Partial Mastectomy
CRITICAL ELEMENTS
Tumor Resection
Tumor Localization
Intraoperative Specimen Imaging
Specimen Orientation
Clip Placement for Radiation Targeting in Oncoplastic Cases
1. TUMOR RESECTION
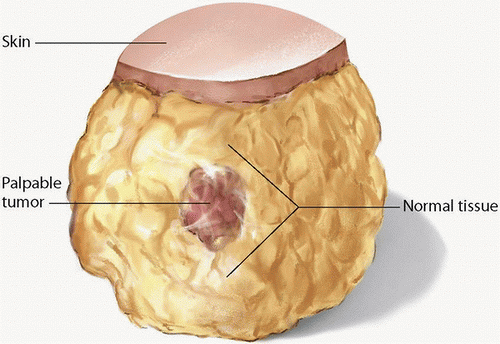
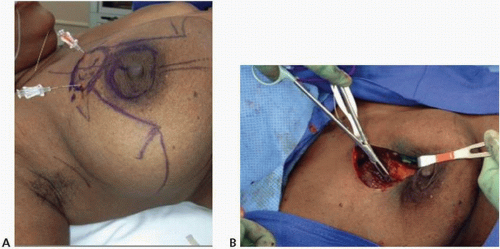
Recommendation: The surgeon should aim to resect all gross disease with microscopically negative margins without violation of the tumor itself during the dissection (Fig. 1-1).
Type of Data: Period after trials.
Strength of Recommendation: The group strongly recommends performing partial mastectomy with the goal of resecting all gross disease with microscopically negative margins. This recommendation, which can be applied to most patients in most circumstances, is based on multiple randomized prospective studies demonstrating the benefits of the approach.
Rationale
In breast cancer patients undergoing partial mastectomy, surgical margins help determine future treatment. Because positive surgical margins are associated with an increased risk of local recurrence and, consequently, systemic disease, the primary goal of partial mastectomy is to remove the tumor with a negative microscopic margin. The primary goal of partial mastectomy in the operating room is to remove the cancer with an envelope of normal tissue around it to obtain negative margins (i.e., to achieve
an R0 resection; see Fig. 1-1) while maintaining optimal breast cosmesis. In the operating room, surgeons must be cognizant of encompassing the tumor with normal tissue around it. For patients with tumors involving the skin and/or chest wall, en bloc resection that includes the overlying skin and/or underlying muscle should be performed. The oncologic safety of breast conservation has been proven over many years, multiple randomized studies having shown that local recurrence rates following lumpectomy with negative margins plus radiotherapy are significantly lower than those following lumpectomy with a positive margin plus radiotherapy.
an R0 resection; see Fig. 1-1) while maintaining optimal breast cosmesis. In the operating room, surgeons must be cognizant of encompassing the tumor with normal tissue around it. For patients with tumors involving the skin and/or chest wall, en bloc resection that includes the overlying skin and/or underlying muscle should be performed. The oncologic safety of breast conservation has been proven over many years, multiple randomized studies having shown that local recurrence rates following lumpectomy with negative margins plus radiotherapy are significantly lower than those following lumpectomy with a positive margin plus radiotherapy.
The width of negative surgical margins necessary to minimize local recurrence in patients undergoing breast cancer surgery, including partial mastectomy, remain controversial. The National Surgical Adjuvant Breast and Bowel Project has long taken the position that unless tumor cells are immediately adjacent to the inked surface of the lumpectomy specimen, no further excision is needed. Long-term results of this approach and recurrence rates have been published.1
For example, the B-06 study, which randomized patients to lumpectomy alone versus lumpectomy plus radiation versus total mastectomy, has shown acceptable local recurrence rates with lumpectomy plus radiation compared to mastectomy with a greater than 20-year follow-up. Other randomized prospective trials from the National Cancer Institute, Veronesi et al, and the European Organization for Research and Treatment of Cancer (trial 10801) have shown similar results, reporting 5-year local recurrence rates of about 10% for patients who undergo lumpectomy with no tumor at the inked margin plus radiotherapy.2,3,4 Unfortunately, these trials’ findings are inconclusive, as the investigators did not know whether the surgeons resected additional tissue in cases involving “close” margins or “ink on tumor.” Some thoughtful meta-analyses suggest that obtaining margins greater than 1 mm does not improve rates of local control.5,6,7 Regardless, virtually all studies investigating surgical margins in breast cancer surgery
have shown that patients in whom positive margins are retained have higher recurrence rates than do patients in whom negative margins are achieved.8 A recent consensus statement from the Society of Surgical Oncology and American Society of Radiation Oncology concluded, “The use of no ink on tumor as the standard for an adequate margin in invasive cancer in the era of multidisciplinary therapy is associated with low rates of ipsilateral breast tumor recurrence and has the potential to decrease re-excision rates, improve cosmetic outcomes, and decrease health care costs.”9,10 Critics of this meta-analysis comment that the majority of the studies used were retrospective and details about adjuvant treatment were lacking, including radiation. Some surgeons still advocate a more individualized approach. When making decisions about treatment after lumpectomy, the care team should carefully consider the final pathologic assessment of surgical margins in a multidisciplinary fashion.11
have shown that patients in whom positive margins are retained have higher recurrence rates than do patients in whom negative margins are achieved.8 A recent consensus statement from the Society of Surgical Oncology and American Society of Radiation Oncology concluded, “The use of no ink on tumor as the standard for an adequate margin in invasive cancer in the era of multidisciplinary therapy is associated with low rates of ipsilateral breast tumor recurrence and has the potential to decrease re-excision rates, improve cosmetic outcomes, and decrease health care costs.”9,10 Critics of this meta-analysis comment that the majority of the studies used were retrospective and details about adjuvant treatment were lacking, including radiation. Some surgeons still advocate a more individualized approach. When making decisions about treatment after lumpectomy, the care team should carefully consider the final pathologic assessment of surgical margins in a multidisciplinary fashion.11
2. TUMOR LOCALIZATION
Recommendation: Image-guided localization is necessary for nonpalpable lesions.
Type of Data: This recommendation is based on data from single-institution studies and small randomized controlled trials.
Strength of Recommendation: The group strongly recommends using imageguided tumor localization in patients with nonpalpable tumors.
Rationale
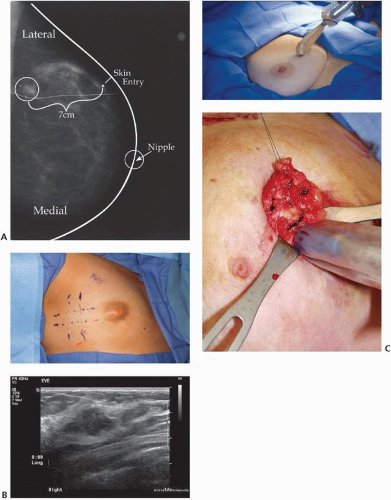
Most breast cancers, many of them nonpalpable, are detected by radiographic screening such as mammography. These nonpalpable lesions require image-guided localization to help maximize the likelihood that negative surgical margins will be achieved. Image-guided tumor localization is guided by the modality used to detect the tumor: mammography,12 ultrasonography,13 or magnetic resonance imaging14 (Fig. 1-2). For lesions that span a large area (a large segment of malignant calcifications), bracketing the area by the use of wires or other markers to delineate the area improves the likelihood that the surgeon is able to completely remove the targeted lesions.15
The most commonly used modality for image-guided tumor localization is wire localization. Despite its very high success rate, wire localization has several drawbacks, including a high positive margin rate,16 wire migration,17 wire transection,18 and surgery scheduling issues because it is performed on the same day, limiting the times and number of procedures performed per day. Alternatives to wire localization (see Fig. 1-2) include radioactive seed localization (RSL),19 radioguided occult lesion localization (ROLL),20 and intraoperative ultrasonography localization. Retrospective studies have shown that these techniques can decrease the volume of tissue excised and the rate of positive margins.21 Single-center institutional series and small randomized trials have shown that, in experienced hands, these techniques can be used to delineate tumors with an accuracy similar to that of wire localization and may have positive margin rates that are lower than that of wire localization.22,23,24,25,26,27 Regardless of the method of localization, the tumor targeted
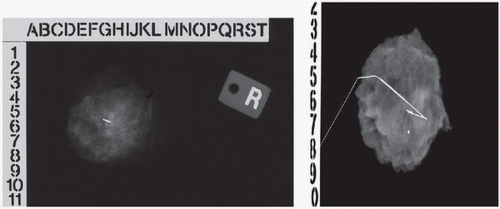
for excision must be documented by specimen radiography28,29 (see Fig. 1-3). Even for palpable lesions, where the surgeon’s physical examination guides the location of the incision and the amount of tissue to be resected, the use of intraoperative ultrasonography has been shown to decrease the volume of tissue excised and the rate of positive margins.30,31
for excision must be documented by specimen radiography28,29 (see Fig. 1-3). Even for palpable lesions, where the surgeon’s physical examination guides the location of the incision and the amount of tissue to be resected, the use of intraoperative ultrasonography has been shown to decrease the volume of tissue excised and the rate of positive margins.30,31
3. INTRAOPERATIVE SPECIMEN IMAGING
Recommendation: Resection of the target lesion must be confirmed with intraoperative imaging studies.
Type of Data: This recommendation is based on data from retrospective studies.
Strength of Recommendation: The group strongly recommends performing intraoperative imaging studies to confirm the resection of the target area. This recommendation is based on data from retrospective studies, which have clearly shown that the benefits of intraoperative specimen imaging outweigh its risks to the patient, and is in agreement with the recommendations of previous consensus panels.
Rationale
The use of intraoperative imaging to confirm the resection of nonpalpable lesions has been the standard of care for the last 10 years. In 2001 (and again in both 2005 and 2009), the Image-Detected Breast Cancer: State-of-the-Art Diagnosis and Treatment Consensus Panel made the following recommendation:
“Specimen radiography or specimen ultrasonography should be routinely performed for all excisions of image-detected abnormalities to help document the success of the procedure in finding the target. Specimen radiography should use two 90-degree orthogonal views. Compression of the specimen is not needed to obtain adequate images and should be avoided. Such compression can fracture the specimen and create false (artifactual) margins after inking.”32
In addition, multiple retrospective studies have shown that the positive margin and re-excision rates of cases in which intraoperative imaging has been performed are lower than those of cases in which intraoperative imaging has not been performed.33,34
In addition, investigators who have examined the utility of the RSL technique have recommended that even in cases in which radioactive seeds have been utilized for tumor localization, specimen radiography is needed to confirm that the marker clip and seeds have been removed with the specimen.35 Finally, the American Society of Breast Surgeons recommends that specimen radiography or specimen ultrasonography be performed routinely during the excision of abnormalities detected with imaging studies to document the removal of the target lesion.36
In addition, investigators who have examined the utility of the RSL technique have recommended that even in cases in which radioactive seeds have been utilized for tumor localization, specimen radiography is needed to confirm that the marker clip and seeds have been removed with the specimen.35 Finally, the American Society of Breast Surgeons recommends that specimen radiography or specimen ultrasonography be performed routinely during the excision of abnormalities detected with imaging studies to document the removal of the target lesion.36
4. SPECIMEN ORIENTATION
Recommendation: During resection, specimen orientation should be achieved either by staining or painting the specimen or by marking the specimen with three sutures to facilitate margin assessment by the pathologist.
Type of Data: Small retrospective studies.
Strength of Recommendation: The procedure has a low burden of risk, but the data supporting its use, which are drawn from retrospective studies, are not strong. The group strongly recommends employing some form of specimen orientation to enable the pathologist to report surgical margins as accurately as possible. This information is important for planning any future surgery to excise remaining positive margins and for planning adjuvant radiotherapy.
Rationale
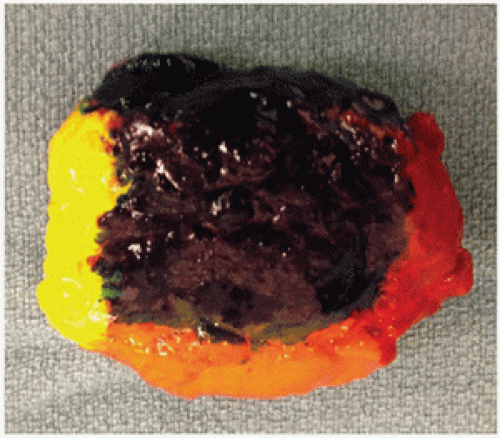
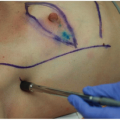
Specimen orientation at the time of excision enables the pathologist to replicate the specimen’s anatomical position during pathologic evaluation. For consistency in reporting and communication, the surface of the lumpectomy specimen is commonly delineated into superficial, deep, superior, inferior, medial, and lateral sides. The orientation method that is least equivocal and most likely to minimize discordance between surgery and pathology settings appears to be intraoperatively marking each of the six sides of the specimen with inks or paints of different colors immediately following resection (Fig. 1-4).
Another option for specimen orientation is to use three sutures to mark the anterior, lateral, and superior sides of the specimen. Specimen orientation with two sutures, which has been reported to result in high rates of discordance between surgery and pathology settings, is not recommended. The recommendation to use three rather than two sutures is primarily based on the findings of a limited prospective analysis of specimen disorientation using the two-suture technique and a retrospective analysis of intraoperative inking versus standard postoperative inking after initial orientation using the three-suture technique.37 Most surgeons rely on the final postoperative pathologic evaluation for final margin assessment in order to decide whether additional tissue must be removed. Regardless of whether the pathologist examines the specimen during or after surgery, reliable specimen orientation is required to facilitate the decision to remove additional tissue of specific margins.38,39
Another option for specimen orientation is to use three sutures to mark the anterior, lateral, and superior sides of the specimen. Specimen orientation with two sutures, which has been reported to result in high rates of discordance between surgery and pathology settings, is not recommended. The recommendation to use three rather than two sutures is primarily based on the findings of a limited prospective analysis of specimen disorientation using the two-suture technique and a retrospective analysis of intraoperative inking versus standard postoperative inking after initial orientation using the three-suture technique.37 Most surgeons rely on the final postoperative pathologic evaluation for final margin assessment in order to decide whether additional tissue must be removed. Regardless of whether the pathologist examines the specimen during or after surgery, reliable specimen orientation is required to facilitate the decision to remove additional tissue of specific margins.38,39
5. CLIP PLACEMENT FOR RADIATION TARGETING IN ONCOPLASTIC CASES
Recommendation: In cases involving oncoplastic techniques, localization of the lumpectomy cavity with surgical clips is critical for the accurate planning of whole- or partial-breast irradiation.
Type of Data: This recommendation is based on observational studies only.
Strength of Recommendation: Placing surgical clips is a low-risk procedure, and it is not burdensome to the patient. The group strongly recommends marking the lumpectomy cavity with surgical clips to facilitate radiotherapy planning in cases involving oncoplastic tissue rearrangement.
Rationale
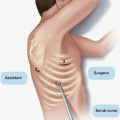
Clip placement in cases where oncoplastic techniques are utilized for immediate reconstruction is essential due to the uncertainty about the location of the excision cavity that can affect the targeting of breast irradiation, as well as the radiation boost delivered as part of whole-breast radiotherapy. Oncoplastic techniques often require that breast tissue adjacent to the tumor bed be detached from the pectoralis major muscle and mobilized to close the excision cavity and subsequently results in little or no seroma formation, making it difficult to visualize the lumpectomy cavity at the time of radiotherapy planning. In addition, because the breast tissue is rearranged, the position of the tumor bed may shift, further complicating radiotherapy planning.39 Furthermore, the surgical scar is a poor indicator of the location of the excision cavity and cannot be reliably used to direct radiotherapy planning. The placement of surgical clips for the localization of the lumpectomy cavity after tumor resection and before breast reconstruction facilitates the accurate delivery of radiation to the tumor bed (Fig. 1-5).
Movement of the tumor bed owing to immediate breast remodeling after lumpectomy is not uncommon. In one retrospective study, the tumor bed, which had been marked with surgical clips, had extended beyond its original breast quadrant or had migrated completely to a different region in 8 (73%) of 11 patients at the time of
radiotherapy planning.40 In another study evaluating 50 patients’ radiation target field plans, which were based on surgical clip location, simulations revealed that the absence of surgical clips would have resulted in inadequate radiation doses in tangent fields in 4 (8%) patients and totally or marginally missed radiation boost targets in 23 (46%) patients.41 Placing surgical clips provides a reliable means of delineating the tumor bed. In a study of 40 breast conservation patients, breast irradiation plans revealed that, compared with no surgical clip placement, the placement of surgical clips at the four cardinal points of the lumpectomy cavity resulted in a higher conformity index of tumor bed delineation and less interobserver variability among radiation oncologists.42 Another study of 30 patients demonstrated that the relative positions of the implanted surgical clips remained consistent throughout the course of radiotherapy.43
radiotherapy planning.40 In another study evaluating 50 patients’ radiation target field plans, which were based on surgical clip location, simulations revealed that the absence of surgical clips would have resulted in inadequate radiation doses in tangent fields in 4 (8%) patients and totally or marginally missed radiation boost targets in 23 (46%) patients.41 Placing surgical clips provides a reliable means of delineating the tumor bed. In a study of 40 breast conservation patients, breast irradiation plans revealed that, compared with no surgical clip placement, the placement of surgical clips at the four cardinal points of the lumpectomy cavity resulted in a higher conformity index of tumor bed delineation and less interobserver variability among radiation oncologists.42 Another study of 30 patients demonstrated that the relative positions of the implanted surgical clips remained consistent throughout the course of radiotherapy.43
REFERENCES
1. Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347(16):1233-1241.
2. van Tienhoven G, Voogd AC, Peterse JL, et al. Prognosis after treatment for loco-regional recurrence after mastectomy or breast conserving therapy in two randomised trials (EORTC 10801 and DBCG-82TM). EORTC Breast Cancer Cooperative Group and the Danish Breast Cancer Cooperative Group. Eur J Cancer 1999;35(1):32-38.
3. Jacobson JA, Danforth DN, Cowan KH, et al. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med 1995;332(14):907-911.
4. Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347(16):1227-1232.
5. Morrow M, Harris JR, Schnitt SJ. Surgical margins in lumpectomy for breast cancer—bigger is not better. N Engl J Med 2012;367(1):79-82.
6. Revesz E, Kahn SA. What are safe margins of resection for invasive and in situ breast cancer? Oncology (Williston Park) 2011;25(10):890-895.
7. Singletary SE. Surgical margins in patients with early-stage breast cancer treated with breast conservation therapy. Am J Surg 2002;184(5):383-393.
8. Kopans DB, Meyer JE. Versatile spring hookwire breast lesion localizer. AJR Am J Roentgenol 1982;138(3):586-587.
9. Houssami N, Macaskill P, Marinovich ML, et al. The association of surgical margins and local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy: a meta-analysis. Ann Surg Oncol 2014;21:717-730.
10. Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with wholebreast irradiation in stages I and II invasive breast cancer. J Clin Oncol 2014;32:1507-1515.
11. Hunt KK, Sahin AA. Too much, too little, or just right? Tumor margins in women undergoing breast-conserving surgery. J Clin Oncol 2014;32:1401-1406.
12. Homer MJ, Pile-Spellman ER. Needle localization of occult breast lesions with a curved-end retractable wire: technique and pitfalls. Radiology 1986;161(2):547-548.
13. Kopans DB, Meyer JE, Lindfors KK, et al. Breast sonography to guide cyst aspiration and wire localization of occult solid lesions. AJR Am J Roentgenol 1984;143(3):489-492.
14. Landheer ML, Veltman J, van Eekeren R, et al. MRI-guided preoperative wire localization of nonpalpable breast lesions. Clin Imaging 2006;30(4):229-233.
15. Liberman L, Kaplan J, Van Zee KJ, et al. Bracketing wires for preoperative breast needle localization. AJR Am J Roentgenol 2001;177(3):565-572.
16. Kurniawan ED, Wong MH, Windle I, et al. Predictors of surgical margin status in breast-conserving surgery within a breast screening program. Ann Surg Oncol 2008;15(9):2542-2549.
17. Davis PS, Wechsler RJ, Feig SA, et al. Migration of breast biopsy localization wire. AJR Am J Roentgenol 1988;150(4):787-788.
18. Homer MJ. Transection of the localization hooked wire during breast biopsy. AJR Am J Roentgenol 1983;141(5):929-930.
19. McGhan LJ, McKeever SC, Pockaj BA, et al. Radioactive seed localization for nonpalpable breast lesions: review of 1,000 consecutive procedures at a single institution. Ann Surg Oncol 2011;18(11):3096-3101.
20. van der Ploeg IM, Hobbelink M, van den Bosch MA, et al. ‘Radioguided occult lesion localisation’ (ROLL) for non-palpable breast lesions: a review of the relevant literature. Eur J Surg Oncol 2008;34(1):1-5.
21. Barentsz MW, van Dalen T, Gobardhan PD, et al. Intraoperative ultrasound guidance for excision of non-palpable invasive breast cancer: a hospital-based series and an overview of the literature. Breast Cancer Res Treat 2012;135(1):209-219.
22. Fortunato L, Penteriani R, Farina M, et al. Intraoperative ultrasound is an effective and preferable technique to localize non-palpable breast tumors. Eur J Surg Oncol 2008;34(12):1289-1292.
23. Lovrics PJ, Cornacchi SD, Vora R, et al. Systematic review of radioguided surgery for non-palpable breast cancer. Eur J Surg Oncol 2011;37(5):388-397.
24. Lovrics PJ, Goldsmith CH, Hodgson N, et al. A multicentered, randomized, controlled trial comparing radioguided seed localization to standard wire localization for nonpalpable, invasive and in situ breast carcinomas. Ann Surg Oncol 2011;18(12):3407-3414.
25. Ngo C, Pollet AG, Laperrelle J, et al. Intraoperative ultrasound localization of nonpalpable breast cancers. Ann Surg Oncol 2007;14(9):2485-2489.
26. Olsha O, Shemesh D, Carmon M, et al. Resection margins in ultrasound-guided breast-conserving surgery. Ann Surg Oncol 2011;18(2):447-452.
27. Rahusen FD, Bremers AJ, Fabry HF, et al. Ultrasound-guided lumpectomy of nonpalpable breast cancer versus wire-guided resection: a randomized clinical trial. Ann Surg Oncol 2002;9(10):994-998.
28. Britton PD SL, Yamamoto AK, Koo B, et al. Breast surgical specimen radiographs: how reliable are they? Eur J Radiol 2011;79(2):245-2459.
29. Cox CE, Furman B, Stowell N, et al. Radioactive seed localization breast biopsy and lumpectomy: can specimen radiographs be eliminated? Ann Surg Oncol 2003;10(9):1039-1047.
30. Davis KM, Hsu CH, Bouton ME, et al. Intraoperative ultrasound can decrease the re-excision lumpectomy rate in patients with palpable breast cancers. Am Surg 2011;77(6):720-725.
31. Fisher CS, Mushawah FA, Cyr AE, et al. Ultrasound-guided lumpectomy for palpable breast cancers. Ann Surg Oncol 2011;18(11):3198-3203.
32. Silverstein MJ, Lagios MD, Recht A, et al. Image-detected breast cancer: state of the art diagnosis and treatment. J Am Coll Surg 2005;201(4):586-597.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree