Benign tumors
Papillary adenoma
Renal oncocytoma
Metanephric adenoma
Metanephric adenofibroma
Malignant tumors
Clear cell renal cell carcinoma
Multilocular clear cell renal cell neoplasm of low malignant potential
Papillary renal cell carcinoma
Chromophobe renal cell carcinoma
Carcinoma of the collecting ducts of Bellini
Renal medullary carcinoma
Xp11 translocation carcinomas (MiT family translocation renal cell carcinoma)
Mucinous tubular and spindle cell carcinoma
Carcinoma associated with neuroblastoma
Unclassified renal cell carcinoma
Table 2.2
International Society of Urological Pathology (ISUP) Vancouver classification of kidney tumors. Renal tumors defined since the 2004 WHO classification are listed below
Proposed new renal epithelial tumors |
Tubulocystic renal cell carcinoma |
MiT family translocation renal cell carcinoma (including t(6;11) renal cell carcinoma) |
Clear cell (tubulo)papillary renal cell carcinoma |
Hereditary leiomyomatosis renal cell carcinoma syndrome-associated renal cell carcinoma |
Acquired cystic disease-associated renal cell carcinoma |
Proposed emerging/provisional tumor entities |
Thyroid-like follicular renal cell carcinoma |
Succinate dehydrogenase (SDH) B deficiency-associated renal cell carcinoma |
ALK translocation renal cell carcinoma |
Table 2.3
Salient morphologic features of the different renal cell carcinoma types
Renal cell carcinoma (RCC) type | Salient morphologic features | |
|---|---|---|
Clear RCC | Solid, nested, or tubular architecture; thin-walled plexiform vasculature; optically clear cytoplasm | |
Papillary RCC | Papillary, tubular, or solid architecture; frequent hemorrhage and necrosis; foamy macrophages and psammomatous microcalcifications | |
Type 1: Cuboidal epithelium with scant cytoplasm and inconspicuous nucleoli | Type 2: Columnar pseudostratified epithelium with voluminous cytoplasm and prominent nucleoli | |
Chromophobe RCC | Solid, tubular, or nested architecture; thick-walled vasculature; clear to eosinophilic cytoplasm with cytoplasmic membrane accentuation; irregular nuclear membrane border with perinuclear clearing | |
Collecting duct RCC | Medullary centered with tubulopapillary architecture; inflammatory and desmoplastic stroma; high-grade nuclear atypia with dysplasia of adjacent collecting ducts | |
Renal medullary carcinoma | Medullary centered with tubulopapillary, reticular, and microcystic architecture; inflammatory and desmoplastic stroma with prominent neutrophilic infiltrate; high-grade nuclear atypia; sickling of erythrocytes | |
Xp11 translocation carcinoma | Papillary and solid architecture with psammomatous microcalcifications; optically clear cytoplasm with eosinophilic inclusions | |
Mucinous tubular and spindle cell carcinoma | Tubular and spindle cell pattern with mucinous extracellular matrix; low-grade nuclei | |
Clear cell papillary RCC | Cystic tumor with tubulopapillary architecture; clear cytoplasm and apically located, low-grade nuclei | |
Tubulocystic RCC | Cystic tumor embedded in fibrous stroma; clear to eosinophilic cytoplasm and prominent nucleoli | |
Primary thyroid-like follicular carcinoma | Tubular architecture containing eosinophilic colloid-like material; nuclear grooves and pseudoinclusions | |
2.2.1 Clear Cell Renal Cell Carcinoma
Clear cell RCC is the most common type, representing 65–75 % of all RCC in most series [5–7]. These often present as a single solid tumor located at the periphery of the renal parenchyma. A bright yellow or light orange color is most characteristic of clear cell RCC. In addition, there may be areas of cyst formation, hemorrhage, and necrosis. The majority of the carcinomas detected today are confined to the kidney; the rest show gross invasion into the perinephric adipose tissue, the renal sinus adipose tissue, or the renal vein. These carcinomas sometimes extend into the inferior vena cava and, rarely, into the right side of the heart. Tumor cells in clear cell RCC are arranged in sheets, nests, or tubules (Fig. 2.1). One of the hallmark histological features is the presence of delicate, interconnecting, sinusoidal type of thin blood vessels, sometimes likened to “chicken wire” (Fig. 2.2). Most tumor cells have optically clear cytoplasm; however, some tumors can have a combination of cells with clear cytoplasm and granular eosinophilic cytoplasm. Clear cell RCCs almost exclusively composed of cells with eosinophilic cytoplasm are rare. The optically clear appearance of these cells is secondary to the lipid and glycogen content in the cell’s cytoplasm. Periodic acid Schiff (PAS) histochemical stain, with and without diastase, is the best method for demonstrating the glycogen in the cytoplasm. The diagnosis of clear cell RCC is based on a combination of architectural pattern, vascular pattern, and the cytoplasmic characteristics of the tumor cells, rather than on just the tinctorial properties of the cell cytoplasm (Fig. 2.3).
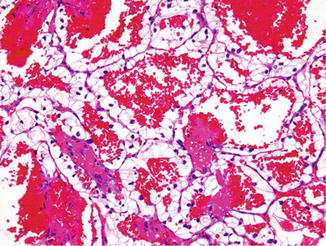
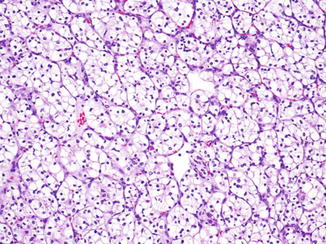


Fig. 2.1
Clear cell renal cell carcinoma, Fuhrman nuclear grade 1, with hemorrhage. H&E stain, 100×

Fig. 2.2
Clear cell renal cell carcinoma, Fuhrman nuclear grade 2, exhibiting typical small nests of clear cells separated by thin sinusoidal blood vessels. H&E stain, 100×

Fig. 2.3
Clear cell renal cell carcinoma, Fuhrman nuclear grade 3, exhibiting clear cells with large nuclei with prominent nucleoli. H&E stain, 100×
2.2.2 Papillary Renal Cell Carcinoma
Papillary renal cell carcinoma accounts for about 10–15 % of all RCC. Multifocal and bilateral tumors are more common in this type of RCC. Grossly, these tumors are soft, friable, have a red-brown cut surface, with abundant hemorrhage and necrosis. Some tumors may appear cystic, with a rind of solid tumor at the periphery and most of the tumor cells in the center suspended within hemorrhagic fluid. Papillary RCC is one of the subtypes of RCC most likely to metastasize to regional lymph nodes. In some cases, the regional lymph node metastases form a larger mass than the primary tumor in the kidney. Papilla formation is the typical histologic feature of this RCC. In addition to the papillae, tumor cells may form tubules, tubulopapillary structures, and, rarely, solid nests. Papillary RCC is divided into type 1 and type 2 tumors based on an array of morphologic features [8, 9]. Type 1 papillary RCCs show thin fibrovascular cores that are lined by a single layer of low cuboidal cells that have scant pale cytoplasm and oval low-nuclear-grade nuclei (Fig. 2.4). In contrast, type 2 papillary RCC has tall columnar pseudostratified cells with abundant eosinophilic cytoplasm and high-grade nuclei (Fig. 2.5). As these RCCs are frequently associated with hemorrhage, hemosiderin pigment may be present in the cell cytoplasm, in adjacent histiocytes, and in the stroma. The presence of foamy macrophages within the fibrovascular stalks and laminated calcifications (psammoma bodies) is more commonly present in type 1 tumors. There is evidence accumulating gradually regarding the genetic and clinical differences of these two types [8, 10].
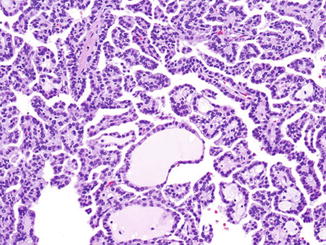
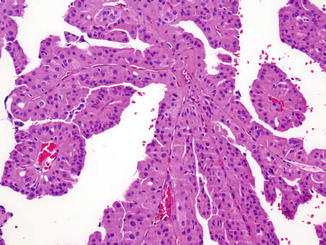

Fig. 2.4
Papillary renal cell carcinoma, type 1. The papillae are lined by cuboidal cells with basophilic cytoplasm and small round nuclei. H&E stain, 100×

Fig. 2.5
Papillary renal cell carcinoma, type 2. The papillae are lined by tall columnar cells with prominent eosinophilic cytoplasm, large nuclei, and prominent nucleoli. H&E stain, 100×
2.2.3 Chromophobe Renal Cell Carcinoma
Chromophobe RCC accounts for about 5 % of all renal carcinomas. This RCC type was first described in 1985 [11, 12] and exhibits distinctive morphologic, biologic, and ultrastructural features that clearly separate it from the other types. There are two morphological variants, typical or classical chromophobe and the eosinophilic variant. This distinction is based on the physical properties of the cell cytoplasm. Tumor cells are arranged in sheets, broad alveoli, or nests, which are separated by variably spaced thick-walled blood vessels. There are two populations of cells, those with clear cytoplasm and some with eosinophilic cytoplasm. Both cell types are usually present in all tumors, with one cell type predominating. Clear cells have abundant clear cytoplasm with a frothy, flocculent, or bubbly appearance (Fig. 2.6). These cells also have a perinuclear halo due to cytoplasmic organelles being pushed to the periphery forming a rim along the cell membrane. This makes the cell membranes appear thick and prominent with a darker hue than the remainder of the cytoplasm; these cells bear a superficial resemblance to plant cells. The eosinophilic cells tend to be smaller and have finely granular eosinophilic cytoplasm, with a variable degree of perinuclear clearing. The nuclei in both cell types are hyperchromatic and frequently binucleated and have a wrinkled nuclear membrane, resembling koilocytes. Hale’s colloidal iron stain is a histochemical stain that is often used for the diagnosis of chromophobe RCC; this stain shows diffuse, reticular staining. At the ultrastructural level, numerous microvesicles are seen in the cell cytoplasm around the nucleus, and the mitochondria have characteristic tubulocystic cristae.
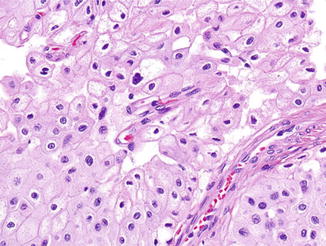

Fig. 2.6
Chromophobe renal cell carcinoma. Tumor cells have pale flocculent cytoplasm, prominent cell membranes, and wrinkled irregular-shaped nuclei. H&E stain, 100×
2.2.4 Collecting Duct Carcinoma
Collecting duct carcinoma is rare, accounting for less than 1 % of all RCCs [13–15]. These tumors arise in the medullary region of the kidney. Microscopically, three features characterize this renal cancer: a tubulopapillary arrangement of cells, desmoplastic reaction of the stroma, and dysplastic changes in the adjacent collecting ducts. Dilated tubules, glands, and solid areas may also be present (Fig. 2.7). These carcinomas tend to be aggressive, and most patients have a short survival time.
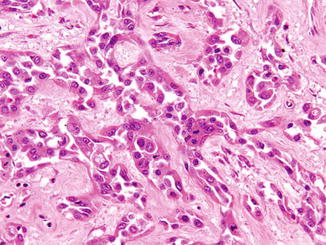

Fig. 2.7
Collecting duct carcinoma of the kidney. The tumor forms glands that are set within dense collagenous stroma. H&E stain, 200×
2.2.5 Renal Medullary Carcinoma
Renal medullary carcinoma is a distinctive type of RCC, which arises in the renal medulla, and is associated with the sickle cell trait [16, 17]. This cancer affects young adults, most of whom present with advanced disease and have an aggressive clinical course. These tumors have distinct morphologic features with reticular, microcystic areas, which resemble testicular yolk sac tumor. Foci of mucin and gland-like areas are also present.
2.2.6 Xp11 Translocation Carcinoma
Translocation carcinomas were first described as papillary RCC with specific translocations; but we now know that these are a separate type of RCC. Xp11 translocation carcinomas are more common in children and young adults, with a female predominance. This RCC type comprises approximately one third of all RCCs affecting the pediatric age group [18]. However, we now know that these are not limited to the younger age group, as numerous cases have been reported in the adult population as well. Translocation RCCs often present as locally advanced tumors with extrarenal disease [19, 20]. As the name suggests, these tumors are characterized by translocation of the TFE3 gene, mapping to the Xp11.2 region, with the following partner genes: PRCC gene t(X;1)(p11.2;q21), ASPL gene t(X;17)(p11.2;q25), and PSF gene t(X;1)(p11.2;p34). Another distinct member of this family of tumors is the RCC with fusion of the alpha and TFEB genes t(6;11)(p21;q12). The typical Xp11.2 translocation RCC has a partially papillary architecture, along with solid nests or sheets of tumor cells. The cells have voluminous clear or pale eosinophilic cytoplasm and may have eosinophilic cytoplasmic inclusions (Fig. 2.8). Psammomatous calcifications may also be present. Regardless of the type of fusion event, there is overexpression of the TFE3 gene, which can be detected by immunohistochemical staining for the nuclear TFE3 protein. Of the confirmatory molecular assays, a break-apart TFE3 fluorescence in situ hybridization (FISH) probe is the most practical since tumor cells can be visualized, and, most importantly, no foreknowledge of the fusion partner is required as with RT-PCR approaches. The TFE family of transcription factors activates downstream targets that results in overexpression of specific proteins, including cathepsin K. Consequently, cathepsin K immunohistochemical stain has been used as a relatively specific immunohistochemical stain for TFE-associated renal carcinomas (both TFE3 and TFEB) as the other common subtypes of RCC, given their different pathogenesis, are cathepsin K negative [21]. The other immunohistochemical stains that may be positive include CD10, cytokeratin, EMA, and vimentin.
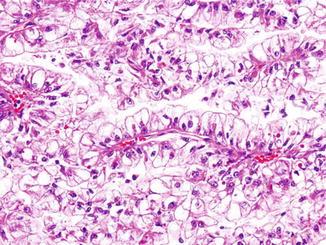

Fig. 2.8
Xp11 translocation carcinoma of the kidney. The tumor forms papillary structures lined by cells with abundant clear cytoplasm. Few cells also have prominent eosinophilic intracytoplasmic inclusions. H&E stain, 100×
2.2.7 Mucinous Tubular and Spindle Cell Carcinoma
Mucinous tubular and spindle cell carcinoma (MTSCC) is a morphologically distinct type of RCC, which superficially resembles papillary RCC. These RCCs are usually small and organ confined; however, sarcomatoid dedifferentiation and metastases have been reported to occur [22–24]. MTSCCs are composed of tubules lined by cuboidal cells that are set within a loose stroma with blue mucin. Foci of bland-appearing spindle cells are also present. The amount of the different components varies from tumor to tumor, with some tumors having more spindle cells than others.
2.2.8 Carcinoma Associated with Neuroblastoma
Pediatric patients who survive childhood neuroblastoma have been reported to have an increased incidence of RCCs. Although only a handful of tumors have been systematically studied, these tumors have a distinctive morphologic appearance. The tumors have varying histologic patterns and have cells with copious eosinophilic or oncocytic cytoplasm [25, 26].
2.2.9 Unclassified Renal Cell Carcinoma
Unclassified RCC is not a distinct type, but rather is a designation for RCC that does not fit into one of the abovementioned categories. As science advances and we develop a better understanding of these rare tumors, other specific types will emerge from the unclassified group. At present, this designation is a sort of “wastebasket” term for tumors that do not neatly fit into any of the usual types listed above. RCCs in this category also include tumors that are composites of the usual types, for example, clear cell RCC and papillary RCC, RCC with extensive necrosis and minimal viable tumor, and RCC with sarcomatoid dedifferentiation where there is a minimal epithelial component that cannot be readily assigned to one of the above categories [3].
2.2.10 Sarcomatoid Dedifferentiation in Renal Cell Carcinoma
The use of the term sarcomatoid RCC was abandoned in the current classification system, as all types of RCC may undergo this transformation. Sarcomatoid dedifferentiation is reported in approximately 2–5 % of all RCC [27], though it is more frequent in advanced disease comprising approximately 20 % of stage IV RCC [28, 29]. The term sarcomatoid dedifferentiation denotes anaplastic transformation of the RCC into a high-grade biphasic tumor that has both malignant elements, that is, carcinomatous and sarcomatous (resembling a mesenchymally derived sarcoma). The carcinomatous component may have any nuclear grade but is usually high grade, usually a Fuhrman nuclear grade 3 (Fig. 2.9a). The sarcomatous component may be undifferentiated, resembling a pleomorphic malignant fibrous histiocytoma (MFH) or an unclassified spindle cell sarcoma (Fig. 2.9b), or rarely may show differentiation (heterologous differentiation) into the bone, cartilage, skeletal muscle, or blood vessels. The differential diagnosis for these tumors also includes primary sarcomas of the kidney, which are rare tumors. The presence of a distinct carcinoma component helps separate primary sarcomas from RCC with sarcomatoid dedifferentiation. Benign spindle cells are sometimes seen in RCC, which need to be differentiated from a true sarcomatoid component. The majority of these tumors present at high stage with poor prognosis. The amount of sarcomatoid dedifferentiation (as a percentage of the entire tumor) has historically shown some prognostic value as patients with more than 50 % sarcomatoid dedifferentiation in their tumor tend to do poorly. This point is controversial, however, as RCCs with even a minor sarcomatoid component (5–15 %) have been reported to result in metastasis and cancer-specific death [30, 31], and some recent data suggest no overall correlation between percentage of sarcomatoid elements and cancer-specific mortality [29].
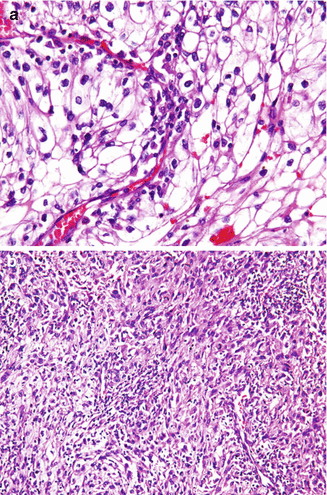

Fig. 2.9
Clear cell renal cell carcinoma with sarcomatoid dedifferentiation. The epithelial component consists of Fuhrman nuclear grade 3 clear cell renal cell carcinoma (a). The sarcomatoid component has high-grade spindle cells resembling a soft tissue sarcoma (b). H&E stain, 100×
2.2.11 New and Rare Renal Cell Carcinoma Types
Since the publication of the WHO RCC classification, newer, rare subtypes have been described. These are briefly described below and are listed in Table 2.2.
Tubulocystic RCC in the past has been referred to as low-grade collecting duct carcinoma. Tubulocystic carcinoma is a circumscribed, exclusively cystic tumor with interspersed fibrous stroma. Neoplastic cells with clear or eosinophilic cytoplasm and prominent nucleoli line tubules and cysts of varying caliber. Metastatic disease has been documented to occur with these tumors [32, 33].
Clear cell (tubulo) papillary RCC is another novel type seen in association with ESRD, though it can as well be seen in patients without ESRD. This tumor, arising in a cystic background, is arranged in a predominantly papillary pattern with neoplastic cells containing clear cytoplasm and whose nuclei are low grade (Fuhrman grade 2) and oriented toward the apex of the cell. All clear cell papillary RCCs have been organ confined, with no metastases reported [34, 35].
Hereditary leiomyomatosis RCC (HLRCC) syndrome-associated RCC is another rare tumor seen in patients with the HLRCC familial syndrome, an autosomal dominant syndrome with germ line mutations of the fumarate hydratase gene. These patients also have uterine and subcutaneous leiomyomas. This tumor superficially resembles type 2 papillary renal cell carcinoma with a tubulopapillary architecture and large cells with eosinophilic cytoplasm. However, the characteristic morphologic feature is the large prominent cherry red nucleolus surrounded by a clear halo. This RCC type is highly aggressive, with most patients presenting with metastatic disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree