for differential diagnosis.
12.5 Breast Cancer Precursors
The introduction of mammographic breast cancer screening programs has dramatically increased the detection of risk indicators, premalignant/preinvasive lesions, thus posing important issues related to patient management. A multitude of proliferative hyperplastic and premalignant alterations have been documented not uncommonly occurring synchronously with invasive breast cancer.
Observational and correlative studies have identified some of these lesions as risk indicators or breast cancer precursors [51–55]. A risk indicator can be defined as lesions that have been reported to be associated with increased risk of breast cancer development, whereas breast cancer precursors are those preinvasive lesions with the potential to progress to an overtly malignant phenotype [14]. Typically, the latter group includes risk indicators that have been shown to be clonal, neoplastic proliferations and to have histologic, IHC, and molecular features identical to those of matched invasive breast cancers, either synchronous or metachronous [14]. Based on the observation that the chances of one of these precursors progressing to invasive breast cancer rarely, if ever equates to 100 %, these lesions are best named nonobligate precursors [14].
Observational and molecular studies have recently demonstrated that a family of in situ lesions of the breast coexist at frequencies that could not be justified by chance alone [56, 57]. This family encompasses CCLs, flat epithelial atypia (FEA), ADH, atypical lobular hyperplasia (ALH), lobular carcinoma in situ (LCIS) and low-grade DCIS, and invasive lesions reported to be found in association with these nonobligatory precursors (i.e., invasive tubular carcinoma, invasive cribriform carcinoma, classic invasive lobular carcinoma, and low-grade invasive ductal carcinoma) [14]. These lesions are characterized by low histologic grade, expression of hormone receptors, lack of HER2 expression or HER2 gene amplification, lack of high molecular weight cytokeratin expression, and by the presence of genetic aberrations usually found in low-grade breast cancers (i.e., deletions of 16q and gains of 1q) [14]. In fact, frequent co-existence of tubular carcinoma, lobular carcinoma in situ, and CLLs (the so-called “Rosen triad” [58]) is on record since late 1990s., the Nottingham group coined the term “low-grade breast neoplasia family” to refer to these lesions [56, 57, 59].
Although different stages of progression have been identified for low-grade lesions, until recently only high-grade DCIS was recognized as a precursor of high-grade breast cancer [24, 60]. It has been demonstrated, however, that microglandular adenosis, a lesion considered by many only to be hyperplastic and an incidental finding, is often associated with high-grade breast cancer and harbors genomic aberrations identical to those found in adjacent synchronous invasive cancers [61–63].
Based on the observations above, we can subdivide low-grade precursors from high-grade precursors [14]. Low-grade precursors are almost exclusively of luminal phenotype (ER-positive/HER2-negative) and give rise to luminal invasive breast cancers. By contrast, high-grade precursors are more heterogeneous, including luminal, HER2-positive, and triple negative (TN) lesions. Microarray-based gene expression analyses have confirmed that breast cancer evolution follows two main pathways according to histologic grade, as preinvasive and invasive lesions when subjected to hierarchical clustering analysis cluster together according to grade rather than stage [64]. Nevertheless, it has been later documented that progression from low to high grade is not an uncommon event within the luminal subtype [65]. Therefore, given that ER defines two fundamentally different large subgroups of breast cancer, a modified hypothetical model of breast cancer evolution has been proposed, which follows two main pathways according to ER pathway activation [14]. In this model, the ER-positive branch includes all well-established low-grade ER-positive preinvasive lesions and their low-grade ER-positive invasive counterparts, which may progress to high-grade ER-positive lesions. The ER-negative branch includes ER-negative DCIS and microglandular adenosis as preinvasive lesions, and invasive carcinomas, which are mostly high-grade, frequently HER2-positive and display high levels of genetic instability. It should be mentioned that a subset of low-grade ER- and HER2-negative carcinomas do exist and display low levels of genetic instability, such as adenoid cystic and secretory carcinomas. Of note, these special types of ER-negative carcinomas are often driven by specific fusion genes.
12.5.1 Low-Grade ER-Positive Precursors
12.5.1.1 Columnar Cell Lesions
CCLs of the breast encompass a spectrum of lesions that feature distended acini lined by tightly packed columnar epithelial cells with apical snouts [66–68]. They can display varying degrees of cytological and/or architectural atypia, ranging from columnar cell change and hyperplasia to FEA and are frequently associated with microcalcifications (Table 12.3), thus justifying their increasing incidence as a result of mammographic screening [67]. Importantly, their behavior and significance are still poorly understood [69, 70] and this impacts on therapeutic decisions about interventions. Of note, complex architectural atypia such as well-defined micropapillae, rigid arches, and secondary lumen formation cannot be present in FEA; whenever these features are present, a diagnosis of ADH or low-grade DCIS according to the size of the lesion should be rendered.
Table 12.3
Morphological details of columnar cell lesions
CCC | CCC-a | CCH | CCH-a | |
|---|---|---|---|---|
Architecture | TDLUs with variably dilated acini lined by one or two layers of epithelial cells Flocculent secretion and luminal calcifications may be present | TDLUs with variably dilated acini with stratification of with more than two cell layers. Small mounds, tufts, or abortive micropapillations may be formed. Abundant flocculent secretion is frequent, as well as luminal calcifications that may have the configuration of psammoma bodies | ||
Cytology | 1. Columnar epithelial cells with uniform, ovoid to elongated nuclei oriented in a regular fashion perpendicular to the basement membrane 2. Evenly dispersed chromatin, no conspicuous nucleoli 3. Mitotic figures rarely encountered | 1. Increase in the nuclear/cytoplasmic ratio (akin to that seen in DCIS) 2. Nuclei may show stratification 3. Nuclear chromatin may be evenly dispersed or slightly marginated 4. Nucleoli variably prominent 5. Mitotic figures may be seen but uncommon | 1. Nuclei are ovoid to elongated and for the most part oriented perpendicular to the basement membrane 2. Some cells may have hobnail appearance 3. Crowding or overlapping of nuclei may give appearance of nuclear hyperplasia | 1. Increase in the nuclear/cytoplasmic ratio (akin to that seen in DCIS) 2. Nuclei may show stratification 3. Nuclear chromatin may be evenly dispersed or slightly marginated 4. Nucleoli variably prominent 5. Mitotic figures may be seen but uncommon |
A striking feature of CCLs is the constant expression of ER and PR and low Ki67 labeling indices. They also lack expression of HER2 and “basal” keratins [26, 66, 69, 70]. In accordance with this homogeneous immunoprofile, molecular studies have provided evidence that the majority of CCLs are clonal and neoplastic rather than hyperplastic [24, 26, 69, 71, 72]. Interestingly, the degree of genetic changes appears to mirror the degree of architectural and cytological atypia found in different types of CCLs [26, 71]. Allelic imbalances are most commonly located at 3p, 9q, 10q, 11q, 16q, 17p, and 17q [71, 72], while recurrent copy number alterations include losses of 16q and chromosome X and gains of 15q, 16p, 17q, and 19q [26]. These molecular analyses, combined with morphological and IHC observations have provided strong circumstantial evidence to suggest that CCLs are part of the above described “low-grade breast neoplasia family” [56, 57], and they constitute the first morphologically identifiable precursor of low-grade ER-positive breast cancers [69]. Indeed, CCLs frequently coexist with ADH, DCIS, and lobular neoplasia (LN) in the same breast or even in the same TDLU [56, 73] with whom they also share similar IHC profiles [57] and identical genomic aberrations [26, 71, 72].
Although there is scientific evidence that CCLs, in particular those with atypia (aka FEA), are nonobligate precursors of breast cancer, their rate of progression and their relative risk of subsequent invasive cancer is still a matter of debate. There is some evidence that the risk of developing invasive cancer conferred by a diagnosis of FEA as the most advanced lesion in a breast biopsy is low and perhaps comparable to that of UDH [74, 75]. Therefore, the best therapeutic option in this setting is yet to be demonstrated. It is unknown if surgical excision and/or hormonal prophylaxis should be offered. Adequate radiological–pathological correlation plays an important role in this setting, however, FEA on its own should not be routinely interpreted in the same way as ADH or LN [16].
12.5.1.2 Lobular Neoplasia
The initial classifications for preinvasive lesions assumed that some breast cancers would arise from the ducts and others would arise from the lobules and therefore in situ proliferations were named “ductal” carcinoma in situ and “lobular” carcinoma in situ [14]. It is important to note that the terms “ductal” and “lobular” carcinoma have no specific implications with regard to the site of origin within the mammary ductal-lobular system. The seminal works of Wellings et al. [76, 77] have documented that the vast majority of preinvasive lesions of the breast would arise in the TDLUs. Despite this, which constituted the beginning of the end of the histogenetic implications of the ductal and lobular terminology [14, 78], the terms ductal and lobular have been perpetuated, as they do identify lesions with distinct morphological and molecular features, and, more importantly, different therapeutic implications.
Lobular carcinoma in situ (LCIS) is composed of a monomorphic population of generally small and loosely discohesive cells that expand the TDLUs with or without pagetoid involvement of terminal ducts [79–81]. ALH was coined to refer to a morphologically similar but less well-developed lesion, i.e., a partial involvement of acini by LN cells [82, 83]. Morphological distinction of these lesions, however, is somewhat arbitrary and partly subjective [14]. In 1978, Haagensen et al. [80] retrospectively analyzed 211 examples of in situ lobular proliferations and introduced the term LN to encompass both ALH and LCIS. The rationale for combining these lesions into a single category was based on the observation that in that study the microscopic distinction of ALH from LCIS based on morphological parameters was not found to have any value in predicting subsequent carcinoma [80]. Importantly, the levels of genetic instability found in ALH and LCIS appear to be similar and both ALH and LCIS display similar patterns of recurrent unbalanced chromosomal aberrations, including losses of 16q, 16p, and 17p, and gains of 6q [84]. In fact, the molecular data available to date provide support for the LN concept and for the notion that distinction between ALH and LCIS is largely quantitative (i.e., extent of disease) rather than qualitative. A recent study [85], however, demonstrated a surprisingly greater amount of copy number alterations in ALH than in LCIS. It is important to specify that even though the term LN helps to avoid somewhat arbitrary diagnostic decisions, there is strong evidence to suggest that LCIS carries a higher risk of breast cancer development than ALH (8–10 vs. 4–5, respectively [86]).
In a way akin to other lesions of the “low-grade breast neoplasia family,” LN is characterized by ER and PR expression and is typically HER2-negative [81, 87]. From a genetic standpoint, LN harbors recurrent deletions of 16q and gains of 1q [84, 85].
ALH and LCIS have been accepted as risk indicators of breast cancer development, either in the ipsi-or contralateral breast; however, the risk is higher in the ipsilateral breast [88]. Conversely, their role as nonobligate precursors of ILC has been a matter of contention [89, 90]. The identification of CDH1 (E-cadherin gene) as the target gene of 16q deletions in lobular carcinomas [51, 91, 92] was crucial in elucidating the role of LN in breast cancer progression. Vos et al. [93] demonstrated the presence of identical CDH1 truncating mutations in matched LCIS and adjacent ILCs, providing strong circumstantial evidence to suggest that at least some LNs may evolve to ILCs [87]. This hypothesis is further corroborated by the similarity between LN and matched ILCs at the genetic level [94–96]. A transcriptomic analysis focused on normal epithelium, LCIS, and ILC has recently identified differentially expressed genes between the three groups and identified 169 candidate precursor genes, which likely play a role in LCIS progression, including PIK3R1, GOLM1, and GPR137B. Interestingly, these potential precursor genes map significantly more frequently to 1q and 16q, regions frequently targeted by gene copy number alterations in LCIS and ILC [97].
Taken together, the clinical and molecular evidence available suggests that ALH and LCIS are clonal and neoplastic, and that these lesions are both risk indicators and nonobligate precursors of breast cancer. It should be noted, however, that as a group the proclivity of LN to progress to invasive breast cancer is low. Conservative management of these lesions remains the mainstay of treatment [98].
LCIS can be subclassified as classic and variants, of which the most important are the florid and pleomorphic. Classic LCIS refers to the lesion above described, and should not display marked pleomorphism, comedonecrosis, or extreme distension of the involved TDLUs, and is best managed as an indolent disease. The pleomorphic variant of LCIS is defined by the presence of marked nuclear pleomorphism and is described below. More recently, a florid variant has been cataloged, which is cytologically similar to classic LCIS, but induces extreme distension of the TDLUs, with frequent comedonecrosis. Observational analyses have stressed that florid LCIS is often associated with invasive lobular carcinoma, while molecular analyses have documented that this lesion is molecularly as advanced as the pleomorphic variant [99]. Therefore, it has been suggested that florid LCIS should be treated more aggressively and completely resected. It should be noted, however, that histologic subtyping of LCIS has not been fully integrated in the last World Health Organization (WHO) classification of breast tumors, in part due to its subjective nature and the lack of prospective validation of its clinical relevance.
12.5.1.3 Atypical Ductal Hyperplasia (ADH) and Low-Grade DCIS
Low-grade DCIS is defined by a proliferation of monomorphic cells with uniform-sized nuclei and rare mitotic figures growing in arcades, micropapillae, cribriform, or solid patterns (Fig. 12.4; Table 12.4) [16]. Lesions classified as ADH display some but not all morphological features of low-grade DCIS. Similarly to the distinction between ALH and LCIS, the distinction between low-grade DCIS and ADH is mainly quantitative and somewhat subjective [16]; however, the risk of invasive breast cancer development reported for low-grade DCIS is higher than that for ADH [14, 37, 52, 83, 100, 101]. Whenever asked, breast pathology experts do acknowledge that they use both qualitative and quantitative criteria for this distinction in their practice. Despite being arbitrarily set, quantitative criteria are important to avoid overtreatment of minute lesions in the era of mammographic screening.
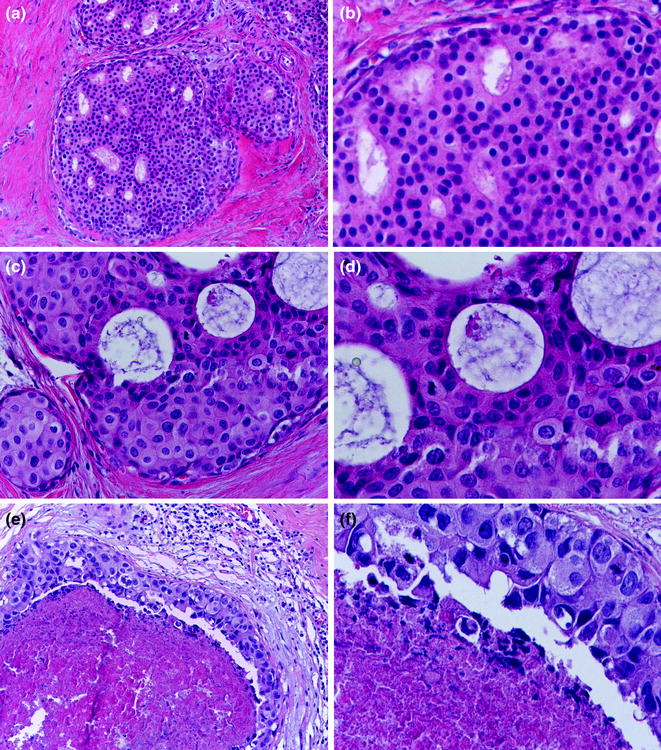

Fig. 12.4
DCIS (a, b low nuclear grade; c, d intermediate nuclear grade; e, f high nuclear grade)
Table 12.4
Morphological features for diagnosis of ductal carcinoma in situ (DCIS)
Low-grade DCIS | DCIS of intermediate grade | High-grade DCIS | |
|---|---|---|---|
Architecture | Any (very frequently cribriform pattern; solid and/or micropapillary pattern may also be present) | Any (solid, cribriform or micropapillary pattern) | Any (solid, cribriform or micropapillary pattern) |
Cytologic features | Monotonous and uniform rounded cell with round nuclei Minimal increase in nuclear-cytoplasmic ratio Regular chromatin pattern, inconspicuous nuclei | Mild to moderate variability in size, shape and placement Cell polarization not well developed as in low nuclear grade Nuclei with variably coarse chromatin and variably prominent nucleoli | Highly atypical cells Markedly pleomorphic nuclei, poorly polarized with irregular contour Coarse, clumped chromatin with prominent nucleoli |
Necrosis | Uncommon but possible* | May be present | Frequently present* |
Mitosis | Absent or rare | May be present | Usually common* |
Typical pattern of calcifications | Calcification of psammomatous type common | Either similar to low grade or amorphous calcifications like in high grade | Amorphous calcification often associated with necrotic intraluminal debris |
ADH has long been recognized as both a risk indicator and a nonobligate precursor of low-grade DCIS and invasive breast cancer [52, 86]. In fact, the similarities between ADH and low-grade DCIS are striking. Immunophenotypically, both lesions are consistently positive for ER and PR, and lack HER2 overexpression and gene amplification, and expression of high molecular weight cytokeratins (Table 12.2), thus are part of the “low-grade breast neoplasia family” [52, 56, 57, 86]. Close similarities have also been repeatedly reported at the genetic level and these data have corroborated the idea that ADH is neoplastic and a nonobligate precursor with an unequivocal role in the development of low-grade DCIS and invasive lesions [20, 102, 103]. ADH and low-grade DCIS harbor allelic imbalances at similar frequencies [104, 105]. In addition, the study of matched samples of ADH, DCIS and invasive lesions identified concordant allelic imbalances. Recurrent regions displaying LOH include loci on 1q, 16q, and 17p [20, 102, 103]. CGH studies have confirmed these observations and demonstrated that ADH and low-grade DCIS are clonal, neoplastic, and have similar number, type, and complexity of unbalanced chromosomal aberrations [22, 24, 106, 107]. Not surprisingly for lesions of low histologic grade, ADH and low-grade DCIS are characterized by recurrent losses of 16q and 17p and gains of 1q. In conclusion, at the molecular level, ADH and low-grade DCIS seem to be nearly, if not completely, identical.
12.5.2 High-Grade Precursors
12.5.2.1 High-Grade DCIS
High-grade DCIS is composed of a population of atypical cells displaying marked nuclear pleomorphism, arranged in multiple architectural patterns, including solid, cribriform, and micropapillary [16] (Fig. 12.4; Table 12.4). Comedonecrosis is often present, but its detection does not necessarily equate to a diagnosis of high-grade DCIS [16]. There is one particular scenario, called Paget disease of the nipple, in which malignant and mostly HER2-positive glandular epithelial cells populate the squamous epithelium of the nipple and this is almost always associated with an underlying high-grade DCIS, which typically involves more than one collecting ducts and also more distant ducts deep in the breast gland (Fig. 12.5). An associated infiltrating carcinoma can also be seen in up to 90 % of cases [16].
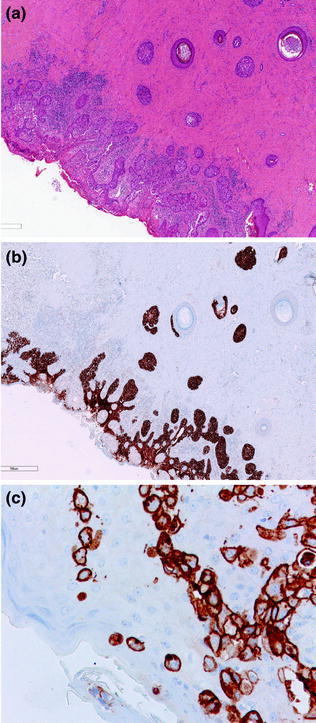

Fig. 12.5
Paget disease of the nipple (a, b H&E; c HER2)
High-grade DCIS is a paradigmatic lesion for the concept of nonobligate precursor of invasive breast cancer, given that it has a proclivity to progress to invasive cancer but does not always [14]. A diagnosis of high-grade DCIS is associated with a significantly higher risk of invasive breast cancer development and with earlier recurrences than low-grade DCIS [37, 52, 86, 108]. In fact, the risk of an ipsilateral breast recurrence either in the form of DCIS or invasive breast cancer after local excision alone of high-grade DCIS is estimated at around 15 % at 5 years [108].
As mentioned above, the immunoprofile and patterns of genetic aberrations of high-grade DCIS are more heterogeneous than those observed in low-grade DCIS [14]. Importantly, despite the greater complexity of the pattern of genetic aberrations found in high-grade than in low-grade DCIS, deletions of the whole of 16q are found in a minority of the former suggesting that the majority of high-grade DCIS arise either de novo or from a precursor other than ADH⁄low-grade DCIS [14]. However, most likely, most ER-positive DCIS may still display 16q whole arm loss and possibly originate from low-grade lesions. In contrast, ER-negative high-grade DCIS probably arise either de novo or from a precursor other than ADH⁄ low-grade DCIS.
As a last remark, intratumor heterogeneity has been definitely documented in DCIS [109, 110]. For instance, it is not uncommon to find different nuclear grades in the same lesion [109], a fact that has been associated with our poor ability to predict clinical behavior of DCIS based on morphological parameters [111, 112]. aCGH and fluorescence in situ hybridization (FISH) analyses of matched DCIS and invasive carcinomas have also demonstrated the existence of subclones in DCIS and that stromal invasion may occur due to a clonal selection process [110].
12.5.2.2 Pleomorphic Lobular Carcinoma In Situ
Pleomorphic lobular carcinoma in situ (PLCIS) typically displays cytological and architectural features of both classic LCIS and high-grade DCIS [113–115]. The growth pattern exhibits large, discohesive cells, often with finely granular apocrine cytoplasm and intracytoplasmic vacuoles with marked nuclear atypia and pleomorphism. Foci of classic LN can be found in association with PLCIS [113, 116–118]. PLCIS is characterized by moderate proliferation index and may show comedonecrosis [113–115]. These lesions often show low levels of ER and PR expression, lack of E-cadherin expression, and occasionally display HER2 overexpression and amplification [115–119]. Although traditionally described in association with its invasive counterpart (pleomorphic invasive lobular carcinoma, ILC) [113, 114, 120], PLCIS can also be found as an isolated lesion [115].
Molecular studies have demonstrated that PLCIS and pleomorphic ILC harbor remarkably similar genetic profiles, and that both have the hallmark features of lobular carcinomas, namely 16q loss, 17p loss, 1q gain, and E-cadherin loss of expression [117, 121]. Of note, PLCIS and pleomorphic ILC display additional genetic aberrations, including amplification of key oncogenes, deletion of 8p and 13q, and gains of 8q. These alterations may account for the higher nuclear grade and reported more aggressive clinical behavior of PLCIS and pleomorphic ILCs [117, 118, 121]. Chen et al. [116] analyzed a large series of PLCIS and classic LCIS confirming the similarities between these lesions at the genetic level. More specifically, PLCIS samples were divided into two groups, nonapocrine and apocrine: the former had similar levels of genetic instability as those observed in classic LCIS, whereas the latter displayed more and specific genomic changes as amplification at 17p11.2–17q12 and 11q.13.3, gain of 16p and losses of 11q and 13q [116]. These alterations were very much similar to those previously described [117].
12.5.2.3 Microglandular Adenosis
Microglandular adenosis (MGA) is an uncommon entity characterized by a haphazard proliferation of homogeneous small and rounded glands lined by a single layer of epithelial cells around lumen containing secretions and/or calcifications (Fig. 12.6) [122–124]. It has long been described to occur in association with invasive carcinomas (Fig. 12.6) [123, 125, 126], however, the precursor nature of this lesion was called into question by some experts in the field [124]. This lesion displays a typical immunophenotype, being strongly positive for S100 protein and negative for ER, PR, and HER2 expression (TN phenotype), an immunophenotype that is also shared by concurrent invasive carcinomas, which are often of high histologic grade and frequently express basal markers [61, 127]. aCGH-based analyses have identified concordant copy number changes in this spectrum (MGA, atypical MGA, associated DCIS, and invasive carcinomas), providing molecular evidence of the neoplastic and nonobligate precursor nature of a subset of MGA [61].
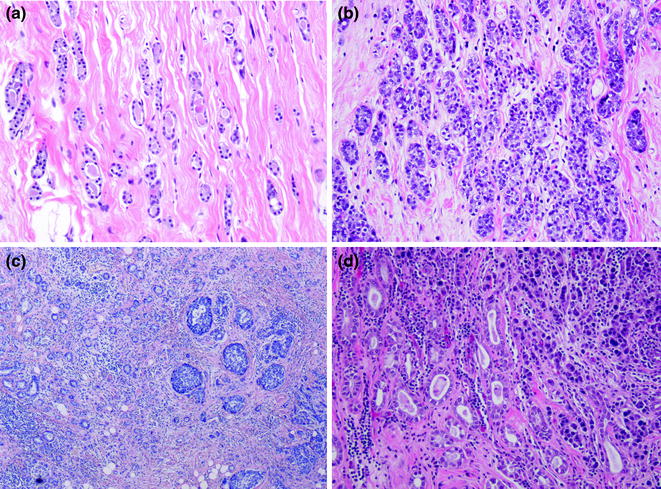

Fig. 12.6
MGA (a typical; b atypical; c, d two examples with concurrent invasive carcinoma)
12.6 Papillary Lesions
Papillary breast lesions encompass a group of lesions that share a typical architectural pattern, being defined as epithelial proliferations supported by fibrovascular stalks with or without a layer of myoepithelial cells occurring anywhere in the ductal system (from the large retroareolar ducts to the TDLU) [16]. They can be benign (intraductal papilloma), atypical (atypical papilloma), or carcinomas (encapsulated papillary carcinoma, solid papillary carcinoma, and invasive papillary carcinoma).
12.6.1 Intraductal Papilloma
An intraductal papilloma is defined as a proliferation of epithelial and myoepithelial cells overlying fibrovascular stalks, thus creating an arborescent structure within the lumina of a duct [16]. They can be central (large ducts involved) or peripheral (arising in the TDLU). Central papillomas usually present with unilateral sanguineous nipple discharge, while palpable masses are less frequent. Mammography can detect a circumscribed retroareolar mass with dilated duct, whereas small lesions can be occult. Calcifications are rare. Galactography, by detecting the irregular filling defect and obstruction may be of help to the surgeon to localize the discharging duct before intervention [16].
Histopathologically, the arborescent structure may present a coexistence of papillary and ductal patterns; whenever the latter predominates and is also associated with marked sclerosis, the term sclerosing papilloma is used.
Papillomas can undergo a series of changes, such as inflammation, necrosis, and metaplasia (of different types: apocrine, squamous, chondroid, osseous, mucinous). In addition, the whole range of atypical/neoplastic proliferations may arise in a papilloma or secondarily involve it.
Peripheral papillomas are often clinically occult and multiple, involve terminal ducts and TDLUs rather than large ducts, and nipple discharge is much less frequent. Morphologically, they share the same architectural pattern and histologic features; the main distinction resides on the more frequent association with concomitant sclerosing adenosis, radial scars, UDH, ADH, and in situ or invasive carcinomas [16].
12.6.2 Encapsulated Papillary Carcinoma and Solid Papillary Carcinoma
Breast papillary carcinomas are histologically defined as malignant neoplasms with a papillary growth pattern, where the epithelial cells reside directly in fine and delicate papillary fronds, with no underlying myoepithelial cell layer. A papillary carcinoma that is clearly intraductal (i.e., surrounded by a ductal wall with a myoepithelial cell layer) is considered as a papillary pattern of DCIS. The spectrum of breast papillary carcinomas encompasses, however, three other histologic entities, namely encapsulated papillary carcinoma (EPC), solid papillary carcinoma (SPC), and invasive papillary carcinoma (IPC) [128, 129]. IPCs will be discussed in the section of special histologic types of invasive carcinoma. Here we discuss the two controversial papillary lesions (EPC and SPC), whose status as intraductal or invasive lesions has been a matter of debate.
EPC is a well-circumscribed nodule of papillary carcinoma surrounded by a thick fibrous capsule. In EPCs, the neoplastic cells are of low to intermediate nuclear pleomorphism and arranged in papillary fronds in the majority of cases; however, areas with cribriform and/or solid patterns can be found [16, 130]. Although initially perceived as a variant of in situ papillary carcinoma, recent studies have demonstrated that EPCs consistently lack a myoepithelial cell layer not only in the papillary fronds, but also at the periphery of the tumor nodules [129, 131–133]. Therefore, what was thought to be a malignant papillary intraductal/intracystic neoplasm, is now best considered as an invasive carcinoma with circumscribed borders, slow growth, and surrounded by a reactive fibrous capsule. In agreement with this notion, recurrence in the chest wall and rare lymph node metastasis with the same papillary architecture have been documented [129, 134]. Despite this invasive nature, its long-term prognosis is excellent and comparable to DCIS; thus, most experts still recommend EPC to be staged and clinically managed as an in situ carcinoma.
SPC is also a well-circumscribed lesion that is densely cellular and composed of one or multiple expansile nodules, sheets, or coalescent papillae of ovoid-to-spindle-shaped cells growing in a solid pattern. Frequently, the fine and delicate fibrovascular cores are not evident at first glance. Neuroendocrine differentiation is a frequent feature, which may be of diagnostic utility [135]. In addition, when SPC is associated with a clearly invasive carcinoma, the latter often displays neuroendocrine features [136]. In a way akin to EPC, the classification of SPCs as invasive or in situ disease remains a matter of controversy. For instance, a myoepithelial cell layer may also be absent in solid-papillary nodules, but if these nodules are histologically well circumscribed, the lesion can still be considered in situ. Nevertheless, an invasive counterpart of solid-papillary carcinoma is accepted by many experts and it has been coded in the last WHO classification of breast tumors [16]. Solid nodules in a geographic, jigsaw-like pattern within a fibrous, focally desmoplastic background, coupled with absence of myoepithelial cells, may suggest an invasive disease. As EPCs, these tumors may also have the potential to disseminate to axillary lymph nodes; distant metastases, although rare, are on record [129, 130]. However, it has been suggested that, if there is uncertainty about invasion, SPCs should be regarded for staging purposes as in situ carcinoma [16].
Finally, EPCs and SPCs are not uncommonly associated with a clearly invasive carcinoma component. This invasive component may be of no special type, usually of low to intermediate histologic grade, or of some special types, in particular carcinomas of mucinous type or with neuroendocrine differentiation. Whenever a clearly invasive carcinoma component is present, its size and features should be used for staging, histologic grading, and IHC profiling purposes.
From a molecular standpoint, a recent exploratory analysis of the transcriptomic profiles of all three subtypes of PCs has revealed that despite displaying similar patterns of gene copy number alterations, transcriptomic profiles showed significant differences among EPCs, SPCs, and IPCs: EPCs expressed a subset of genes involved in cell migration at significantly lower levels than SPCs and IPCs, and SPCs displayed transcriptomic and IHC features consistent with those associated with neuroendocrine differentiation. One may hypothesize that such differences may account for their different histologic features [137].
A recent study described a high-grade variant of EPC, which was frequently ER-negative and, in one case, had a fatal outcome despite the lack of usual stromal invasion in the primary lesion [138]. We argue that perhaps those cases with papillary architecture, marked nuclear pleomorphism, and ER-negative phenotype would be best categorized as invasive carcinomas of no special type (IC-NSTs) with papillary features to avoid under-treatment.
12.7 Invasive Carcinoma
An invasive breast carcinoma is defined by a morphological infiltrative pattern, often coupled with a desmoplastic stromal reaction, and/or, with very few exceptions, the lack of a myoepithelial cell layer.
The introduction of annual screening programs has dramatically changed the presentation and natural history of breast cancer over the past decades. In screening populations smaller lesions are usually diagnosed at lower stages, allowing more conservative surgical interventions. Invasive carcinomas are detected at mammography as opacities, distortions, or stellate lesions with or without calcifications. This pattern largely mirrors what pathologists observe at gross analysis where invasive carcinomas are usually described as opaque whitish nodules, distortions, or lesions. Occasionally, well-circumscribed lesions of soft texture and/or gelatinous appearance can be encountered, typically for mucinous, papillary, and some high-grade carcinomas that at ultrasound examination may be misinterpreted as dense cysts or benign nodules.
The WHO classification [16] is currently based on morphological features and roughly separates invasive carcinoma of no special type (IC-NST), which is the commonest form of breast cancer, from a large group of so-called “special histologic types,” which together account for approximately 25 % of all newly diagnosed breast carcinomas. It is important to note that histologic subtyping holds prognostic significance, accordingly to earlier studies conducted by Elston and Ellis [139], where it was shown that patients affected by some special types of breast cancer have a better or worse outcome than those with an IC-NST (formerly known as invasive ductal carcinoma of no special type, IDC-NST). The St. Gallen International Expert Consensus stressed that attention should be posed on the recognition of histologic types, as some of them, like tubular and cribriform carcinomas, have an excellent prognosis and may be treated by surgery alone [140]. On the other hand, interobserver agreement rates of histologic subtyping are modest and the existence of some entities is controversial [16].
Since the last decade of the twentieth century, the application of high-throughput molecular techniques, in particular microarray-based gene expression profiling, has definitely reshaped our understanding of breast cancer. Studies using this set of techniques have changed some aspects of breast cancer classification, and provided a working model for the taxonomy for breast cancer [141]. Undoubtedly, the most important contribution of microarrays to breast cancer research has been the realization that breast cancer is not a single disease. The seminal expression microarray-based class discovery studies by the Stanford group devised the so-called molecular classification of breast cancer, based on the identification of the “intrinsic gene list” and subsequent hierarchical clustering of cases based on the expression of genes pertaining to this list [142]. This approach divides breast cancers into two main groups: ER-positive and ER-negative disease. The ER-positive group comprises the luminal tumors: these typically show the expression of ER and of genes pertaining to ER pathway, and other transcripts usually found in luminal epithelial cells [141, 142]. Luminal cancers can be further subdivided into two distinct subgroups: luminal A (characteristically showing high levels of ER and ER pathway activation) and luminal B cancers (which show lower levels of ER and high levels of genes pertaining to the proliferation cluster) [141, 142]. The ER-negative cluster encompasses HER2, and basal-like cancers. The microarray-defined group of HER2-expressing tumors is characterized by high levels of expression of genes pertaining to the HER2 amplicon, including HER2, GRB7, GATA4, and a high level of NF-κB activation [141, 142]. ER-positive HER2-amplified cancers often cluster together with luminal B tumors [141]. The last group comprises cancers that lack ER and HER2 expression and have been named basal-like carcinomas, as they are characterized by the expression of genes known to be preferentially expressed by basal/myoepithelial cells, such as CK 5 and 17, integrin 4, laminin, c-KIT, α6 integrin, metallothionein IX, fatty acid binding protein 7, P-cadherin, EGFR, and NF-κB [141, 142].
This molecular classification is not a mere academic exercise, given that it holds prognostic significance, with tumors of luminal A subtype being associated with the best outcome, and tumors of basal-like or HER2 subtype with the worst outcome [143–145].
In addition, subsequent studies have refined this classification and recognized further subgroups. For instance, the ER-negative group beyond the originally described basal-like and HER2-enriched subtypes [142, 146] has been shown to encompass other subgroups. These include claudin-low tumors, of which 60–70 % are of TN phenotype (i.e., lacking ER, PR, and HER2 expression) and are potentially enriched for the so-called cancer stem cells [147] and the molecular apocrine subtype, characterized by the expression of androgen receptor, transcriptomic features consistent with the activation of the androgen receptor pathway and poor clinical outcome [148–150].
It has long been debated about carcinomas of basal-like intrinsic subtype being equal to IHC-defined TN breast cancers (TNBCs). Although there is a wide overlap between the two entities (about 80 %), it has been demonstrated that one is not the surrogate of the other [151–153]. The clinically-defined umbrella “TN subgroup” is more heterogeneous than the basal-like intrinsic subtype [151, 154]. While perhaps the major feature of basal-like subtype is a notable association with BRCA1-mutant patients (“BRCAness”) [155–158], recent studies have shown the existence within TNBCs of either six (basal-like I, basal-like II, mesenchymal, mesenchymal stem-like, immunomodulatory, and luminal androgen receptor) [159] or four subtypes (luminal androgen receptor, mesenchymal, basal-like immune-suppressed, and basal-like immune-activated) [160]. These TNBC classification systems also provide an interesting framework to match subtypes of the disease with specific targeted therapies [160, 161], given that the six-subtype classification is associated with distinct responses to neoadjuvant chemotherapy [162].
A further step about molecular classification of breast cancer has been added by the Molecular Taxonomy of Breast Cancer International Consortium (METABRIC), which has reported on a breast cancer classification based on a genomic analysis that integrates gene expression and genome-wide copy number alterations (CNAs) [163]. From the first study, which included the analysis of approximately 2000 tumors, it has become apparent that the most parsimonious number of molecular subtypes of breast cancer is ten [163]. ER-positive and ER-negative tumors differ also in the pattern and type of gene CNAs: while the majority of ER-positive breast cancers (grade 1, 80 %; grade 3, 50 %) are characterized by concurrent deletions of 16q and gains of 1q, these concurrent alterations appear to be remarkably rare in ER-negative tumors [65]. On the other hand, TNBCs harbor complex patterns of copy number gains and losses throughout the genome [164, 165]. The proponents of this classification system have further developed a gene expression-based approach to classify breast carcinomas into the ten integrative clusters [165]. This methodology, in a way akin to PAM50 for the “intrinsic” subtypes [146] could enable a simpler translation of such integrative analysis that otherwise would require both gene expression and CNA information. The analysis of 7544 BCs with the new classifier has revealed that the METABRIC classification may be more informative in the contextualization of the genomic drivers identified by massively parallel sequencing studies of breast cancer [165] than the “intrinsic” subtypes [166].
Although the molecular classification of breast cancer has been largely adopted by the scientific and clinical community, the histologic classification should not be neglected. The St. Gallen International Expert Consensus has indeed suggested that early breast cancer therapy should be defined according to the intrinsic subtypes [140]. Nevertheless, as mentioned above, St. Gallen experts advised that some special histologic types may be particularly endocrine sensitive and therefore may not need chemotherapy. In addition, in the context of TN disease, proper histologic classification is of extreme importance to avoid unnecessary systemic treatment for patients with some low-grade forms of TN carcinoma, such as acinic cell, adenoid cystic, and secretory carcinoma. Therefore, despite the great value of the molecular classification for treatment decision, histologic classification still plays a role and should not be relegated to an academic exercise.
12.7.1 Invasive Carcinoma of no Special Type (IC-NST)
Invasive carcinoma of no special type (IC-NST), despite accounting for the vast majority (up to 75 %) of all invasive breast carcinomas, represents a diagnosis of exclusion, as it is best defined as any epithelial invasive neoplasm that does not fulfill criteria for any of the special histologic subtypes [16]. This entity was formerly known as invasive ductal carcinoma of no special type. As explained above, the ductal/lobular terminology has no histogenetic implications, thus the WHO committee decided to change the nomenclature.
Due to this negative definition, morphologic heterogeneity is to be expected. An IC-NST can be composed of several growth patterns, such as duct-forming structures of variable size or large solid sheets of cells. To circumvent this histologic heterogeneity and provide some prognostic information, histologic grading according to the Elston and Ellis grading system, also known as Nottingham grading system [167] plays a major role in this subtype of breast carcinoma (see below).
The stromal compartment is usually desmoplastic and may be populated by inflammatory cells, such as lympho-mononuclear cells that infiltrate the tumor (tumor infiltrating lymphocytes, TILs). Recently, due to the increasing knowledge on the immune response elicited by tumors and the availability of immune therapies, attention has been given to the TILs in breast cancer. Presence of TILs has been reported in different breast cancer subtypes: brisk lymphocytic infiltrates represent, for instance, one of the typical features of basal-like and BRCA1-associated breast carcinomas [168], and increased lymphocytic infiltration into tumors has been associated with ductal histology, high grade, absence of expression of hormone receptors or high expression of the proliferation antigen Ki67 [169, 170]. Lymphocyte-rich tumors have been associated with effective therapeutic responses and favorable clinical outcomes [171–174]. Multiple retrospective analyses of prospective clinical trial samples have provided level I evidence for the assessment of TILs as a prognostic factor in breast cancer, and international guidelines for their assessment have been published [175]. It should be noted, however, that TILs are a prognostic factor in patients with TN or HER2-positive tumors treated with chemotherapy. Therefore, for the time being, TIL assessment may not have a clear clinical utility, as the management of these patients is still the same independent of the presence or lack of TILs. Perhaps, the introduction of immune therapies or distinct chemotherapies for those patients that lack an immune response may change this scenario.
In a way akin to the morphological heterogeneity, IC-NSTs is also heterogeneous at the immunophenotypical and molecular level, as highlighted by the molecular classification of breast cancer, which was originally described in a cohort of IC-NSTs and a few lobular carcinomas [142]. The vast majority is ER-positive (approximately 75 %), 15 % of cases may be HER2-positive and the remaining cases fall into the category of TNBC. As for histologic special types, definition of prognosis is based on evaluation of morphologic features (size, histologic grade, mitotic index, lymph node status), immunophenotypical profiling (assessment of ER, PR, proliferation index by Ki67), and genomics (HER2 gene status, use of gene expression signatures), which will be discussed more in detail in the next sections.
IC-NSTs can be admixed to any histologic special type and the WHO classification has defined some arbitrary cut-offs. A 50 % cut-off is adopted (i.e., the special type component has to represent at least 50 % of the lesion to allow for a diagnosis of invasive mixed carcinoma [16]). When the special type makes up for less than 50 % of the tumor, a diagnosis of IC-NST with a focal special type component should be rendered.
12.7.2 Special Types of Invasive Carcinoma
Breast cancer special types account for up to 25 % of all breast cancers. The WHO classification recognizes the existence of at least 20 entities, which are mainly defined by unique histologic features [16]. It is important to note that histologic subtyping holds some prognostic significance: patients affected by some special histologic types do differently than those affected by IC-NST [139].
It should be noted that the molecular classification was originally devised for IC-NSTs and a small subset of ILCs, however, a subsequent study [176] analyzed a series of 113 tumors from 11 special histologic types of breast cancer and provided the first transcriptomic description of histologic special types. Importantly, gene expression [176–181] and aCGH studies [177, 180, 182–184] of special histologic types of breast cancer have revealed that at the genomic and transcriptomic level, tumors from each of the special histologic types of breast cancer are more homogeneous amongst themselves than IC-NSTs. In addition, some special histologic types appear to be almost exclusively ER-positive (micropapillary, mucinous, tubular/cribriform, lobular and papillary carcinomas), whereas others (adenoid cystic, secretory and metaplastic breast cancers) are uniformly ER-negative [176, 185–187].
Genotypic–phenotypic correlations between specific genetic aberrations and special histologic types of breast cancer have emerged [185]. For instance, adenoid cystic carcinomas and secretory carcinomas are underpinned by the recurrent fusion genes MYB–NFIB [188] and ETV6–NTRK3 [189], respectively, and lobular carcinomas are underpinned by loss of function of E-cadherin [187, 190]. Furthermore, micropapillary [183, 184, 191], mucinous [182], and adenoid cystic [192] carcinomas display different patterns of genomic alterations when compared to grade- and ER-matched IC-NSTs. Finally, the genes whose expression determines the clinical behavior of special types of breast cancer may differ from those implicated in the prognosis of IC-NSTs, as exemplified by the relatively poor discriminatory power of prognostic gene signatures observed in special types of breast cancer [182, 193, 194].
Below we provide an overview of some special histologic types where morphologic features and genomic make-ups are described.
12.7.2.1 Lobular Carcinoma
Invasive lobular carcinomas (ILC) is the most frequently diagnosed special histologic type and the second most frequently diagnosed form of invasive breast cancer, accounting for about 10–15 % of all newly diagnosed breast cancers [16]. It is characterized by small discohesive neoplastic cells invading the stroma in a single cell-file (aka “indian file”) pattern (classic type) (Figs. 12.7 and 12.8) [79]. ILC variants have also been described including the solid, alveolar, trabecular, and pleomorphic patterns. The solid pattern features sheets of cells, whereas in the alveolar variant we encounter globular aggregated of up to 20 cells. In both cases cells show the typical discohesiveness of the classic variant, but in the solid variant, more significant pleomorphism and higher mitotic activity may be present [16]. The pleomorphic variant displays the distinctive growth pattern of lobular carcinoma but with a marked degree of cellular atypia and nuclear pleomorphism [16] and may show apocrine or histiocytoid differentiation [16]. Signet ring cells are typically encountered in all ILC variants, if making up most of the tumor volume a signet ring cell variant may be diagnosed.
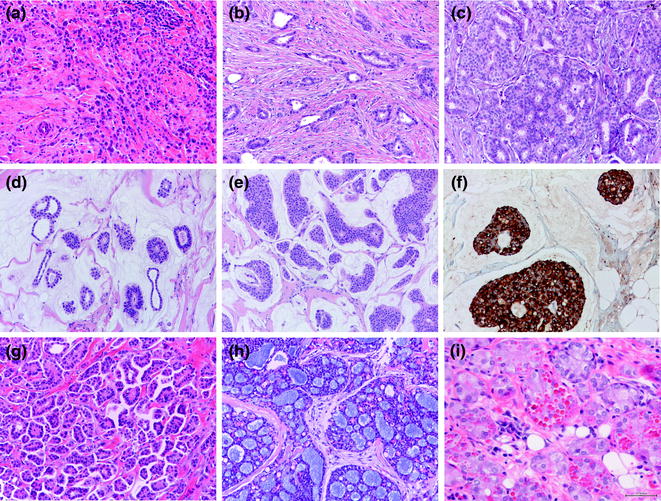
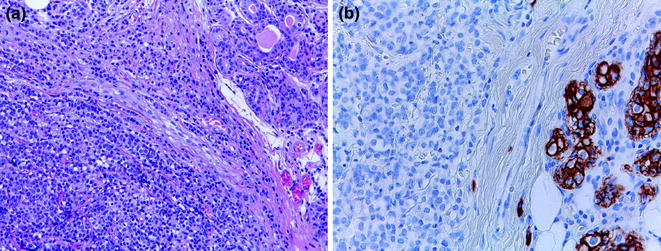

Fig. 12.7
Panel of special histologic types of invasive breast cancer (a lobular carcinoma; b tubular carcinoma; c cribriform carcinoma; d mucinous carcinoma, type A; e mucinous carcinoma, type B; b; f synaptophysin staining in a mucinous carcinoma, type B; g inverted micropapillary carcinoma; h adenoid cystic carcinoma; i acinic cell carcinoma)

Fig. 12.8
Invasive lobular carcinoma (a H&E; b E-CAD staining with positive internal control, i.e. a breast lobule)
Lack of E-Cadherin protein expression is the hallmark feature of ILC (Fig. 12.7), also encountered in the in situ lesion (see section on LN) and represents the underlying reason for the discohesive growth pattern [16, 78]. This phenotype is determined by inactivating CDH1 mutations in the large majority of cases. The CDH1 gene is located on 16q, thus truncating mutations, loss of 16q, gene promoter methylation and/or transcriptional alterations co-occur leading to biallelic inactivation of the gene and loss of protein expression [78]. Interestingly, CDH1 inactivation may not only be responsible for the histologic pattern, but also for the metastatic behavior of ILCs, which more commonly metastasize to anatomical sites such peritoneum, meninges, and gynecological and gastrointestinal organs. In a mouse model where CDH1 and TP53 were inactivated, the animals developed tumors that recapitulated both morphological and metastatic patterns of ILCs [91].
IHC for E-cadherin can be used to confirm the histologic type in lesions with borderline ductal (no special type) versus lobular histologic features, even though it has to be pointed out that aberrant E-cadherin expression in ILCs may be misleading [78]. Another characteristic immunophenotypic feature of ILC is the presence of cytoplasmic expression of p120 catenin [78]. p120 catenin, normally bound to the intracytoplasmic domain of E-cadherin, becomes upregulated when E-cadherin is lost [195]. Cytoplasmic p120 staining can be of help to resolve discordance between morphology and the E-cadherin pattern of staining.
A diagnosis of ILC on presurgical biopsy material should be made whenever possible; indeed, this detail may be of help to clinicians since: (i) ILC is frequently multicentric and bilateral, and careful study of both mammary glands with magnetic resonance may be considered (lobular carcinoma represents one of the indications in the guidelines of the EUSOMA working group for magnetic resonance imaging of the breast [196]); (ii) it has been reported that lobular carcinomas show a low rate of pathologic complete response (pCR) when subjected to primary systemic treatment, therefore, this histologic type does not represent a good candidate for neoadjuvant treatment [197, 198].
These carcinomas are typically ER-positive (only 5 % of ILCs are actually ER-negative) [16] and most of them are of luminal A subtype [176]. However, some degree of molecular heterogeneity is still observed in ILCs, in particular in the pleomorphic form, which is not uncommonly ER-negative, HER2-positive by IHC or ISH, and/or harbor HER2 gene mutation [199]. Notably, gene expression analyses have confirmed that pleomorphic ILCs are closer to classic ILCs than grade- and ER-matched IC-NSTs [190].
Very recently, The Cancer Genome Atlas (TCGA) consortium has carried out the most comprehensive genomic comparison between ILCs and IC-NSTs and identified, in addition to the best-known ILC genetic hallmark E-cadherin loss, some ILC-enriched features, including mutations targeting PTEN, TBX3, and FOXA1 [200]. HER2 mutations were also detected, at a lower frequency (4 %) than reported before (23 % in [201]). A striking difference between ILCs and IC-NST was a higher PI3K/Akt activation in ILCs as compared with IC-NSTs within the luminal A subtype, a finding that may have therapeutic implications as anti-PI3K therapy (i.e., Everolimus) is a therapeutic option for ER-positive/HER2-negative metastatic breast cancer [202]. Interestingly, contrary to the previous notion that CDH1 gene promoter methylation could be causative of loss of E-cadherin expression in ILCs, the results of the ILC TCGA study failed to observe an impact of methylation on E-cadherin protein expression [202]. Further studies to determine the mechanism by which E-cadherin is lost in ILCs lacking biallelic inactivation of CDH1 are warranted.
12.7.2.2 Tubular Carcinoma
Tubular carcinoma (TC) accounts for approximately 2–4 % of all invasive breast cancers [16]. Histologically, TCs feature well-defined round, ovoid or angulated tubules with open lumina dispersed in a cellular fibrous or fibroelastotic cellular stroma (Fig. 12.7), which is characteristically reproduced also in rare lymph node metastasis. The tubules are lined by a single layer of relatively uniform epithelial cells with little nuclear pleomorphism and low proliferation [16]. Given the high percentage of tubule formation, low levels of nuclear pleomorphism, and low mitotic rates, TCs always fall within histologic grade 1 carcinomas [16]. It is important to adhere to stringent criteria when diagnosing TC to ascertain its excellent prognosis.
TC is typically hormone receptor positive, HER2-negative, and low proliferative (Ki67 < 10 %), and the great majority of these tumors are classified as luminal A molecular subtype [176], which has been consistently shown to display a better outcome than the remaining molecular subtypes [143–146]. We have observed, however, morphological bona fide examples of TCs that are PR-negative, despite a very low Ki67 index. This may have some clinical impact as PR levels below 20 % have been suggested to correlate with luminal B subtype [203–205], whereas the identification of TCs denotes a particularly good prognosis [16, 206]. A large retrospective study carried out by Rakha et al. [206] has demonstrated that the outcome of patients with pure TCs is significantly better than that of patients with grade 1 IC-NSTs. Pure TCs, low-grade IC-NSTs, and low-grade ILCs have been demonstrated to share immunophenotypic and genetic similarities, and pertain to the so-called “low-grade breast neoplasia family” [56, 57]. At the transcriptomic level, TCs are indeed very similar to histologic grade- and molecular subtype-matched IC-NSTs, supporting the concept that these two entities may evolve through common molecular pathways and have similar precursor lesions. However, subtle transcriptomic differences between these two entities were detected. Pure TCs were shown to be characterized by an upregulation of several components of the ER canonical pathway, including ESR1, CREBBP1, and NCOR1. Furthermore, TCs were shown to display higher expression levels of INPP4B, a tumor suppressor gene with inhibitory effect on PI3K pathway. It should be noted, however, that differences were small and it is very unlikely that markers or a gene signature for TC could be developed. Therefore, the correct histologic identification of TCs remains an important prognostic factor within the luminal A subtype. For instance, patients with TCs may not need a good-prognosis gene expression signature to forgo chemotherapy.
12.7.2.3 Cribriform Carcinoma
Similar to TCs, this lesion represents an invasive carcinoma with an excellent prognosis that shows a cribriform growth pattern similar to that of the in situ carcinoma, with which it is frequently associated (Fig. 12.7). A mixture of cribriform and tubular components may be appreciated in some cases. As in TCs, tumor cells are small and nuclei show a low or moderate degree of pleomorphism. Mitotic figures are rare. Cribriform carcinoma is invariably (100 %) ER positive and frequently (about 70 %) PR positive. The main and most important differential diagnosis is with adenoid cystic carcinoma, which mimics the growth pattern but is consistently of TN phenotype [16].
12.7.2.4 Mucinous Carcinoma
Also called mucin-producing or colloid carcinoma, this histologic type includes a variety of carcinomas accounting for about 2 % of all breast cancers characterized by production of abundant extracellular and/or intracellular mucin [16]. Mucinous carcinomas preferentially affect older women and are usually associated with a good clinical outcome [207–209].
Histologically, mucinous carcinomas feature small cell clusters floating in large amounts of extracellular mucin (Fig. 12.7). Nuclear atypia and mitotic figures are uncommon. There is some controversy on how to diagnose breast carcinomas with mucin production but with marked nuclear pleomorphism. Some advocate that these cases should be called “IC-NSTs with mucin production,” while the term mucinous carcinoma should be used only for those with low-grade histology. The WHO classification, however, accepts the existence of some high-grade mucinous carcinomas. Capella et al. [210] have described two variants, based on cellularity, size of the clusters and cellular patterns: (i) type A, or hypocellular; (ii) type B, or hypercellular. Type B is more frequently associated with neuroendocrine differentiation as a cellular/nuclear pattern and at IHC [181, 211].
Mucinous tumors are typically ER-positive and classified as “luminal” tumors at the transcriptomic level. The histologic variants, type A and type B, have been shown to harbor significantly different gene expression profiles. Mucinous B tumors often display features of neuroendocrine differentiation and transcriptomic profiles remarkably similar to those of neuroendocrine breast cancers [181].
Mucinous carcinomas have been shown to be genomically distinct from IC-NSTs. In a comparative study with grade- and ER-matched IC-NSTs, these tumors significantly less frequently harbored gains of 1q and 16p and losses of 16q and 22q. Notably, no pure mucinous carcinoma displayed concurrent 1q gain and 16q loss, a hallmark genetic feature of low-grade ER-positive IC-NSTs [182]. In addition, mucinous carcinomas have been shown to completely lack PIK3CA mutations, another feature of low-grade ER-positive IC-NSTs [212]. Taken together, these findings suggest that mucinous carcinomas may evolve from a different molecular pathway as compared to usual ER-positive breast cancers.
12.7.2.5 Micropapillary Carcinoma
Pure micropapillary carcinomas (MPCs) account for 0.7–3 % of all breast carcinomas [16]. MPCs display a distinctive growth pattern, featuring clusters of cells with “inverted polarity” [213] surrounded by empty spaces in a spongy stroma [16] (Figs. 12.7 and 12.9). A more objective identification of this subtype can be achieved with the help of IHC analysis with antibodies against epithelial membrane antigen (EMA, aka MUC-1) [214–216], which typically decorate the stroma-facing border of the cell clusters (Fig. 12.9). Importantly, pure MPCs of the breast are associated with a peculiar proclivity for lymphovascular invasion (Fig. 12.9), a high incidence of lymph node metastases and arguably a poorer prognosis than unselected IC-NSTs [214, 217, 218]. Importantly, when compared with IC-NSTs matched for age, tumor size, and grade, peritumoral vascular invasion, IHC-defined molecular subtype, number of positive lymph nodes and year of surgery, micropapillary histologic type did not add any independent information to the risk of locoregional or distant relapse, or overall survival [219]; however, MPCs more frequently presented as locally advanced disease than IC-NSTs [219].
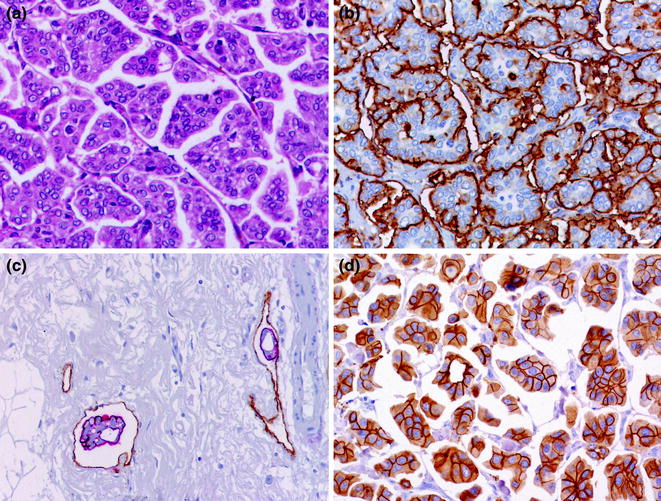

Fig. 12.9
MPC (a H&E; b EMA; c tumor emboli, double staining for EMA (red) and for D2-40 (brown); d HER2)
MPCs are usually ER-positive/HER2-negative with a minority being ER-positive/HER2-positive and accordingly classified at the transcriptomic level as luminal carcinomas [176]. We have observed, however, some rare cases of MPCs with apocrine differentiation, which are ER-negative and frequently HER2-positive. Interestingly, positivity for HER2, even when strong, is typically not complete and is typically mutually exclusive with EMA (Fig. 12.9). This pattern has been taken into account in the latest ASCO/CAP guidelines as a particular category that needs ISH testing to confirm the HER2 status, in case membrane positivity is moderate/intense but incomplete in more than 10 % of cells [220]. Proliferation indices are usually medium to high, leading to the concept MPCs may be closer to luminal B than luminal A carcinomas [184]. This has been corroborated by results obtained with aCGH analysis, which have revealed that: (i) pure and mixed MPCs are remarkably similar at the genetic level [183]; (ii) they harbor a constellation of genetic aberrations that is distinct from that of grade- and ER-matched IC-NSTs [183, 184], and (iii) they typically display genomic features of luminal B breast carcinomas, such as gains of 1q, 8q, 17q, and 20q and losses of 1p 8p, 13q, 16q, and 22q [183, 184, 191].
In a small series of pure MPCs subjected to targeted capture sequencing for genes recurrently mutated in breast cancer and DNA repair-related genes, single cases harbored mutations described in luminal B carcinomas corroborating the concept that MPCs have a constellation of genetic aberrations similar to those of luminal B breast cancers [191]. No pathognomonic mutation or expressed fusion gene underpinning the micropapillary morphology has so far been identified.
12.7.2.6 Invasive Papillary Carcinoma
Invasive papillary carcinoma (IPC) of the breast is a histologic special type that accounts for approximately 1 % of all invasive breast cancers. This is a poorly-characterized histologic special type of breast cancer; thus, clinical features and outcome features are unknown. As already described in general for papillary lesions, histologically they feature arborescent fibrovascular stalks lined by a layer of neoplastic epithelial cells devoid of an intervening myoepithelial cell layer, frankly invading surrounding tissue [128]. Whenever facing a clearly invasive papillary carcinoma in the breast parenchyma, a metastatic carcinoma from another organ to the breast should be ruled out [16].
At the genomic level, one study revealed papillary carcinomas constitute a relatively homogeneous entity displaying a luminal phenotype and lacking HER2 gene amplification. They display relatively simple genomes [185], consistent with those of low-grade ER-positive breast cancers (i.e., 16q losses, 16p gains, and 1q gains) [14], with a significantly lower number of gene copy number aberrations than grade- and ER-matched IC-NSTs. It should be noted that this study included EPCs and SPCs. IPCs have been reported to harbor PIK3CA mutations in about 40 % of cases, a feature of ER-positive IC-NSTs of good prognosis [221]. A recent transcriptomic analysis has revealed that IPCs display lower levels of expression of proliferation-related, cell assembly and organization and cellular movement and migration genes when compared to IC-NSTs of the same histologic grade and ER status [137].
12.7.2.7 Carcinoma with Neuroendocrine Features
Primary neuroendocrine breast carcinoma (NBC) was originally described in 1977 by Cubilla and Woodruff [222], based on the recognition of carcinoid-like structures in breast cancer showing a solid/alveolar pattern. In 2003, the WHO included a category of neuroendocrine tumors that in the latest edition has been rephrased as carcinoma with neuroendocrine features [16]. These lesions, which account for 2–5 % of all BCs, are defined as carcinomas exhibiting morphological features similar to those of neuroendocrine neoplasms of other organs, including the gut [16]. Typically, tumor cells are arranged in solid nests and/or trabeculae separated by delicate fibrovascular stroma; rosettes, peripheral palisading, and solid papillary formations are also considered features of neuroendocrine cancers [16]. Some variants have been described, i.e., solid/carcinoid-like type, usually of low to intermediate grade, the small/oat cell variant and the large cell type, which are both poorly differentiated NBCs [211]. In addition to morphological features, the diagnosis of NBCs relies on the expression of neuroendocrine markers, which ought to be present in >50 % of tumor cells. These apparently easy-to-apply criteria are equivocal and controversial (except for the extremely rare classical small/oat cell carcinomas), since NBC morphology may be highly heterogeneous displaying features shown by non-NBCs. Of note, other histologic types can show neuroendocrine differentiation, such as the cellular mucinous type B (Fig. 12.7) and SPC [16].
These lesions usually affect elderly women and have an indolent clinical behavior; they are typically ER-positive/HER2-negative and at the transcriptomic level have been shown to pertain to luminal A subtype more frequently than to the luminal B subgroup [181]. Interestingly, in a transcriptomic study of mucinous and NBCs, where it was demonstrated that both are transcriptionally distinct from IC-NST, mucinous carcinomas type B and NBCs shared similar transcriptomic profiles [181].
12.7.2.8 Carcinoma with Apocrine Differentiation
Apocrine carcinoma of the breast is a rare subtype of breast carcinoma constituting approximately 1 % of all mammary carcinomas [16]. In routine work, these tumors are often diagnosed using standard hematoxylin and eosin (H&E) staining because of their characteristic cytological features, i.e., cells with abundant eosinophilic and granular cytoplasm, large nuclei with prominent nucleoli, and visible cell membrane (Fig. 12.7). Some authors, however, have suggested that (pure) apocrine carcinoma should be diagnosed only when morphology correlates with the expected immunoprofile, that is, ER-, PR- and HER2-negative and androgen receptor (AR)-positive [223, 224]. The cytomorphological appearance of apocrine differentiation in a breast tumor does not always correspond to a classic apocrine immunophenotype reported in the literature as specific for apocrine tumors [225, 226]. In fact, only 73 % of these carcinomas fulfill the IHC criteria of true apocrine breast tumors (i.e., ER-/PR-/AR+) [226]. Diffuse expression of the 15-kDa gross cystic disease glycoprotein (GCDFP-15) is also a common feature [226], whereas in usual breast carcinomas GCDFP-15 expression is usually focal.
Transcriptomic analyses [176] have suggested that apocrine carcinomas hold heterogeneous gene expression profiles and pertain to multiple molecular subtypes, consistent with the notion that these tumors are unlikely to constitute a distinct entity. As breast carcinomas of any type and grade may display features of apocrine differentiation, these data suggest that it might be more clinically and biologically relevant to identify the group of “molecular apocrine” tumors, which show not only features of apocrine differentiation at the histologic level, but also increased androgen signaling [176]. Recently, Lehmann-Che et al. [227] have described the morphological and IHC features of a series of “molecular apocrine”-defined tumors. From the histologic point of view, the retrospective analysis described them all as IC-NSTs, but only 7 % presented with morphological apocrine features. From the IHC point of view, the signature “HER2(3+) OR GCDFP-15(+)” had a sensitivity and a specificity for molecular apocrine tumors of 94 and 100 %, respectively, and displayed the best possible marker combination to discriminate molecular apocrine carcinomas from basal-like carcinomas in the context ER negative breast cancer [227].
Targeted sequencing analysis on a small subset of apocrine carcinomas (7 cases) has revealed mutations affecting mTOR signaling pathway genes including PIK3CA (2/7) and PTEN genes (3/7) (coupled to PTEN loss of protein expression), and TP53 (2/7) [225]. PIK3CA mutations have been previously described in a small series of benign and malignant apocrine lesions, whereas germline PTEN mutations have been described in patients with Cowden syndrome that are prone to develop breast cancer with apocrine differentiation. Single cases also harbored KRAS and BRAF gene mutations [225].
Given that apocrine differentiation can occur in any histologic type and is associated with heterogeneous molecular profiles, and that, at this moment, the clinical significance of apocrine differentiation is not clear, apart from a definite association with androgen signaling and possible benefit from antiandrogen therapy, the latest WHO classification does not recognize pure apocrine carcinoma. It has been recommended to type an invasive carcinoma according to its structural type and mention the apocrine differentiation. Thus, for instance, according to the WHO classification, a case with non special morphology, but with diffuse apocrine cytology, should be diagnosed as IC-NST with apocrine differentiation [16].
12.7.2.9 Metaplastic Carcinoma
Metaplastic breast carcinoma (MBC) accounts for 0.2–5 % of all invasive breast cancers and is an aggressive histologic type of breast cancer. The adjective metaplastic is actually an “umbrella term” for a heterogeneous group of cancers that are characterized by the presence of neoplastic cells displaying differentiation toward squamous epithelium and/or mesenchymal components, such as spindle, chondroid, osseous, or rhabdoid cells [16, 228]. They are perceived clinically as a single entity, however, there is evidence to demonstrate that these tumors are heterogeneous in regards to their biological characteristics and, potentially, clinical behaviors [16, 228, 229]. For the time being, the WHO adopted a descriptive histologic classification of MBC. Thus, when diagnosing a MBC, one should clearly describe the distinct morphological components present. In fact, this may have some clinical implications, as, for instance, MBCs with complete (>95 % of the tumor) sarcomatoid differentiation display a significantly worse prognosis and seem not to benefit from conventional chemotherapy offered for TNBC patients [230].
As a group, MBCs (>90 %) display a TN phenotype, and frequent expression of basal-markers [228]. Like TNBCs, MBCs are generally characterized by high levels of genetic instability and these two entities also share a similar pattern of gene copy number alterations [231]. At the transcriptomic level, MBCs preferentially have a basal-like or claudin-low molecular subtype and frequently harbor TP53 mutations [231–233]. MBCs with spindle cell morphology are the ones more likely to be classified as claudin-low [228, 234–236], which is not surprising given that these cases display overt mesenchymal differentiation akin to epithelial-to-mesenchymal transition phenotype, a defining-feature of the claudin-low subtype. In addition, it has been shown that MBCs were preferentially of mesenchymal-like and mesenchymal stem-like using the six molecular subtype classification of TNBC [159]. When applying the integrative clustering approach, MBCs were of IntClust 4, IntClust 1, IntClust 8, and IntClust 9 [229].
As a further level of complexity, it has recently been shown that different histologic components of MBCs are associated with specific molecular features [229]. Samples exclusively or predominantly composed of areas of spindle cell metaplasia or chondroid metaplasia were of claudin-low intrinsic molecular subtype and of mesenchymal-like TNBC subtype, respectively, whereas those samples exclusively or predominantly composed of squamous metaplasia were more heterogeneous. These findings imply that the molecular subtype of a metaplastic breast carcinoma may be different depending on the morphologic component sampled and subjected to molecular analysis. It is currently unknown, however, whether the different histologic components of metaplastic breast carcinomas are underpinned by distinct constellations of point mutations, structural genetic rearrangements, and/or epigenetic alterations; however, a previous study has demonstrated that histologically distinct components of a given metaplastic breast carcinoma may harbor different somatic genetic alterations [237].
The information on the mutational landscape of MBCs is still scarce. No pathognomonic mutation or recurrent fusion gene that accounts for the histologic characteristics of these cancers has been identified, and it is unknown whether different repertoires of somatic mutations drive the distinct subtypes of MBCs. The morphologically and phenotypically distinct components of individual MBCs have been shown to be clonal on the basis of the presence of identical TP53 mutations [237]. However, intratumor genetic heterogeneity (i.e., differences in the somatic genetic alterations between phenotypically distinct components of individual cases) is also evident [237]. Mutations in TP53 and PIK3CA, and losses of PTEN and p16 cyclin-dependent kinase inhibitor 2A (CDKN2A) have been recurrently found in MBCs; however, they do not seem to be restricted to tumors of specific phenotypes [231, 237] and are not unique to MBCs. Overexpression and amplification of the epidermal growth factor receptor (EGFR) gene have been reported in a subset of MBCs and seem to be more prevalent in tumors with squamous and/or spindle cell metaplasia [238].
Although MBCs are mostly high-grade and display an aggressive behavior, which appears to be worse than usual TNBCs, there are two low-grade MBC subtypes, namely, fibromatosis-like spindle cell carcinoma and low-grade adenosquamous carcinoma. Both are also of TN phenotype, but carry distinct clinical implications. First, from a histologic point of view, fibromatosis-like spindle cell carcinoma has to be distinguished from desmoid-type fibromatosis and other benign spindle cell lesions of the breast, as, though less than high-grade MBCs, they still have a long-term metastatic potential and must be surgically treated as carcinomas [41, 239]. Low-grade adenosquamous carcinoma display infiltrative borders and a tendency for local recurrence, thus extreme attention to surgical margins are needed. In contrast, the metastatic potential is minimal and therefore chemotherapy should be avoided.
12.7.2.10 Adenoid Cystic Carcinoma
Adenoid cystic carcinomas (AdCCs) are malignant tumors most commonly affecting the salivary glands, but they can also be found in other anatomical sites, including the breast, lungs, and prostate [240]. AdCCs provide a clear example of genotypic–phenotypic correlation, as they display similar histologic characteristics, irrespective of the site of origin, and often harbor the recurrent t(6, 9)(q22–23;p23–24) translocation that results in the formation of the MYB–NFIB fusion gene [188, 240].
The existence of AdCC in the breast has been questioned, given its morphological similarity with cribriform carcinoma [241]. In fact, misdiagnosis between these two entities is not an uncommon event. IHC and ultrastructural studies, however, have proven the existence of true breast AdCC [16, 240]. AdCCs account for 0.1–1 % of all breast cancers [16, 241–243] and their most striking feature is the excellent long-term prognosis, which is in stark contrast with that of salivary gland AdCCs or common-type TNBCs (i.e., IC-NSTs) [240, 241, 243].
At the morphological level AdCCs can feature a variety of growth patterns, including cribriform, trabecular, and solid variants (Fig. 12.7) [243]. Not uncommonly, single cases display all growth patterns. A sebaceous differentiation is found in up to 14 % of cases, and foci of adenosquamous differentiation may also be encountered [243, 244]. The proportion of solid growth has been reported to be clinically meaningful, as Ro et al. [245] have reported tumors with solid components were more likely to develop recurrences; however, independent validation of the prognostic impact of this classification has yet to be reported. In addition, in the same cohort, the only patient who experienced metastases was affected by a high-grade tumor.
Phenotypically, AdCCs display a TN phenotype and express basal CKs (CK5/6). Not surprisingly, at the transcriptomic level AdCCs share similar gene expression patterns with metaplastic and medullary carcinomas within the ER-negative subgroups. From a molecular standpoint, AdCCs often display the recurrent chromosomal translocation t(6, 9)(q22–23;p23–24), which generates a fusion transcript involving the genes MYB and NFIB [188]. The prevalence of the MYB–NFIB fusion gene ranges from 23 to 100 % [188, 192, 246–248]. Differences may be partly explained to sample type (frozen vs. FFPE) and methodology (FISH vs. PCR), and the potential inclusion of other mimics of AdCC (e.g., breast cylindroma) [249]; however, the clinical significance of this observation remains unclear. Although a substantial proportion of AdCCs lack the MYB–NFIB fusion gene, the majority of MYB–NFIB fusion gene-negative salivary gland AdCCs likely displays activation of MYB due to mechanisms other than the t(6, 9) chromosomal translocation [250].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree